Abstract
Cancer chemotherapeutics like paclitaxel and oxaliplatin produce a dose-limiting chronic sensory peripheral neuropathy that is often accompanied by neuropathic pain. The cause of the neuropathy and pain is unknown. In animal models, paclitaxel-evoked and oxaliplatin-evoked painful peripheral neuropathies are accompanied by an increase in the incidence of swollen and vacuolated mitochondria in peripheral nerve axons. It has been proposed that mitochondrial swelling and vacuolation are indicative of a functional impairment and that this results in a chronic axonal energy deficiency that is the cause of the neuropathy’s symptoms. However, the significance of mitochondrial swelling and vacuolation is ambiguous and a test of the hypothesis requires a direct assessment of the effects of chemotherapy on mitochondrial function. The results of such an assessment are reported here. Mitochondrial respiration and ATP production were measured in rat sciatic nerve samples taken 1–2 days after and 3–4 weeks after induction of painful peripheral neuropathy with paclitaxel and oxaliplatin. Significant deficits in Complex I-mediated and Complex II-mediated respiration and significant deficits in ATP production were found for both drugs at both time points. In addition, prophylactic treatment with acetyl-L-carnitine, which inhibited the development of paclitaxel-evoked and oxaliplatin-evoked neuropathy, prevented the deficits in mitochondrial function. These results implicate mitotoxicity as a possible cause of chemotherapy-evoked chronic sensory peripheral neuropathy.
Keywords: acetyl-L-carnitine, bioenergetics, mitotoxicity, neuropathic pain, sensory neuropathy
Introduction
Cancer chemotherapeutics in the taxane and platinum-complex classes have the same dose-limiting side effect – a chronic, distal, symmetrical, sensory peripheral neuropathy that is often accompanied by a neuropathic pain syndrome (Binder et al., 2007; Cata et al., 2006; Dougherty et al., 2004, 2007; Quasthoff and Hartung, 2002). In the rat, paclitaxel-evoked and oxaliplatin-evoked chronic painful peripheral neuropathies are associated with a significant increase in the incidence of swollen and vacuolated mitochondria in peripheral nerve axons (Flatters & Bennett, 2006; Jin et al., 2008; Xiao et al., 2011a).
It has been hypothesized that the swelling and vacuolation are signs of impaired mitochondrial function, and that this results in a chronic axonal energy deficit that is the proximate cause of all of the neuropathy’s symptoms (Bennett et al., 2011; Flatters & Bennett, 2006; Jin et al., 2008). However, the significance of mitochondrial swelling and vacuolation is ambiguous. Mitochondrial swelling and vacuolation are known to be reversible (Nowikovsky et al., 2009), they are not uncommonly seen in the axons of normal animals (Flatters & Bennett, 2006; Jin et al., 2008; Xiao et al., 2011a,b), and there is evidence that they may result when mitochondria operating at a high rate of activity are exposed to aldehyde fixatives (Brewer & Lynch, 1986). Thus, a clear test of the hypothesis requires direct measurement of mitochondrial function in peripheral nerve samples from chemotherapy-treated animals.
In the experiments reported here, mitochondrial function was assessed directly via assays for respiration and ATP production in sciatic nerves obtained from rats with paclitaxel-evoked and oxaliplatin-evoked painful peripheral neuropathy. In addition, the hypothesis predicts that a pharmacological intervention that protects mitochondria should protect against the chemotherapy-evoked neuropathy. Prophylactic dosing with acetyl-L-carnitine (ALCAR) prevents paclitaxel-evoked and oxaliplatin-evoked neuropathic pain and it has been proposed that this is due to a mitochondrial protective action (Flatters et al., 2006; Jin et al., 2008; Xiao et al., 2011a). Here, we confirmed ALCAR’s behavioral effect and determined whether it was indeed accompanied by protection against chemotherapy-evoked mitotoxicity. Preliminary results have appeared in abstract form (Zheng et al., 2010).
Method
These experiments conformed to the ethics guidelines of the International Association for the Study of Pain (Zimmermann, 1983), the National Institutes of Health (USA), the Canadian Institutes of Health Research, and the Canadian Council on Animal Care. All experimental protocols were approved by the Animal Care Committee of the Faculty of Medicine, McGill University.
Animals
Adult male Sprague-Dawley rats (175–250 g, Harlan Inc., Indianapolis, IN; Frederick, MD breeding colony) were housed on sawdust bedding in plastic cages. Artificial lighting was provided on a fixed 12 hour light-dark cycle with food and water available ad libitum.
Chemotherapy models
Paclitaxel (Taxol®) was administered as described previously: a stock solution (6 mg/ml in Cremophor/EL; Biolyse Pharma Corp.; St. Catherines, ON, Canada) was diluted with saline to a concentration of 2 mg/ml and injected IP at 2 mg/kg on four alternate days (D0, D2, D4 and D6) for a cumulative dose of 8 mg/kg (Flatters and Bennett, 2004; Polomano et al., 2001). Control animals received matched injections of the vehicle.
Oxaliplatin (Eloxatin®; Sanofi-Aventis) was administered as described elsewhere (Xiao et al., 2011a). The stock solution (5 mg/ml) was diluted to 2 mg/ml with 5% dextrose in distilled water and injected IP at 2 mg/kg on five consecutive days (D0–D4) for a cumulative dose of 10 mg/kg. Control animals received matched injections of vehicle.
Behavioral measures
Assays for chemotherapy-evoked mechano-allodynia and mechano-hyperalgesia used von Frey hair stimuli as described previously (Flatters and Bennett, 2004). Briefly, 4 g and 15 g von Frey hairs were applied to the plantar hind paws (5 times per side) and the percentage of applications that resulted in a withdrawal reflex were recorded. Normal rats rarely respond to the 4 g stimulus and this evokes a touch sensation when applied to ourselves (at the volar wrist, where the skin thickness is comparable to the rat’s hind paw skin). An increase in withdrawal responses to the 4 g stimulus is thus indicative of mechano-allodynia. In the normal rat, the 15 g stimulus evokes a withdrawal reflex about 20% of the time; this stimulus evokes a pricking pain sensation when applied to ourselves. An increase in withdrawal responses to the 15 g stimulus is thus indicative of mechano-hyperalgesia.
Mitochondrial respiration assay
In preliminary studies, we found that it was possible to measure respiration in a preparation of isolated mitochondria obtained from sciatic nerves via homogenization and centrifugation (Frezza et al., 2007). However, we feared that if there were a mitotoxic effect, then damaged mitochondria might be relatively unlikely to survive the harsh procedures required for isolation. Instead, we developed a tissue preparation generated by mincing the sciatic nerves into 1–1.5 mm segments and then teasing each segment apart into microfilaments using #5 watchmaker’s forceps (Fig. 1).
Fig. 1.
Nerve preparation for mitochondrial respiration and ATP assays. The sciatic nerves were excised and minced into 1–1.5 mm segments (inset) which were then teased apart into microfilaments (stained here with toluidine blue for illustration).
Rats were deeply anesthetized with isoflurane (2% in 95% oxygen; flow rate 250 ml/min) and their sciatic nerves were exposed bilaterally from the sciatic notch to the popliteal fossa. The nerves were excised and placed in ice-cold mitochondria preservation medium (MiP02; see below) and the animal was then overdosed with sodium pentobarbital. The minced and teased nerve preparation was prepared in the MiP02 medium and the microfilaments were then transferred to the temperature controlled (37° C) recording chamber of a high-resolution respirometer (Oxygraph 2K; Oroboros Instruments; Innsbruck, Austria) and the chamber sealed. The chamber contained 2 ml of respiration medium (MiR05; see below) that had been equilibrated for 30 min at 37° C in air such that a stable O2 concentration was obtained. Stable O2 consumption rates were established within 5 min of adding the preparation and sealing the chamber. The MiR05 respiration medium (Gnaiger et al., 2000) contained: EGTA, 0.5 mM; MgCl26H2O, 3.0 mM; K-lactobionate, 60 mM; taurine, 20 mM; KH2PO4, 10 mM; sucrose, 110 mM; BSA, 0.1%; and HEPES, 20 mM. The MiP02 preservation medium contained the following additional compounds: histidine, 20 mM; vitamin E succinate, 20 µM; glutathione, 3 mM; leupeptine, 1 µM; glutamate, 2 mM; malate, 2 mM; Mg-ATP, 2 mM (all chemicals from Sigma; St. Louis, MO).
A pair of animals, one drug-treated and one vehicle-treated control, was examined on each day, with a random order of testing. Nerves were assayed at two post-treatment times, selected on the basis of previously published studies of the time courses of the sensory abnormalities (Flatters & Bennett, 2006; Bennett et al., 2011; Xiao et al., 2011a). The early time point was one day (paclitaxel) or two days (oxaliplatin) after the last injection, when the pain symptoms are not yet present. The late time point was from the plateau phase of peak pain severity. For paclitaxel, these times were day 7 (D7) and D28; for oxaliplatin, D7 and D35.
The four respiratory Complexes in the mitochondrial electron transport system generate a proton gradient across the mitochondrial inner membrane; the proton gradient drives ATP production via ATP synthase. We focused on Complex I (NADH:ubiquinone oxidoreductase) and Complex II (succinate dehydrogenase) activity because a large body of evidence indicates that damage to these Complexes is a key event in various neurodegenerative diseases (Calvo and Mootha, 2010; Wallace, 2010).
A baseline rate of O2 consumption was determined after the addition of substrates for Complex I-mediated respiration (5 mM glutamate and 5 mM maleate); there is evidence that this combination is more effective than either substrate alone (Puchovicz et al., 2004; Rasmussen & Rasmussen, 2000). Respiration was then stimulated by the addition of ADP (1.0 mM). Next, a new baseline condition was created by inhibiting the activity of Complex I via the addition of rotenone (0.5 µM). Finally, substrate for Complex II-mediated respiration (5 mM succinate) was added. The amounts noted above are the final concentrations and each addition was in a volume of 5 or 10 µl. The responses to substrate additions were measured when the response had reached a stable plateau, which occurred in 4–5 min.
Respiration studies using whole cells or teased muscle fibers require pre-incubation with digitonin to create pores in the plasma membrane so that exogenous substrates can reach the mitochondria (Kuznetsov et al., 2008). In pilot studies we found that there was no increase in the response to additions of Complex I and II substrates and ADP following pre-incubation with 50 µM digitonin and that pre-incubation with 100 µM digitonin caused a small decrease in the responses (data not shown). Excessive exposure to digitonin is known to permeabilize the mitochondrial outer membrane and thus impair respiration. The absence of increased respiration in the presence of 50–100 µM digitonin in our preparation indicates that substrates have ready access to axoplasmic mitochondria via the axon’s cut ends.
ATP assay
Sciatic nerve samples were prepared as described above from paclitaxel-treated and oxaliplatin-treated animals sacrificed at early and late time points (paclitaxel: D7 and D28; oxaliplatin: D7 and D35). As before, a pair of rats was examined on each day, one from a chemotherapy-group and one from the respective control group.
The samples were placed in the respirometer chamber and O2 consumption was measured five minutes before and five minutes after the simultaneous addition of substrates for Complex I and Complex II and ADP (5.0 mM glutamate and 2.5 mM maleate, 5.0 mM succinate, 1.0 mM ADP; combined in a volume of 20 µl). Samples (100 µl) for ATP measurement were removed from the recording chamber immediately after recording the baseline and stimulated O2 consumption rates.
Samples were kept on ice (maximum 10 min.) until ATP levels were determined with a glow-type luciferase assay (CellTiter-Glo; Promega, Madison, WI) using luminometer detection (GloMax; Promega, Madison, WI). ATP sample values were computed relative to a standard curve of known ATP concentrations that was prepared daily.
Citrate synthase assay
Respiration and ATP values were normalized relative to the levels of the activity of the mitochondrion-specific enzyme, citrate synthase. Immediately after the respiration or ATP assay, the tissue sample was removed from the recording chamber and stored at −80° C until assayed. The tissue was homogenized in 0.8 ml lysis medium (CelLytic TM; Sigma, St. Louis, MO) and centrifuged at 12,000 g for 12 min at 4° C. The supernatant was removed and enzyme activity was determined (Citrate Synthase Assay Kit; Sigma, St. Louis, MO) and normalized relative to the amount of protein in the sample (Protein Assay; Bio-Rad Laboratories, Hercules, CA). We expressed the results as citrate synthase units (CSU) of activity (10−7 mol/min/mg protein).
Effects of acetyl-L-carnitine (ALCAR) on mitochondrial respiration
The prophylactic dosing protocols used here have been previously shown to significantly block the development of paclitaxel-evoked and oxaliplatin-evoked neuropathic pain. For the paclitaxel group, ALCAR (100 mg/kg/ml, PO) or vehicle (corn oil) was given daily, beginning on the day of the first paclitaxel dose and daily thereafter for a total of 21 daily administrations (Flatters et al., 2006; Jin et al., 2008). The oxaliplatin group was treated in the same way except that dosing began the day before the first dose of oxaliplatin, yielding a total of 22 daily administrations (Xiao et al., 2011a). When ALCAR and the chemotherapeutic were scheduled to be given on the same day, ALCAR was administered at 0900h and the chemotherapeutic at 1200h. For both the paclitaxel and oxaliplatin experiments, we included a normal control group that received neither chemotherapy nor ALCAR. Groups for the paclitaxel experiment were n = 12, and for the oxaliplatin experiment n = 10.
ALCAR’s effect on the development of mechano-allodynia and mechano-hyperalgesia were assessed at the expected time of peak pain severity (D28 for paclitaxel and D35 for oxaliplatin) by an observer who was blind as to group assignment. Subsequently, sets of animals (one for each treatment group: naïve, chemotherapeutic alone, chemotherapeutic + ALCAR) were sacrificed during the following two weeks (paclitaxel: D28–D40; oxaliplatin: D35–D45) and their sciatic nerves harvested for analysis. It is known that the prophylactic ALCAR treatment protocols used here in paclitaxel-treated and oxaliplatin-treated rats yield a long-lasting (at least several weeks) or permanent inhibition of mechano-allodynia and mechano-hyperalgesia (Flatters et al., 2006; Jin et al., 2008; Xiao et al., 2011a). Respiration was assessed with the sequential additions of substrates for Complex I and Complex II as described above.
Results
Paclitaxel and oxaliplatin effects on mitochondrial respiration
Complex I-mediated respiration
Baseline respiration rates after the addition of substrates for Complex I (Fig. 2) were similar in all groups: pmol/s/CSU (mean ± SEM): control vs. paclitaxel D7: 42.9 ± 1.5 vs. 47.0 ± 3.2; control vs. paclitaxel D28: 33.9 ± 1.7 vs. 30.6 ± 2.5; control vs. oxaliplatin D7: 43.0 ± 2.6 vs. 41.0 ± 3.7; control vs. oxaliplatin D35: 42.9 ± 1.8 vs. 41.7 ± 2.0. The between-group differences were not statistically significant.
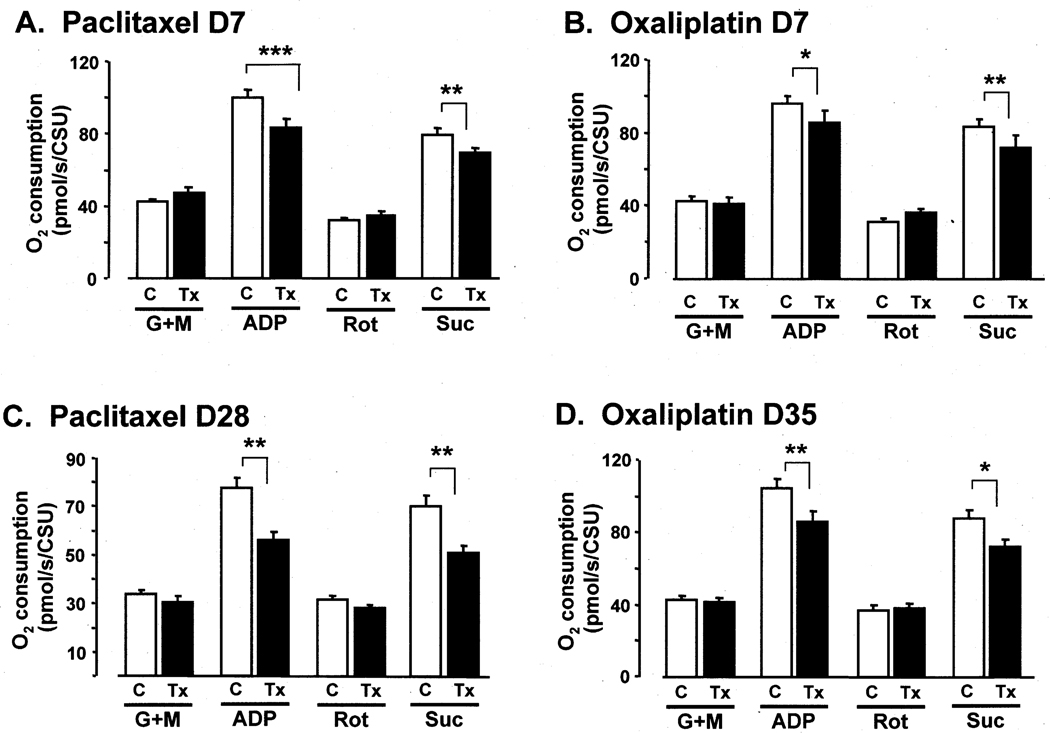
Fig. 2.
Effects of paclitaxel on (A) day 7 and (C) day 28 and of oxaliplatin (B) on day 7 and (D) day 35 on mitochondrial respiration. Vehicle-injected controls (C) compared to groups receiving chemotherapy treatment (Tx). G+M: glutamate + maleate (Complex I substrates), ADP: adenosine diphosphate (stimulates oxidative phosphorylation); Rot: rotenone (inhibits Complex I), and Suc: succinate (Complex II substrate). CSU: units of citrate synthase activity. *, **, *** p < 0.05, 0.01, 0.001 vehicle-injected control group vs. chemotherapy-treated group, percent increases relative to baseline; Bonferroni-corrected t-tests.
Stimulating Complex I respiration via the addition of ADP produced the expected large increases in respiration rate in all groups (Fig. 2: pmol/s/CSU (mean ± SEM): control vs. paclitaxel D7: 99.9 ± 4.5 vs. 83.1 ± 5.1; control vs. paclitaxel D28: 77.5 ± 4.4 vs. 56.3 ± 3.1; control vs. oxaliplatin D7: 95.9 ± 4.3 vs. 85.3 ± 7.1; control vs. oxaliplatin D35: 104.6 ± 5.0 vs. 86.1 ± 5.4. The percent increases in Complex I-mediated respiration rates (relative to baseline) were significantly less in the paclitaxel-treated and oxaliplatin treated groups (Fig. 2): control vs. paclitaxel D7: 234 ± 7% vs. 179 ± 11% (a 55% deficit); control vs. paclitaxel D28: 230 ± 8% vs. 188 ± 8% (a 42% deficit); control vs. oxaliplatin D7: 227 ± 9% vs. 194 ± 9% (a 33% deficit); control vs. oxaliplatin D35: 247 ± 14% vs. 205 ± 7% (a 42% deficit).
Complex II-mediated respiration
The addition of rotenone returned respiration rates to baseline levels in all groups (Fig. 2): pmol/s/CSU (mean ± SEM): control vs. paclitaxel D7: 32.3 ± 1.3 vs. 34.9 ± 2.2; control vs. paclitaxel D28: 31.8 ± 1.6 vs. 28.0 ± 1.2; control vs. oxaliplatin D7: 31.3 ± 2.2 vs. 36.6 ± 2.2; control vs. oxaliplatin D35: 37.2 ± 2.3 vs. 38.3 ± 2.3). The between-group differences were not statistically significant.
The addition of Complex II substrate (with ADP already present) increased O2 consumption in all groups (Fig. 2): pmol/s/CSU (mean ± SEM): control vs. paclitaxel D7: 79.8 ± 3.3 vs. 69.4 ± 3.0; control vs. paclitaxel D28: 70.3 ± 4.7 vs. 50.9 ± 2.7; control vs. oxaliplatin D7: 83.4 ± 4.1 vs. 72.2 ± 6.5; control vs. oxaliplatin D35: 87.4 ± 5.0 vs. 72.2 ± 3.8. The percent increases in Complex II-mediated respiration rates (relative to baseline) were significantly less in the paclitaxel-treated and oxaliplatin treated groups (Fig. 2): control vs. paclitaxel D7: 249 ± 12% vs. 204 ± 11% (a 45% deficit); control vs. paclitaxel D28: 221 ± 8% vs. 182 ± 8% (a 39% deficit); control vs. oxaliplatin D7: 276 ± 17% vs. 222 ± 10% (a 55% deficit); control vs. oxaliplatin D35: 241 ± 15% vs. 193 ± 11% (a 48% deficit).
Oxygen levels
O2 concentration data are not directly comparable to the O2 consumption rate data. The latter are point estimates, while O2 concentration measured at the end of the protocol reflects the cumulative effects of the several additions of substrates, ADP, and rotenone. Nevertheless, O2 concentration data serve as a check on the respiration measurements.
There were no significant between-group differences for the O2 concentrations in the recording chamber at the start of the experiment: nmol/ml (mean ± SEM): control vs. paclitaxel D7: 182.8 ± 2.1 vs. 183.3 ± 2.1; control vs. paclitaxel D28: 197.8 ± 0.9 vs. 196.5 ± 2.1; control vs. oxaliplatin D7: 185.4 ± 2.1 vs. 187.4 ± 2.1; control vs. oxaliplatin D35: 188.9 ± 2.1 vs. 188.6 ± 2.2. The O2 concentrations present at the conclusion of the protocol (i.e., after the addition of succinate) were significantly greater (all p < 0.05; t-tests) in the paclitaxel-treated and oxaliplatin-treated groups relative to their control groups: nmol/ml (mean ± SEM): control vs. paclitaxel D7: 83.4 ± 4.3 vs. 101.6 ± 7.3 (a 21.8% excess); control vs. paclitaxel D28: 122.6 ± 5.2 vs. 139.2 ± 4.4 (a 13.5% excess); control vs. oxaliplatin D7: 90.7 ± 5.5 vs. 110.2 ± 7.2 (a 21.5% excess); control vs. oxaliplatin D35: 86.3 ± 5.2 vs. 109.0 ± 7.4 (a 25.9% excess). The higher O2 concentrations in the chemotherapy-treated groups at the end of the experiment are consistent with the respiration measurements showing impaired O2 consumption in these groups.
Citrate synthase and protein levels
There were no between-group differences in the levels of citrate synthase activity (mean ± SEM CSU): control vs. paclitaxel D7: 1.12 ± 0.04 vs. 1.09 ± 0.05; control vs. paclitaxel D28: 1.21 ± 0.13 vs. 1.33 ± 0.14; control vs. oxaliplatin D7: 1.10 ± 0.03 vs. 1.15 ± 0.03; control vs. oxaliplatin D35: 1.09 ± 0.03 vs. 1.12 ± 0.03. This suggests that neither paclitaxel nor oxaliplatin had any effect on the total number (or total mass) of mitochondria in the peripheral nerve. Protein levels also did not differ significantly between treatment and control groups.
Paclitaxel and oxaliplatin effects on ATP production
Baseline ATP levels were very low and not significantly different between groups (Fig. 3). The simultaneous addition of substrates for Complex I and Complex II and ADP evoked very large increases in ATP levels in all groups. The increases in the paclitaxel-treated and oxaliplatin-treated groups were significantly smaller than in their respective control groups at both time points: nmol/ml/CSU (mean ± SEM): control vs. paclitaxel D7: 1257.4 ± 155.1 vs. 843.7 ± 9.2 (a 32.9% deficit); control vs. paclitaxel D28: 1626.3 ± 173.4 vs. 1091.1 ± 102.4 (a 33.0% deficit); control vs. oxaliplatin D7: 2094.1 ± 215.5 vs. 1615.7 ± 161.1 (a 22.9% deficit); control vs. oxaliplatin D35: 1471.1 ± 112.3 vs. 1111.6 ± 110.4 (a 24.4% deficit).
Fig. 3.
Effects of paclitaxel treatment on (A) day 7 and (B) day 28 and oxaliplatin treatment on (C) day 7 and (D) day 35 on ATP production and O2 consumption before (Pre) and after (Post) the simultaneous addition of substrates for Complex I & II and ADP. CSU: units of citrate synthase activity. *, **: p < 0.05, 0.01 vs. vehicle-injected control group; t-tests.
O2 consumption rates were also obtained in these experiments (Fig. 3). Prior to the addition of substrates and ADP, the rates for all groups were very low and there were no significant between-group differences. The addition of substrates and ADP evoked the expected large increase in respiration. The increases in O2 consumption in the paclitaxel-treated and oxaliplatintreated groups were significantly smaller than in their respective control groups at both time points: pmol/s/CSU (mean ± SEM): control vs. paclitaxel D7: 122.2 ± 4.7 vs. 96.3 ± 3.8 (a 21.2% deficit); control vs. paclitaxel D28: 144.4 ± 6.0 vs. 121.8 ± 8.1 (a 15.7% deficit); control vs. oxaliplatin D7: 138.0 ± 5.1 vs. 112.4 ± 5.4 (18.6% deficit); control vs. oxaliplatin D35: 126.9 ± 9.0 vs. 97.6 ± 6.9 (a 23.1% deficit).
ALCAR effects on paclitaxel-evoked and oxaliplatin-evoked pain and mitochondrial impairment
Pain assays
Rats treated with paclitaxel or oxaliplatin alone developed the expected statistically significant mechano-allodynia and mechano-hyperalgesia (Fig. 4A,B). Also as expected from prior work (Flatters et al., 2006; Jin et al., 2008; Xiao et al., 2011a), prophylactic administration of ALCAR significantly inhibited the development of mechano-allodynia and mechano-hyperalgesia in both paclitaxel-treated and oxaliplatin-treated rats. With one exception, the inhibition was complete in the sense that the response rates in the ALCAR-treated groups were not significantly from those of the naïve control groups. The exception was a partial, but statistically significant, inhibition of mechano-hyperalgesia in oxaliplatin-treated animals that received ALCAR.
Fig. 4.
Effects of a prophylactic treatment with acetyl-L-carnitine (ALCAR) on (A) paclitaxel-evoked and (B) oxaliplatin-evoked mechano-allodynia (4 g VFH) and mechano-hyperalgesia (15 g VFH) and (C) paclitaxel-evoked and (D) oxaliplatin-evoked deficits in Complex I-mediated and Complex II-mediated respiration. A, B: When given alone both paclitaxel and oxaliplatin evoked significant mechano-allodynia and mechano-hyperalgesia (vs. the naïve control group that received neither chemotherapeutic nor ALCAR). ALCAR treatment completely prevented paclitaxel-evoked mechano-allodynia and mechano-hyperalgesia, and oxaliplatin-evoked mechano-allodynia (** p < 0.01, chemo-only group greater than naïve control and chemo + ALCAR groups; Bonferroni-corrected t-tests). ALCAR produced a partial but significant inhibition of oxaliplatin-evoked mechano-hyperalgesia (# p < 0.05, greater than naïve control group). C, D: When given alone, both paclitaxel and oxaliplatin evoked significant deficits in stimulated Complex I-mediated and Complex II-mediated respiration at both time points. ALCAR treatment completely blocked these deficits in both paclitaxel-treated and oxaliplatin-treated groups at both time points. There were no significant between-group differences in the post-G+M and post-rotenone conditions. G+M: glutamate + maleate (Complex I substrates); ADP: adenosine diphosphate (stimulates respiration); Rot: rotenone (inhibits Complex I); Suc: succinate (Complex II substrate). CSU: units of citrate synthase activity. * p < 0.05 (or better), chemo-only group less than naïve control and chemo + ALCAR groups, percent increases relative to baseline; Bonferroni-corrected t-tests.
Complex I-mediated respiration
Respiration rates were similar in all groups after the addition of Complex I substrates: pmol/s/CSU (mean ± SEM): paclitaxel naive control: 41.7 ± 1.8; paclitaxel alone: 40.0 ± 3.4; paclitaxel+ALCAR: 40.3 ± 2.7; oxaliplatin naïve control: 57.4 ± 4.4; oxaliplatin alone: 55.8 ± 4.5; oxaliplatin+ALCAR: 46.6 ± 3.8); none of the between-group differences are statistically significant (Fig. 4C,D). Stimulating Complex I-mediated respiration via the addition of ADP evoked large increases in all groups: pmol/s/CSU (mean ± SEM): naïve control vs. paclitaxel alone vs. paclitaxel+ALCAR: 92.8 ± 4.1 vs. 70.9 ± 5.9 vs. 94.1± 6.6; naïve control vs. oxaliplatin alone vs. oxaliplatin+ALCAR: 107.8 ± 4.2 vs. 85.1 ± 6.6 vs. 106.1 ± 9.4 (Fig. 4C, D).
The percent increases in Complex I-mediated respiration rates were significantly less in the groups treated with paclitaxel alone or oxaliplatin alone: naïve control vs. paclitaxel alone: 223 ± 6% vs. 178 ± 8% (a 45% deficit); naïve control vs. oxaliplatin alone: 195 ± 13% vs. 156 ± 10% (a 39% deficit). ALCAR treatment completely prevented the chemotherapy-evoked deficits in Complex I-mediated respiration. The percent increases in the groups receiving chemotherapeutic + ALCAR were significantly greater than those in the groups receiving the chemotherapeutic alone and not significantly different from those seen in the naïve control groups: paclitaxel+ALCAR vs. paclitaxel alone: 237 ± 12% vs. 178 ± 8%; oxaliplatin+ALCAR vs. oxaliplatin alone: 236 ± 9% vs. 156 ± 10%. (Fig. 4C,D).
Complex II-mediated respiration
Respiration rates were similar in all groups after Complex I-mediated respiration was blocked with rotenone: pmol/s/CSU (mean ± SEM): paclitaxel naïve control: 38.4 ± 1.0; paclitaxel alone: 38.7 ± 2.3; paclitaxel+ALCAR: 37.9 ± 2.1; oxaliplatin naive control: 44.6 ± 2.6; oxaliplatin alone: 42.9 ± 3.1; oxaliplatin+ALCAR: 37.3 ± 3.3; none of the between-group differences are statistically significant (Fig. 4C,D).
The addition of succinate (with ADP already present) evoked large increases in all groups: naïve control vs. paclitaxel alone vs. paclitaxel+ALCAR: pmol/s/CSU (mean ± SEM): 90.6 ± 3.8 vs. 77.9 ± 6.1 vs. 94.6 ± 6.6; naïve control vs. oxaliplatin alone vs. oxaliplatin+ALCAR: 89.7 ± 4.8 vs. 73.6 ± 6.9 vs. 84.8 ± 7.4.
The percent increases in Complex II-mediated respiration rates were significantly less in the groups treated with paclitaxel alone or oxaliplatin alone: naïve control vs. paclitaxel alone: 236 ± 7% vs. 199 ± 7% (a 15.7% deficit); naïve control vs. oxaliplatin alone: 204 ± 9% vs. 164 ± 7% (a 19.6% deficit). ALCAR treatment completely prevented the chemotherapy-evoked deficits in Complex II-mediated respiration. The percent increases in Complex II-mediated respiration in the groups receiving chemotherapeutic + ALCAR were significantly greater than those in the groups receiving the chemotherapeutic alone and not significantly different from those seen in the naïve control groups: paclitaxel+ALCAR vs. paclitaxel alone: 251 ± 12% vs. 199 ± 7%; oxaliplatin+ALCAR vs. oxaliplatin alone: 230 ± 12% vs. 164 ± 7%. (Fig. 4C,D).
Discussion
The data reported here are the first direct evidence of functional impairment of peripheral nerve mitochondria obtained from animals with confirmed paclitaxel-evoked and oxaliplatin-evoked painful peripheral neuropathies. Observations of paclitaxel-evoked and oxaliplatin-evoked mitochondrial swelling and vacuolation in peripheral nerve axons suggested the mitotoxicity hypothesis, which proposes that these drugs cause a chronic sensory axonal energy deficiency that is the primary cause of the neuropathies’ symptoms (Bennett et al., 2011; Flatters & Bennett, 2006; Jin et al., 2008). The results reported here support this hypothesis.
There are a large number of neurodegenerative conditions where impaired mitochondrial function has a relatively selective effect on only a subset of neurons (Duffy et al., 2011; Wallace 2010). For example, the mutation in mitochondrial DNA that is responsible for Leber’s hereditary optic neuropathy affects optic nerve axons but rarely involves oculomotor nerve axons and apparently does not affect the axons in any other nerve (Milea et al., 2010). Energy deficiencies due to mitotoxins are also implicated in the pathogenesis of neurodegenerative diseases (Gubellini et al., 2010). For example, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) and the herbicide, paraquat, preferentially damage the mitochondria in the dopaminergic neurons of the substantia nigra and produce the symptoms of Parkinson’s disease, while 3-nitroproprionic acid and methlymalonic acid preferentially damage the mitochondria in the spiny neurons of the striatum and produce the symptoms of Huntington’s chorea. The results shown here suggest that chemotherapeutics preferentially damage mitochondria in somatosensory primary afferent axons and produce a painful peripheral sensory neuropathy. Why different kinds of mitochondrial insults preferentially affect different neuronal subsets is unknown. For paclitaxel and oxaliplatin, the preferential effect on primary afferent sensory neurons may involve a high and persistent exposure to the drug in the dorsal root ganglia (Cavaletti et al., 2000; Xiao et al., 2011b).
Impaired mitochondrial respiration and ATP production
The respiration rates seen after adding Complex I substrates (in the absence of exogenous ADP) did not differ between groups. Similarly, there were no between-group differences in the post-rotenone values (a re-established baseline condition where Complex I was inhibited). The lack of between-group differences in these cases is probably due to the low levels of respiration that exist in the non-stimulated state. Pathology is often not apparent at rest, but clearly reveals itself under conditions of physiological demand. For mitochondria, physiological demand is signalled by ADP levels, and respiration in the stimulated (ADP + substrate) condition revealed the paclitaxel-evoked and oxaliplatin-evoked deficits. It is theoretically possible that compensatory mechanisms could yield normal ATP production despite deficits in Complex I-mediated and/or Complex II-mediated respiration. Moreover, respiration and ATP production can be uncoupled under physiological conditions. It is therefore important that we also found significantly decreased levels (23%–33%) of ATP production in both paclitaxel-treated and oxaliplatin-treated animals at both the early and late time points.
The key point here is that nerves from rats with paclitaxel-evoked and oxaliplatin-evoked peripheral neuropathy had significantly lower rates of stimulated respiration for both Complex I and Complex II, and a significantly lower stimulated production of ATP. It is particularly noteworthy that significant deficits were seen as early as D7 (1–2 days after the last injection) and as late as 3–4 weeks after treatment (D28 for paclitaxel, D35 for oxaliplatin). This indicates that the paclitaxel and oxaliplatin treatment protocols produce mitochondrial injuries with an acute onset and chronic duration. We found no decrease in citrate synthase levels after treatment with paclitaxel or oxaliplatin. The absence of any change in this mitochondrion-specific enzyme suggests that the mitotoxic effect damages, but does not kill, mitochondria.
Magnitude of the effects
It is possible that the deficits that we observed are underestimates of the severity of paclitaxel’s and oxaliplatin’s effects. Our results reflect the summed contribution of respiration and ATP production in the mitochondria of several cell types in the sciatic nerve: sensory axons, motor axons, and Schwann cells. There is evidence that paclitaxel causes significant deficits in Complex I-mediated and Complex II-mediated respiration in dorsal root sensory axons, but not in ventral root motor axons (Xiao et al., 2011b). Thus, a deficit in mitochondrial function in sensory axons may have been partly obscured by unaffected function in mitochondria in motor axons. In addition, neither paclitaxel nor oxaliplatin produce swelling and vacuolation of the mitochondria in myelinating and non-myelinating Schwann cells (Xiao et al., 2011a,b). Thus, the activity of unaffected mitochondria in Schwann cells may have also partly obscured a mitochondrial impairment in sensory axons. In the sural nerve of man and macaque, most mitochondria are in axons and only a small minority are in Schwann cells (about 7% in the monkey and about 16% in man) (Lehmann et al., 2011). However, in the rat saphenous nerve we find (unpublished observations) that 68% of mitochondria are axonal and 32% are in Schwann cells; mitochondria in other cell types (e.g., vascular endothelial cells) make a negligible contribution to the total.
On the mechanism of the mitotoxic effects of paclitaxel and oxaliplatin
The mechanism(s) whereby paclitaxel and oxaliplatin induce mitochondrial dysfunction is unknown. The mitotoxic effect of paclitaxel may be due to its binding to a β-tubulin moiety that is associated with the voltage dependent anion channel (VDAC) that spans the mitochondrial outer membrane (Rostovtseva et al., 2008; Shosan-Barmatz, et al., 2010). Paclitaxel opens VDAC and this causes mitochondrial swelling and vacuolation (Andre et al., 2000; Vabiro et al., 2001). Oxaliplatin and the other platinum-complex chemotherapeutics induce platinum adjuncts in mitochondrial DNA (Ta et al., 2006) and this is likely to reduce the synthesis of essential mitochondrial proteins. Platinum adjuncts are also formed between proteins, and in cancer cells cisplatin induces protein-protein platinum adjuncts involving VDAC. This is a very large effect: the amount of platinum-VDAC adjuncts isolated from the mitochondrial protein fraction is more than 200 fold greater than the amount of platinum found in the whole cell protein fraction (Cullen et al., 2007). Olesoxime, which prevents VDAC opening, protects against the painful peripheral neuropathies produced by oxaliplatin and paclitaxel (Bordet et al., 2007, 2008; Xiao et al., 2009). Alpha-lipoic acid protects sensory neurons against the toxic effects of both paclitaxel and cisplatin, and the effects are likely to be mediated by mitochondrial mechanisms of action (Melli et al., 2008).
ALCAR effects on mitochondria
In animal models, prophylactic treatment with ALCAR inhibits the development of paclitaxel-evoked (Flatters et al., 2006; Ghirardi et al., 2005b; Jin et al., 2008; Pisano et al., 2003; Xiao & Bennett, 2008) and oxaliplatin-evoked (Ghirardi et al., 2005a; Xiao et al., 2011a) mechano-allodynia and mechano-hyperalgesia. It also prevents the paclitaxel-evoked increase in swollen and vacuolated mitochondria in peripheral nerve C-fibers (Jin et al., 2008), and significantly reduces the incidence of paclitaxel-evoked spontaneous discharge in peripheral nerve A-fibers and C-fibers (Xiao & Bennett, 2008).
Here we confirmed that prophylactic treatment with ALCAR significantly inhibited the development of paclitaxel-evoked and oxaliplatin-evoked neuropathic pain and showed, in the same animals, that ALCAR completely prevented the associated deficits in Complex I-mediated and Complex II-mediated respiration. This supports the hypothesis that ALCAR’s effect is indeed due to an anti-mitotoxicity action and suggests that other types of drug that protect mitochondria have the potential to prevent chemotherapy-evoked peripheral neuropathy (Doyle et al., 2011; Xiao et al., 2011a; Xiao et al., 2009). However, ALCAR has many non-mitochondrial mechanisms of action (Jones et al., 2010) and the possibility that its efficacy against chemotherapy-induced neuropathy may be related to multiple mechanisms can not be excluded.
Implications
Chronic, distal, symmetrical, sensory peripheral neuropathies are associated with several toxins and with diabetes. All of these neuropathies have similar sensory symptoms. It is possible that these conditions resemble one another because they all have mitotoxicity as a final common pathway. Vinca alkaloid anti-neoplastic agents like vincristine may be mitotoxic because they (like the taxane agents) bind to the β-tubulin moiety that is associated with mitochondrial VDAC. Vincristine-evoked neuropathy is blocked by the VDAC-binding agent, olesoxime (Bordet et al., 2008). Proteasome-inhibitor anti-neoplastic agents like bortezomib cause mitochondrial calcium dysregulation and an increase in intra-mitochondrial reactive oxygen species in cancer cells (Landowski et al., 2005; Ling et al, 2003; Pei et al., 2003). The cytosolic ubiquitin-proteasome system is known to regulate the import and export of mitochondrial proteins (Livnat-Levanon and Glickman, 2011). The false nucleoside chemotherapeutics used to treat HIV block DNA polymerase γ. This prevents viral replication, but also inhibits the transcription of mitochondrial DNA, which encodes key subunits of Complexes I and II and ATP synthase (Moyle, 2005). Recent data support the hypothesis that the peripheral neuropathy seen in patients with HIV infection involves axonal mitochondrial dysfunction (Lehmann et al., 2011). Accumulating evidence (Fernyhough et al., 2010) suggests that mitochondria in primary afferent neurons may be key targets for the toxic effects of high glucose levels. Thus, mitochondrial dysfunction may be important in the genesis of the distal symmetrical sensory peripheral neuropathy that is seen with diabetes.
Highlights.
Chemotherapy-induced peripheral neuropathy (CIPN) is modeled in the rat.
CIPN-evoked axonal mitochondrial swelling and vacuolation suggest a bioenergetic deficit.
Mitochondrial respiration and ATP production is impaired in rats with CIPN.
Chemotherapy-evoked mitotoxicity is a likely cause of CIPN.
Acknowledgements
This work was supported by the National Institutes of Health (R01-NS052255), the Canada Research Chairs Program, the Canada Foundation for Infrastructure, and the Louise and Alan Edwards Foundation of Montreal. GJB is a Canada Senior Research Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has any conflict of interest with respect to the contents of this report.
References
- Andre N, Braguer D, Brasseur G, Goncalves A, Lemesle-Meunier D, Guise S, Jordan MA, Briand C. Paclitaxel induces release of cytochrome c from mitochondria isolated from human neuroblastoma cells. Cancer Res. 2000;60:5349–5353. [PubMed] [Google Scholar]
- Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration (TAD): a novel lesion produced by the antineoplastic agent, paclitaxel. Eur. J. Neurosci. 2011;33:1667–1676. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A, Stenge M, Maag R, Wasner G, Schoch R, Moosig F, Schommer B, Baron R. Pain in oxaliplatin-induced neuropathy – Sensitization in the peripheral and central nociceptive system. Eur. J. Cancer. 2007;43:2658–2663. doi: 10.1016/j.ejca.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Bordet T, Buisson B, Michaud M, Drouot C, Galéa P, Delaage P, Akentieva NP, Evers AS, Covey DF, Ostuni MA, Lacapère JJ, Massaad C, Schumacher M, Steidl EM, Maux D, Delaage M, Henderson CE, Pruss RM. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 2007;322:709–720. doi: 10.1124/jpet.107.123000. [DOI] [PubMed] [Google Scholar]
- Bordet T, Buisson B, Marchand F, Grist J, Malcangio M, Pruss RM. Antinociceptive activity of the neuroprotective agent TRO19622 in experimental models of diabetic and chemotherapy-induced neuropathy. J. Pharmacol. Exp. Ther. 2008;326:623–632. doi: 10.1124/jpet.108.139410. [DOI] [PubMed] [Google Scholar]
- Brewer PA, Lynch K. Stimulation-associated changes in frog neuromuscular junctions. A quantitative ultrastructural comparison of rapid-frozen and chemically fixed nerve terminals. Neuroscience. 1986;17:881–895. doi: 10.1016/0306-4522(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Calvo SE, Mootha VK. The mitochondrial proteome and human disease. Ann. Rev. Genomics Hum. Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Lee BN, Reubgen JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006;72:151–169. [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D'Incalci M, Zucchetti M, Marmiroli P, Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicol. 2001;21:389–393. [PubMed] [Google Scholar]
- Cullen KJ, Yang Z, Schumaker L, Guo Z. Mitochondria as a critical target of the chemotheraputic agent cisplatin in head and neck cancer. J. Bioenerg. Biomembr. 2007;39:43–50. doi: 10.1007/s10863-006-9059-5. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J. Pain Symp. Manage. 2007;33:166–179. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Salvemini D. Peroxynitrite decomposition catalyst blocks paclitaxel-induced neuropathic pain: microarray analysis of spinal cord gene expression. J. Pain. 2011;12 Suppl 2:37. [Google Scholar]
- Duffy LM, Chapman AL, Shaw PJ, Grierson AJ. The role of mitochondria in the pathogenesis of amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 2011 doi: 10.1111/j.1365-2990.2011.01166.x. In press. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Roy-Chowdhury SK, Schmidt RE. Mitochondrial stress and the pathogenesis of diabetic neuropathy. Expert Rev. Endocrinol. Metab. 2010;5:39–49. doi: 10.1586/eem.09.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain. 2006;122:247–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters SJL, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful neuropathy. Neurosci. Lett. 2006;397:219–223. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nature Protocols. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Ghirardi O, Lo Giudice P, Pisano C, Vertechy M, Bellucci A, Vesci L, Cundari S, Miloso M, Rigamonti LM, Nicolini G, Zanna C, Carminati P. Acetyl-L-carnitine prevents and reverts experimental chronic neurotoxicity induced by oxaliplatin, without altering its antitumor properties. Anticancer Res. 2005a;25:2681–2687. [PubMed] [Google Scholar]
- Ghirardi O, Vertechy M, Vesci L, Canta A, Nicolini G, Galbiati S, Ciogli C, Quattrini G, Pisano C, Cundari S, Rigamonti LM. Chemotherapy-induced allodinia: neuroprotective effect of acetyl-L-carnitine. In Vivo. 2005b;19:631–637. [PubMed] [Google Scholar]
- Gnaiger E, Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Steurer W, Margreiter R. Mitochondria in the cold. In: Heldmaier G, Klingenspor M, editors. Life in the cold. Berlin: Springer; 2000. pp. 431–442. [Google Scholar]
- Gubellini P, Picconi B, Di Filippo M, Calabresi P. Downstream mechanisms triggered by mitochondrial dysfunction in the basal ganglia: from experimental models to neurodegenerative diseases. Biochim. Biophys. Acta. 2010;1802:151–161. doi: 10.1016/j.bbadis.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Jin HW, Flatters SJL, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: Effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exptl. Neurol. 2008;210:229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog. Lipid Res. 2010;49:61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nature Protocols. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65:3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- Lehmann HC, Chen W, Borzan J, Mankowski JL, Höke A. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann. Neurol. 2011;69:100–110. doi: 10.1002/ana.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling YH, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J. Biol. Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- Livnat-Levanon N, Glickman MH. Ubiquitin-proteasome system and mitochondria - reciprocity. Biochim. Biophys. Acta. 2011;1809:80–87. doi: 10.1016/j.bbagrm.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, Taroni F, Lauria G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp. Neurol. 2008;214:276–284. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Milea D, Amati-Bonneau P, Reynier P, Bonneau D. Genetically determined optic neuropathies. Curr. Opin. Neurol. 2010;23:24–28. doi: 10.1097/WCO.0b013e3283347b27. [DOI] [PubMed] [Google Scholar]
- Moyle G. Mechanisms of HIV and nucleoside reverse transcriptase inhibitor injury to mitochondria. Antivir. Ther. 2005;10 Suppl 2:M47–M52. [PubMed] [Google Scholar]
- Nowikovsky K, Schweyen RJ, Bernardi P. Pathophysiology of mitochondrial volume homeostasis: potassium transport and permeability transition. Biochim. Biophys. Acta. 2009;1787:345–350. doi: 10.1016/j.bbabio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Pei XY, Dai Y, Grant S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia. 2003;17:2036–2045. doi: 10.1038/sj.leu.2403109. [DOI] [PubMed] [Google Scholar]
- Pisano C, Pratesi G, Laccabue D, Zunino F, Lo Giudice P, Bellucci A, Pacifici L, Camerini B, Vesci L, Castorina M, Cicuzza S, Tredici G, Marmiroli P, Nicolini G, Galbiati S, Calvani M, Carminati P, Cavaletti G. Paclitaxel and cisplatin-induced neurotoxicity: a protective role of acetyl-L-carnitine. Clin. Cancer Res. 2003;9:5756–5767. [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Puchovicz MA, Varnes ME, Cohen BH, Frieman NR, Kerr DS, Hoppel CL. Oxidative phosphorylation analysis: assessing the integrated functional activity of human skeletal muscle mitochondria – case studies. Mitochondrion. 2004;4:377–385. doi: 10.1016/j.mito.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J. Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Rasmussen HN. Human quadriceps muscle mitochondria: A functional characterization. Mol. Cell Biochem. 2000;208:37–44. doi: 10.1023/a:1007046028132. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shosan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 2010;30:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicol. 2006;27:992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Varbiro G, Veres B, Gallyas F, Sumegi B. Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic. Biol. Med. 2001;31:548–558. doi: 10.1016/s0891-5849(01)00616-5. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008;135:262–270. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zheng H, Bennett GJ. Oxaliplatin-evoked chronic painful peripheral neuropathy in the rat. 2011a doi: 10.1016/j.neuroscience.2011.10.010. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zheng H, Zheng FY, Nuydens R, Meert TF, Bennett GJ. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. 2011b doi: 10.1016/j.neuroscience.2011.10.010. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM. Olesoxime (cholest-4-en-3-one, oxime): Analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain. 2009;147:202–209. doi: 10.1016/j.pain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Xiao WH, Bennett GJ. Mitotoxicity as the cause of the painful peripheral neuropathies evoked by the chemotherapeutics, paclitaxel and oxaliplatin; 13th World Congress on Pain; 2010. Abstr # PW 097. [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]