Abstract
The initial line of defense against infection is sustained by the innate immune system. Together, membrane-bound TLR and cytosolic NLR receptors play key roles in the innate immune response by detecting bacterial and viral invaders as well as endogenous stress signals. NLRs are multi-domain proteins with varying N-terminal effector domains that are responsible for regulating downstream signaling events. Here, we report the structure and dynamics of the N-terminal pyrin domain of NLRP12 (NLRP12 PYD) determined using NMR spectroscopy. NLRP12 is a non-inflammasome NLR that has been implicated in the regulation of TLR-dependent NF-κB activation. NLRP12 PYD adopts a typical 6-helical bundle death domain fold. By direct comparison with other PYD structures, we identified hydrophobic residues that are essential for the stable fold of the NLRP PYD family. In addition, we report the first in vitro confirmed non-homotypic PYD interaction between NLRP12 PYD and the pro-apoptotic protein FAF-1, which links the innate immune system to apoptotic signaling. Interestingly, all residues that participate in this protein:protein interaction are confined to the α2-α3 surface, a region of NLRP12 PYD that differs most between currently reported NLRP PYD structures. Finally, we experimentally highlight a significant role for tryptophan 45 in the interaction between NLRP12 PYD and the FAF-1 UBA domain.
Keywords: NLR proteins, death domain, pyrin domain, NLRP12, FAF-1, innate immune system, apoptosis, NMR spectroscopy, protein:protein interaction
Introduction
Vertebrates have evolved numerous strategies to defend against environmental pathogens, including the innate and adaptive immune responses. Innate immunity is the first line of host defense against pathogenic infection and relies on the detection of pathogen- and danger-associated molecular patterns (PAMPs and DAMPs, respectively) by a set of germ-line encoded receptors collectively called pattern-recognition receptors (PRRs). Among PRRs, the membrane-anchored Toll-like receptors (TLRs) have emerged as essential components of innate immunity. They recognize diverse pathogen-derived molecules, causing the activation of intracellular signaling cascades that ultimately lead to an inflammatory response1; 2; 3; 4.
During the last 10 years it has become clear that TLRs are not the only pathogen- and danger-associated signals sensors in innate immunity. Nucleotide-binding and leucine-rich repeat-containing receptors (NLRs) are expressed in the cytoplasm and their importance in PAMP and DAMP recognition is rapidly growing5; 6. Additionally, numerous human auto-inflammatory disorders have been associated with mutations in NLRs, emphasizing their role as central regulators of immunity and inflammation7. NLRs possess a tripartite architecture. They are composed of a C-terminal leucine-rich repeat domain, necessary for ligand binding; a central NACHT domain, capable of ATP binding and responsible for oligomerization; and an N-terminal effector domain, linking the NLR to downstream signaling cascades8. The human genome encodes for 22 NLRs, which are further grouped into subfamilies according to their N-terminal effector domain (PYRIN, CARD - caspase activation and recruitment domain, or BIR - baculovirus inhibitor repeat domain). NLRPs contain an N-terminal effector PYRIN domain and constitute the largest subfamily of NLRs (NLRP1-14)9.
Two members of the NLRP family, NLRP1 and NLRP3, activate the innate immune response via their interaction with the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) and subsequently procaspase-1. This multi-component complex is called the inflammasome10; 11, a macromolecular machine that activates the cleavage of proinflammatory cytokines like pro-IL-1β and pro-IL-18 into their mature, secreted forms2; 11.
Other members of the NLRP family regulate key functions in the immune system without forming an inflammasome. For example, some NLRPs including NLRP12 (previously called Monarch-1 or PYPAF-7) regulate NF-κB activation12; 13; 14. NLRP12 was one of the earliest identified NLRPs and its expression is restricted to myeloid cells15. Furthermore mutations in NLRP12 are linked to hereditary periodic fevers and atopic dermatitis, stressing its role in immunity and inflammation16; 17. Using gene silencing, it was recognized that NLRP12 fine-tunes downstream signaling via down-regulation of TLR-dependent NF-κB activation. Subsequently, NLRP12 suppresses the production of pro-inflammatory cytokines by inhibiting non-canonical NF-κB activation. This regulation is likely achieved via the inhibition of necessary processing kinases, especially the non-canonical MAP3 kinase NIK (NF-κB inducing kinase)13; 15. In addition, NLRP12 has been reported to interact with the chaperone Hsp90 and postulated to play a role in the proteasome pathway18. Although the precise mechanism by which NLRP12 inhibits NIK-induced NF-κB activation is unknown, it is thought that NLRP12 drives the degradation of NIK via a proteasome-dependent pathway. Recent reports speculate that this modulation can be achieved via the interaction of effector domains with “adaptor-like” proteins, such as Fas-associated factor 1 (FAF-1)19; 20; 21, and thus links the innate immune system to apoptotic signaling22.
We23; 24 and others25; 26; 27; 28; 29 have recently shown that NLRP PYRIN domains (PYDs) can vary in structure and dynamics and, as a result, in surface charge, a critical parameter directing their protein:protein interactions. Thus, to understand the biological function of PYDs in general, and NLRP12 PYD specifically, we determined the structure and auto-correlated fast time scale backbone dynamics of NLRP12 PYD. Interestingly, we identified differences among all currently published NLRP PYD structures in regions described to be central for homotypic PYD:PYD interactions. Finally, we tested the previously proposed heterotypic interaction of NLRP12 PYD with the pro-apoptotic protein FAF-1 in vitro. By testing several different FAF-1 single and multi-domain fragments, we identified a direct interaction of NLRP12 PYD with the UBA domain of FAF-1.
Results
Protein Expression and Purification
The N-terminal effector PYRIN domain of NLRP12 (residues 1–98, two N-terminal cloning artifacts; herein referred as NLRP12 PYD) showed high levels of soluble overexpression in E. coli and was readily purified to homogeneity. NLRP12 PYD is a monomer in solution as verified by size-exclusion chromatography. In low salt conditions (100 mM NaCl) NLRP12 PYD precipitates at a concentration higher than 0.2 mM. This is not surprising, as PYRIN domains are known for their poor solubility and tendency to aggregate26. To achieve concentrations of NLRP12 PYD sufficient for accurate structural and dynamics studies, 500 mM NaCl was necessary. In these conditions, NLRP12 PYD is readily concentrated to 0.6 mM without precipitation or aggregation and stable during the course of several weeks.
FAF-1 constructs (FAF-11–99, FAF-11–57 and FAF-199–180) were overexpressed in E. coli and purified to homogeneity. An MBP tag was used to enhance the solubility of FAF11–57 during expression. All FAF-1 constructs are monomers in solution as verified by size-exclusion chromatography.
Three-dimensional structure of NLRP12 PYD
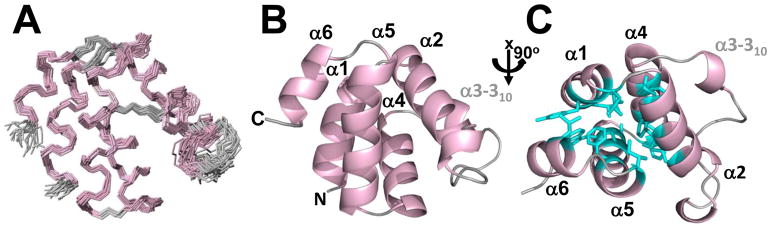
The solution structure of NLRP12 PYD was determined using heteronuclear NMR spectroscopy. Assignments were obtained for 95% of the backbone nuclei (N, HN, C′, Cα, Hα) and 95% of the side chain 13CHn moieties. Of the 96 expected backbone amide NH pairs (3 prolines), 93 were identified; the missing assignments correspond to the N-terminal two-residue cloning artifact Gly-2 and His-1, as well as Thr4 (Supplemental Figure S1). All aliphatic and aromatic side chain resonances that are routinely observed were assigned, except those of residues Gly-2, His-1, Arg3, Ser13, Lys29 and Glu100, where confident assignments were uncertain. An ensemble of 100 structures was calculated from 1842 NOESY-derived distance constraints (~18 NOE constraints/residue) using a simulated annealing protocol within the program CYANA30 and refined in explicit solvent using CNS31. The 20 lowest-energy structures of NLRP12 PYD are shown in Figure 1A. All structures have excellent geometry, with no violations of distance restraints greater than 0.5 Å and no dihedral angle violations greater than 5º (Table 1). In addition, all structures have excellent stereochemistry, with 98.8% of residues in the most favored and additionally allowed regions of the Ramachandran diagram, 0.8% of residues in the generously allowed region, and 0.5% of residues in the disallowed region (Table 1). The NLRP12 PYD structure is well defined, with the exception of the N- and C-termini, residues −2–9 and 92–100, respectively, which are flexible (Supplemental Figure S2A, B). The root-mean-square deviation (RMSD) value about the mean coordinate positions of the backbone atoms for residues 10–91 of NLRP12 PYD is 0.62 ± 0.11 Å (Table 1).
Figure 1.
NMR structure of NLRP12 PYD. (A) Ensemble of the 20 lowest-energy structures calculated for NLRP12 PYD superimposed on the backbone atoms of residues 10–91 (PDBID 2L6A). The 6 helices, characteristic of the death domain fold, are highlighted in light pink, while loops are highlighted in grey. (B) Ribbon representation of the lowest-energy conformer of NLRP12 PYD in an orientation identical to that shown in A. The N- and C-termini, as well as the 6 helices are labeled. (C) Top view of NLRP12 PYD (rotated by 90° about the x axis relative to A and B). Residues forming the central hydrophobic core are shown as cyan sticks.
Table 1.
Structural and CNS refinement statistics
| NLRP12 PYD | |
|---|---|
| Number of restraints | |
| Unambiguous distance restrains (all) | 1859 |
| Intra-residue | 490 |
| Short range | 458 |
| Medium Range | 466 |
| Long Range | 445 |
| Deviations from idealized covalent geometry | |
| Bonds (Å) | 0.009 ± 0.0004 |
| Angles (deg.) | 1.24 ± 0.05 |
| Impropers (deg.) | 1.39 ± 0.09 |
| Structural quality | |
| Ramachandran plot (10–91; NMR-PROCHECK) | |
| Most favored region (%) | 89.1 |
| Additionally allowed region (%) | 9.7 |
| Generously allowed region (%) | 0.8 |
| Disallowed region (%) | 0.5 |
| Pairwise RMSD (Å) | |
| Backbone (N, Cα, C and O) (10–91) | 0.62 ± 0.11 |
| All heavy atoms (10–91) | 1.20 ± 0.14 |
As expected, NLRP12 PYD folds into a tightly packed helical bundle (residues 10–91) consisting of 6 helices (α1-α6) arranged in an anti-parallel fashion (Figure 1B), characteristic of pyrin domains. However, unlike other PYD structures, NLRP12 PYD helix α3 only forms a short 310 helix. The residues forming the 6 helices are: 10–19 (α1), 22–34 (α2), 47–50 (α3 - 310 helix), 53–64 (α4), 66–80 (α5), and 83–91 (α6).
The NLRP12 PYD 6-helical bundle is stabilized by a central hydrophobic core formed by residues Leu13, Tyr16, Leu17, Leu20 from helix α1; Leu25, Phe28, Leu32 from helix α2; Met56, Leu60, Phe64 from helix α4; Ala69, Trp70, Ala73, Phe77, Ile80 from helix α5; and Leu85 from helix α6 (Figure 1C). All α-helices in the structure of NLRP12 PYD are connected by short well-defined loops, with the exception of the loop that connects helices α2 and α3 (310 helix), herein called α2-α3 loop. The α2-α3 loop comprises residues 35–46. Despite the fact that it does not contain any regular secondary structure, the α2-α3 loop is quite ordered in the structure of NLRP12 PYD. This order in the α2-α3 loop is due to hydrophobic interactions of residues Leu38 and Ile43 both within the α2-α3 loop itself and with neighboring residues, especially residue Phe64 in helix α4 (Supplemental Figure S3).
Backbone Dynamics of NLRP12 PYD
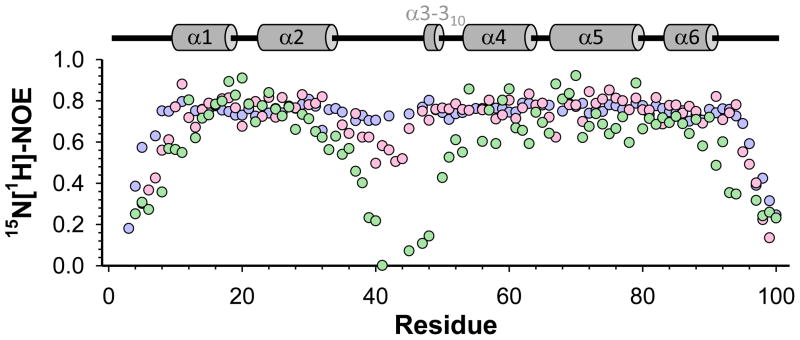
The PYDs of NLRP1 and NLRP7 show distinct dynamics behaviors, especially in the α2-α3 loop, which exhibits substantially increased fast time scale dynamics in NLRP1 PYD (ps-ns as detected by 15N[1H]-NOE measurement), and slow time scale dynamics in NLRP7 PYD (μs as detected by 15N CSA, 15N-1H dipole-dipole cross-correlation relaxation rates and on-resonance R1ρ measurements)24; 26. To investigate the dynamics in NLRP12 PYD, we measured auto-correlated 15N-relaxation data, including measurements of 15N[1H]-NOE, as well as 15N longitudinal (R1) and transverse (R2) relaxation rates. Using a model-free analysis, we calculated a correlation time (τc) of ~7 ns for NLRP12 PYD at 298 K. Comparison of the 15N[1H]-NOE data, which reports on fast time scale (ps-ns) backbone motions, of the PYDs of NLRP1, NLRP7 and NLRP12 is shown in Figure 2. The dynamics of the α2-α3 loop in NLRP12 PYD is much more similar to the dynamics of NLRP7 PYD than that of NLRP1 PYD. NLRP12 PYD shows a small increase in fast time scale dynamics in the α2-α3 loop, but no slow-intermediate time scale dynamics, when compared to NLRP7 PYD. Indeed, the dynamics detected for NLRP12 PYD seems to be most similar to the dynamics reported for the PYD of the inhibitor protein ASC227.
Figure 2.
Comparison of fast time scale backbone dynamics among the PYDs of NLRP12, NLRP7 and NLRP1. Comparison of 15N[1H]-NOE (hetNOE) values measured for the PYDs of NLRP12 (light pink), NLRP7 (light blue) and NLRP1 (light green). Experimentally derived secondary structure elements of NLRP12 PYD are depicted by grey cylinders above the figure. A hetNOE of ~0.8 is typical for well-formed, stable secondary structural elements. While the α2-α3 loop and helix α3 in NLRP1 PYD show a substantial increase in fast time scale backbone dynamics, these motions are missing in NLRP7 PYD. The α2-α3 loop and helix α3 in NLRP12 PYD show increased flexibility when compared to NLRP7 PYD.
A ~10% cis/trans proline isomerization for Pro33 and Pro42 in NLRP7 PYD and Pro42 in NLRP1 PYD was reported. These proline residues flank the α2-α3 loop. In contrast, no significant cis/trans proline isomerization was detected in NLRP12 PYD.
Structural and dynamics comparison of NLRP12 PYD with members of the NLRP family
Despite a relatively low sequence identity between NLRP12 PYD and the PYDs of NLRP7 (29% sequence identity, PDBID 2KM6) and NLRP1 (36% sequence identity, PDBID 1PN5), their structures superimpose well with an RMSD value of 2.4 Å for the backbone atoms between NLRP12 and NLRP7 PYDs, and 3.1 Å between NRLP12 and NLRP1 PYDs. Interestingly, a structure-based sequence alignment identified that the conserved residues between all three PYD structures form the hydrophobic core of the PYD fold. This hydrophobic core is highly conserved among the entire NLRP family (NLRP1-14) and, therefore, defines the overall fold of NLRP PYDs (Figure 3).
Figure 3.
Sequence alignment of human NLRP PYDs. Residues contributing to the hydrophobic core of NLRP12 PYD are conserved among the entire NLRP family and are highlighted by grey boxes. Experimentally derived secondary structure elements of NLRP12 PYD are depicted by grey cylinders on top of the figure.
Superposition of the structure of NLRP12 PYD with those of NLRP1 and NLRP7 PYDs revealed small differences in the length and orientation of the helices. In particular, helices α1 and α6 differ in overall length between the three PYD structures. Furthermore, the relative angle of helix α2 with respect to the overall helical bundle shows the largest difference between the structures of NLRP12 and NLRP7 PYDs.
Clearly, the most significant differences among the three PYD structures are helix α3 and the preceding α2-α3 loop. The overall length of NLRP12 PYD is shorter than that of NLRP7 and NLRP1 PYDs, and the structure-based sequence comparison (Figure 3) identified a two amino acid deletion in the α2-α3 loop region of NLRP12 PYD. This deletion has multiple consequences. First, helix α3 (2 turns) in NLRP7 PYD is replaced by a 310 helix in NLRP12 PYD. Furthermore, this secondary structure element is entirely missing in NLRP1 PYD, where α3 is replaced by a flexible disordered loop that connects helix α2 to helix α4. Second, the dynamics of the α2-α3 loop in all three NLRP PYDs differ. While the α2-α3 loop in NLRP1 PYD shows significantly increased fast time scale dynamics, the α2-α3 loop is rigid in NLRP7 PYD. In NLRP12 PYD, the α2-α3 loop is slightly more flexible than in NLRP7 PYD, but much more rigid than in NLRP1 PYD. Previously, we identified a hydrophobic cluster (consisting of 6 hydrophobic residues) that stabilizes both the α2-α3 loop and helix α3 in NLRP7 PYD. Interestingly, this hydrophobic cluster only contains 3 hydrophobic residues in NLRP12 PYD. The other 3 hydrophobic residues in NLRP12 PYD are replaced by two glycines and one alanine, smaller non-polar residues, that likely account for the reduced rigidity of the α2-α3 loop. Strikingly, NLRP1 PYD lacks all 6 hydrophobic residues entirely (Figure 4A, B). Thus, the dynamics of the α2-α3 loop in NLRP12 PYD is more similar to NLRP7 PYD than to NLRP1 PYD and these differences are directly correlated with the size of the hydrophobic cluster.
Figure 4.
Structural comparison of NLRP PYDs. (A) NLRP12 PYD (light pink) overlaid with the PYDs of NLRP7 (light blue; RMSD 2.4 Å) and NLRP1 (light green; RMSD 3.1 Å). The pair wise RMSD between NLRP12 PYD and all other PYDs was calculated by superposition of helices α1-α6. The largest structural difference is localized to the α2-α3 loop as well as helix α3, which is illustrated in the current orientation. (B) Hydrophobic residues in the α2-α3 loop as well as helix α3 are highlighted as dark blue sticks and labeled. A six-residue hydrophobic cluster stabilizes the α2-α3 loop as well as helix α3 in NLRP7 PYD. The corresponding cluster in NLRP12 PYD consists only of 2 hydrophobic residues. NLRP1 PYD lacks all 6 hydrophobic residues. (C) Residues Gly33 and Trp45 in NLRP12 PYD, as well as Trp30 and Trp43 in NLRP7 PYD, are highlighted as dark red sticks and labeled. While Trp43 in NLRP7 PYD is buried and forms stacking interactions with Trp30, the corresponding Trp45 in NLRP12 PYD is surface exposed.
The hydrophobic cluster in NLRP7 PYD that stabilizes the α2-α3 loop is anchored by two stacking Trp side chains (residue Trp30 and Trp43). However, Trp30 of NLRP7 PYD is replaced in NLRP12 PYD by a Gly residue (Gly33). This substitution has two effects. First, it plays a key role in the increased flexibility of the α2-α3 loop displayed by NLRP12 PYD. Second, it also changes the role of NLRP12 PYD Trp45 (Trp43 is the corresponding residue in NLRP7 PYD). Instead of being highly buried, as it is in NLRP7 PYD, it becomes surface exposed in NLRP12 PYD (Figure 4C).
Structural Comparison of NLRP12 PYD among the death domain superfamily
The most similar structures to NLRP12 PYD, based on DALI z-scores, are the structures of ASC2 PYD (z-score: 10.5) and ASC PYD (z-score: 10.4). The overall length of NLRP12 PYD is shorter than the ASC2 PYD and a structure-based sequence comparison also identified a two amino acid deletion in the α2-α3 loop. Similar assessments are possible for the ASC PYD. Nevertheless, this has very little influence in the overall structure, as well as dynamics of the α2-α3 loop.
The electrostatic surface of PYDs has two distinct faces. One surface is formed by helices α2 and α3 and a second surface is composed of helices α1 and α4. In both the ASC and ASC2 PYDs, these surfaces are highly charged and complementary with one another. The α2-α3 electrostatic surface of NLRP12 PYD is also charged, but also has a significant hydrophobic patch clustered around Trp45 on helix α3.
Figure 5 compares the electrostatic surface of NLRP12 PYD to those of ASC, ASC2, NLRP7 and NLRP1. We used the Protein Interaction Property Similarity Analysis server32 to quantify the similarities between the electrostatic surfaces of the PYDs from NLRP1, NLRP7, NLRP12, ASC and ASC2. The PIPSA server uses the UHBD program to calculate the electrostatic potential of a protein. Pair wise calculations comparing the NLRP12 PYD to the PYDs of ASC, ASC2, NLRP7 and NLRP1 were used to assess overall electrostatic similarity, where 0 indicates identical and 2 completely different electrostatic surfaces. Based on an overall electrostatic distance, NLRP12 PYD is most similar to ASC PYD (electrostatic distance: 0.634) followed by NLRP7 PYD (electrostatic distance: 0.994), ASC2 PYD (electrostatic distance: 1.324) and NLRP1 PYD (electrostatic distance: 1.339). Therefore, based on this analysis, ASC PYD can be classified as identical/highly similar to the NLRP12 PYD (i.e., an electrostatic distance between 0 and 0.75).
Figure 5.
Electrostatics surface potential of NLRP12 PYD and other PYDs with known structures. (A) Top row: ribbon representation of the PYDs of NLRP12, and the ones of the adaptor protein ASC and its inhibitor, ASC2, facing the α2-α3 surface. Bottom row: electrostatic surface representation of the PYDs of NLRP12, ASC and ASC2. Positive surface charge is colored blue; negative surface charge is colored red; and neutral surface, white. (B) Corresponding electrostatic surface representation of the PYDs of NLRP12, NLRP7 and NLRP1.
Numerous reports have argued that differences in surface charge/hydrophobicity are the key drivers for the interaction specificity of NLRP PYDs28; 33; 34. We have tested the direct interaction of NLRP12 PYD with ASC PYD at various ratios using NMR spectroscopy (data not shown), but no chemical shift perturbations were identified in the titration experiments, showing that there is, as expected, no interaction between NLRP12 PYD and ASC PYD.
NLRP12 PYD interacts directly with the UBA domain of FAF-1
Fas-associated factor 1 (FAF-1) is a 74 kDa multi-domain protein that has been shown to function in diverse biological processes, such as the regulation of apoptosis and NF-κB activity, as well as ubiquitination and proteasomal degradation. FAF-1 consists of multiple protein-interaction domains, including a Fas-interacting domain (FID), a death effector domain interacting domain (DEDID), and multiple ubiquitin-related domains. Recently, Kinoshita et al. used yeast two-hybrid screening to search for novel FAF-1 interacting proteins and identified the PYDs of NLRP3, NLRP7 and NLRP12 as potential FAF-1 targets19. In their work, they used the entire FAF-1 FID domain (residues 1–180) as bait. The FID domain of FAF-1 consists of a UBA (ubiquitin-associated) domain (residues 1–57) and an UB1 (ubiquitin-related) domain (residues 99–180) connected by a flexible linker sequence (residues 58–98). We recently tested the interaction of NLRP7 PYD with the FAF-1 FID (residues 1–180) using NMR titrations, but were unable to detect a direct interaction24. This was expected, as no heterotypic interactions had been previously reported for a PYD domain.
Nevertheless, we performed the identical titration study with NLRP12 PYD and FAF-1 FID. Surprisingly, we detected small chemical shift perturbations, indicating a direct interaction between the two proteins. In order to investigate this interaction in more detail, the FAF-1 FID was subcloned into multiple shorter, structurally and biologically meaningful sub-domains: 1) FAF-11–57 (UBA domain only); 2) FAF-11–99 (UBA domain and flexible linker) and 3) FAF-199–180 (UB1 domain only). All FAF-1 constructs expressed solubly, were purified to homogeneity and 1D 1H NMR spectra recorded to confirm their folded state (Supplemental Figure S5). Because, it has been reported that death domain interactions are more robustly identified in higher pH solution34, all titrations were performed at pH 7 or 7.5. NMR chemical shift perturbations (detected using 15N-labeled NLRP12 PYD) showed that NLRP12 PYD interacts with the N-terminal UBA domain of FAF-1 (residues 1–57) (Supplemental Figure S6). Interestingly, when the NMR titration experiment was carried out with FAF-11–99 (UBA domain and linker region), the resulting spectrum was identical to that of the FAF-11–57 titration (Supplemental Figure S7). This shows that the UBA domain of FAF-1 is both necessary and sufficient for the interaction with NLRP12 PYD, and that the residues comprising the flexible linker region (58–98) do not play a role in this interaction. We also show that this interaction is specific, as no shifts were observed when NLRP12 PYD was titrated with FAF-199–180 (UB1 domain) (Supplemental Figure S8).
Using the results from the NMR titrations, we identified the residues of NLRP12 PYD that mediate binding with FAF-11–57. The majority of the perturbed residues (greater than two standard deviations from the mean) were located exclusively to the α2-α3 surface, comprised of helix α2, the α2-α3 loop and helix α3 of NLRP12 PYD. It is interesting to note that this surface has been previously implicated in mediating homotypic interactions of PYDs. Specifically, the interaction between NLRP12 PYD and FAF1 UBA is mediated by residues Lys27, Thr34, Thr36, Glu40, Lys42, Ile43, Trp45, G46 and Lys50 (Figure 6). The largest chemical shift changes were detected for the residues Glu40, Lys42 and Trp45. Based on the poor solubility of both proteins and the limitations of sample concentrations necessary for reliable NMR measurements, the highest feasible titration ratio used was 1:10 (NLRP12 PYD: FAF-11–57). This ratio does not identify a plateau in the titration curve that is characteristic of saturation of the interaction and necessary for a defined calculation of a Kd value. Nevertheless, based on these chemical shift changes, we estimated a dissociation constant (Kd) ≥ 50 μM between NLRP12 PYD and FAF-11–57 as well as FAF-11–99 (Supplemental Figure S9).
Figure 6.
Mapping the interaction of NLRP12 PYD with FAF-11–57. (A) Chemical shift perturbations (CSPs) calculated for NLRP12 PYD upon titration of FAF-11–57 at a molar ratio of 1:10 (10 mM Na-phosphate buffer pH 7.0, 100 mM NaCl, 0.5 mM TCEP). The color scheme denotes the intensity of the shifts observed in the titration experiment. CSP values higher than two standard deviations from the mean are colored light blue; three standard deviations, marine; and four standard deviations, purple. Experimentally derived secondary structure elements of NLRP12 PYD are depicted by grey cylinders on top of the figure. (B) NLRP12 PYD structure displaying the residues that show the highest CSP values upon titration with FAF-11–57. Side chains are depicted in stick model, labeled and colored according to A. (C) Surface representation of the NLRP12 PYD structure in the same orientation as B, displaying the residues that show the highest CSP values. These residues are clustered on the α2-α3 surface, which has been previously implicated in homotypic interactions of PYDs.
To elucidate the importance of hydrophobic contacts in the interaction between NLRP12 PYD and FAF-11–57, numerous point mutants of NLRP12 PYD residue Trp45 were generated (W45A, W45E, W45R, W45F and W45I). The ability of the NLRP12 PYD mutants to bind FAF-11–57 was then assayed using NMR titration experiments. Substitution of NLRP2 PYD W45 by an A, E or R residue completely abolished binding with FAF-11–57, as no chemical shifts changes were observed in the titration experiments (Supplemental Figures S10, S11 and S12). In contrast, chemical shifts changes were identified for W45F and W45I mutations (Supplemental Figures S13 and S14). This highlights a role for the surface-exposed hydrophobic Trp45 residue in the binding of NLRP12 PYD to FAF-11–57.
Recently, a crystal structure of FAF-15–47 was reported (PDBid 3E21), as were the chemical shift assignments for FAF-11–8121. While the domain boundaries for these constructs differ from those used in our studies, we were nevertheless able to rapidly transfer the reported sequence specific backbone assignments to FAF-11–57, allowing the detection of NLRP12 PYD caused chemical shift perturbations on 15N-labeled FAF-11–57 in a 2D [1H,15N] HSQC spectrum. However, these reverse titrations experiments (unlabeled NLRP12 PYD into 15N-labeled FAF-11–57), did not result in any significant chemical shift perturbations, up to the protein ratios technically possible (Supplemental Figures S15). The most likely explanation for the lack of detectable chemical shift perturbations is that this interaction occurs in an intermediate exchange regime when detected by FAF-11–57. Therefore, it does not lead to progressive chemical shift changes, which are the hallmarks of chemical shift perturbation mapping experiments in a fast or slow exchange regime.
In order to test this hypothesis, we measured the peak intensities of free FAF-11–57 and FAF-11–57 bound to NLRP12 PYD (at the highest titration ratio of 1:10) under identical buffer, concentration and NMR conditions. If NLRP12 PYD binds to FAF-11–57, the intensities of the peaks corresponding to those residues which interact directly with NLRP12 PYD will change. As shown in Figure 7A, I/I0 intensity changes that are 2× or 3× higher than the standard deviation were detected. These changes, which define the NLRP12 PYD interaction surface on FAF-1, occur in three α-helices that form the core structure of FAF-11–57. FAF-1 also interacts with ubiquitin and ubiquitin-like proteins and the residues involved in these interactions differ from those that interact with NLRP12 PYD21, showing that these proteins bind to distinct surfaces on FAF-1.
Figure 7.
Mapping the interaction of FAF-11–57 with NLRP12 PYD. (A) Chemical shift intensity differences for unbound FAF-11–57 and NLRP12 PYD bound FAF-11–57 (1:10 ratio; 10 mM Na-phosphate buffer pH 7.0, 100 mM NaCl, 0.5 mM TCEP; black bars). Cyan denotes values of I/I0 2× higher than the standard deviation (SD) and blue I/I0 values 3× higher than SD. The color scheme denotes the intensity of the shifts observed in the titration experiment. Experimentally derived secondary structure elements of FAF-11–57 are depicted by grey cylinders above the figure. (B) FAF-15–47 structure (PDBid: 3E21) displaying the residues that show the largest intensity changes upon titration with NLRP12 PYD. Side chains are depicted in stick model, labeled and colored according to A. (C) Surface representation of the FAF-11–57 structure, with residues that show the highest I/I0 differences colored according to A.
Discussion
PYDs are the most common member of the death domain (DD) superfamily35. PYDs play a key role in the control of signaling pathways in the immune system, as well as in many apoptotic pathways36. While numerous structures of PYDs have been reported during the last few years, the differences, both in structure and dynamics, between them are larger than the similarities. Moreover, the currently available structures have neither revealed the differential specificities for their homotypic interactions, nor have heterotypic interactions of these domains been reported in vitro. Here we report the 3-dimensional structure of human NLRP12 PYD, one of the first NLRPs discovered that functions to down-regulate TLR-dependent NF-κB activation via direct interaction with processing kinases. Furthermore, NLRP12 plays also a role in the proteasome pathway13; 15. Thus, NLRP12 is considered as an essential regulatory bridge between innate immunity and apoptotic signaling pathways.
The structure of NLRP12 PYD folds into an expected death domain fold. Using a structure-based sequence alignment of the currently three available NLRP PYD structures (NLRP1, NLRP7 and NLRP12), it was possible to identify conserved residues that form the hydrophobic core of these three PYDs. Critically, this hydrophobic core is nearly perfectly conserved among the NLRP family (NLRP1-14) and, thus, enabled us to define the overall hydrophobic core of the NLRP PYD family. However, because of a two amino acid deletion in the α2-α3 loop, helix α3 is replaced by a 310 helix in NLRP12 PYD. Furthermore, analysis of the dynamics in the α2-α3 loop of NLRP12 PYD shows slightly increased fast time scale dynamics, when compared to NLRP7 PYD. Interestingly, the PYD domain that is structurally most similar to that of NLRP12 is that of ASC2. However, the ASC2 PYD has highly charged electrostatic surfaces formed by helices α2/α3 (positive) and α1/α4 (negative), which are indicated to be essential for the interaction with ASC PYD25; 27; 37. These oppositely charged surfaces are mostly missing in NLRP PYDs. NLRP12 PYD has a higher surface charge than NLRP7 PYD (which explains why it was necessary to add 500 mM NaCl to stabilize the NLRP12 PYD sample for NMR measurements). However, NLRP12 PYD has fewer hydrophobic patches than NLRP7 PYD. Based on the differential electrostatic surfaces, it seems unlikely that the charge driven NLRP1 PYD:ASC PYD interaction can be formed between NLRP12 PYD:ASC PYD, which is typically required for the formation of an inflammasome38.
While numerous homotypic and heterotypic interactions have been reported for NLRP PYDs, very few have been confirmed in vitro. Here we tested the previously reported interaction with Fas-associated factor 1 (FAF-1), a vital protein involved in ubiquitination and proteasomal degradation regulation19. We were able to use NMR chemical shift mapping to detect a very weak interaction (estimated a Kd of ≥150 μM based on chemical shift analysis) with the N-terminal UBA (ubiquitin-associated) domain (residues 1–57) of FAF-1. This is the first in vitro confirm heterotypic interaction of a pyrin domain. The inflammatory response to danger signals is a difficult balancing act for the host and therefore must be tightly regulated. Whereas the most prominent NLRPs, including NLRP1 and NLRP3, are positive regulators of inflammatory responses, NLRP2, NLRP4 and NLRP12 were shown to be negative regulators of pro-inflammatory signaling by inhibiting NF-κB activation12; 13; 18; 39; 40.
As FAF-1 itself is indicated in inhibiting NF-κB signaling, it is likely that this interaction can lead to a synergistic effect between NLRP12 PYD and FAF-1 UBA. Thus, NLRP12 together with FAF-1 modulate the pro inflammatory response, where NF-κB possesses a key role. Therefore, to understand the molecular basis of this interaction, we performed a number of mutagenesis experiments and showed that the hydrophobic nature of a surface-exposed Trp (Trp45) is critical for the interaction. Interestingly, the identical Trp residue in NLRP7 PYD is completely buried in the hydrophobic core of this protein. This likely explains the differential interaction with the FAF-1 UBA domain.
Taken together, our analysis revealed initial insights into a non-homotypic PYD interaction between NLRP12 PYD and the FAF-1 UBA domain. In contrast to homotypic PYD:PYD interactions, which are mainly driven by electrostatic contacts and anchored by hydrophobic residues, this interaction is mainly driven by a weak hydrophobic contact. The interaction between NLRP12 PYD and FAF-1 indicates a possible mechanism for inhibition of TLR-dependent NF-κB activation.
Material and Methods
Protein Expression and Purification
NLRP12 PYD (residues 1–98) was subcloned into pHisparallelSTOP41, which encodes an N-terminal His6-purification tag and a TEV protease cleavage site. FAF-1 constructs FAF-11–99 (UBA domain and linker region; residues 1–99) and FAF-199–180 (UB1 domain; residues 99–180) were subcloned into RP1B42, which encodes a Thio6His6 expression/purification tag and a TEV protease cleavage site. FAF-11–57 (UBA domain; residues 1–57) was cloned into pETM30-MBP, which encodes an N-terminal His6-MBP purification/solubility tag and a TEV cleavage site. The plasmids were transformed into Escherichia coli BL21-Codon-Plus (DE3)-RIL (Stratagene) cells. The expression of uniformly 13C/15N-labeled and 15N-labeled protein was carried out by growing freshly transformed cells in M9 minimum medium containing 4 g/L [13C]-glucose and/or 1 g/L 15NH4Cl (Cambridge Isotope Laboratory) as the sole source of carbon and nitrogen, respectively. Cell cultures were grown at 37°C under vigorous shaking (250 rpm) in the presence of 34 μg/mL chloramphenicol and 50 μg/mL kanamycin in the case of all FAF-1 constructs, or in the presence of 34 μg/mL chloramphenicol and 50 μg/mL ampicillin in the case of NLRP12 PYD, until they reached an OD600 of 0.6. Expression of all proteins was induced by addition of 1 mM IPTG to the culture medium, and cultures were allowed to grow overnight (18 h) at 18°C under vigorous shaking (250 rpm). Cells were harvested by centrifugation and stored at −80°C.
Purification of NLRP12 PYD and all FAF-1 constructs was performed as follows. Cells were resuspended in lysis buffer (50 mM Tris pH 8.0, 500 mM NaCl, 5 mM imidazole, 0.1% Triton-X 100, supplemented with EDTA-free protease inhibitor tablets (Roche)) and lysed by high-pressure homogenization (Avestin C-3 Emulsiflex). Cell debris was removed by centrifugation at 35000 × g for 40 minutes at 4°C, and the supernatant containing soluble proteins was loaded onto a HisTrap HP column (GE Healthcare) equilibrated with 50 mM Tris pH 8.0, 500 mM NaCl, 5 mM imidazole. His6-tagged proteins were eluted with a 5–500 mM imidazole gradient. Fractions containing the protein of interest, as identified by SDS-PAGE, were pooled, incubated with His6-TEV NIa (S219V) protease, and dialyzed at 4°C against 50 mM Tris pH 7.5, 200 mM NaCl, until cleavage was complete. The untagged proteins were separated from the enzymatically-cleaved His6- or His6-MBP tag, as well as from His6-TEV by Ni+2-affinity subtraction purification. Proteins were subsequently purified by size-exclusion chromatography using a Superdex 75 26/60 column (GE Healthcare). Fractions containing the pure proteins, as identified by SDS-PAGE, were pooled and concentrated. All purifications were performed at 4°C.
NMR Spectroscopy
NMR experiments were acquired at 298 K on a Bruker Avance 500 MHz spectrometer. In addition, a 3D 13C-resolved [1H,1H] NOESY spectrum was recorded on a Bruker Avance 800 MHz spectrometer. Both spectrometers are equipped with a TCI HCN z-gradient cryoprobe. Proton chemical shifts were referenced directly to internal 3-trimethyl-sylil-1-propanesulfonic acid sodium salt (DSS). 13C and 15N chemical shifts were referenced indirectly to DSS using the absolute frequency ratios.
Chemical Shift Assignments and Structure Calculation
All NMR experiments for chemical shift assignments and structure determination were performed with either a 15N- or a 15N/13C-labeled NLRP12 PYD sample at a final concentration of 0.6 mM in 20 mM sodium phosphate pH 6.5, 500 mM NaCl, 0.5 mM tris(2-carboxiethyl-phosphine) (TCEP). The following spectra were used to achieve the sequence-specific backbone and side chain resonance assignments of NLRP12 PYD: 2D [1H,15N] HSQC, 3D HNCA, 3D HNCACB, 3D CBCA(CO)NH, 3D HNCO, 3D HN(CA)CO, 3D CC(CO)NH (CC-TOCSY mixing time of 12 ms), 3D HBHA(CO)NH, 3D HC(C)H-TOCSY (CC-TOCSY mixing time of 12 ms). TopSpin 2.1 (Bruker) was used for data acquisition and processing. NMR spectra were analyzed using the program CARA (http://www.nmr.ch).
The following spectra were used for the structure calculation of NLRP12 PYD: 3D 15N-resolved [1H,1H] NOESY (mixing time of 80 ms), 3D 13C-resolved [1H,1H] NOESY (mixing time of 80 ms), and 2D [1H,1H] NOESY (mixing time of 80 ms; acquired in 100% D2O solution). NOESY peak picking, NOESY peak assignment, and 3D structure calculation were performed automatically using the ATNOS/CANDID module in the Unio software43; 44. The input for the structure calculations of NLRP12 PYD was the amino acid sequence, the complete chemical shift lists, and the 3- and 2-dimensional NOESY spectra45. Default program parameters were used for all calculations. Constraints for backbone dihedral angles derived from 13C chemical shifts were only used in the initial structure calculation. The 20 conformers from the final CYANA30 cycle with the lowest residual CYANA target function values were energy-minimized in a water shell using CNS31 and the RECOORD46 script package (Table 1). The structure quality was assessed by the “Protein Structure Validation Suite” (PSVS http://psvs-14-dev.nesg.org). All structural comparisons throughout the manuscript were performed using the lowest-energy conformer of the ensemble of structures.
Relaxation Measurements and Analysis
15N relaxation experiments were performed in the exact same conditions as described for the structure determination of NLRP12 PYD. 15N longitudinal (R1) and transverse (R2) relaxation rates, and 15N[1H]-NOE (hetNOE) measurements were acquired using sensitivity-enhanced pulse sequences. T1 experiments were acquired with relaxation delays (T) of 20, 100, 200, 400, 550, 700, 850, and 1000 ms. T2 experiments were acquired with relaxation delays (T) of 20, 80, 110, 140, 180, 220, 250, and 350 ms. A recycle delay of 3 s between scans was used for all T1 and T2 experiments. 15N[1H]-NOEs were measured from a pair of spectra acquired with and without presaturation recorded in an interleaved manner. A recycle delay of 5 s between scans was used for the heteronuclear NOE experiments.
All spectra were processed with NMRPipe47 and analyzed with NMRView48. R1 and R2 relaxation rates were determined by fitting the peak intensities as a function of the relaxation delays using an exponential decay function, I(T) = Ae(−r/T), where I(T) is the peak intensity after a time delay T, A is the intensity at time zero and r = R1 or R2. 15N[1H]-NOEs were calculated by dividing the intensity of the peaks in the spectra recorded without presaturation by the intensity of the peaks in the presaturated spectra.
NLRP12 PYD: FAF-1 Interactions
The interaction of NLRP12 PYD with FAF-1 was investigated by NMR titration experiments using a 15N-labeled NLRP12 PYD or FAF-11–57 sample at a final concentration of 35 μM in 10 mM Na-phosphate buffer pH 7.0 or 7.5, 100 mM NaCl, 0.5 mM TCEP (pH varied depending on the pI of the FAF-1 constructs used). Titration experiments were performed with three different FAF-1 constructs (FAF-11–99, FAF-199–180 and FAF-11–57) at 1:2, 1:5, 1:10 and 1:15 molar ratios, with the highest concentration ratio dependent on the solubility of the FAF-1 construct in the case of detection by 15N-labeled NLRP12 PYD. Chemical shift perturbations (CSPs) in the 2D [1H,15N] HSQC spectrum of NLRP12 PYD upon titration with FAF-1 constructs were used to monitor binding. CSPs were calculated using the following equation: CSP = [(ΔNH)2 + (Δ15N/10)2]1/2, where ΔNH and Δ15N represent the difference between free and bound 1H and 15N chemical shifts, respectively. A dissociation constant (KD) for the interaction between NLRP12 PYD and FAF-11–57/99 were calculated using the chemical shift changes in the NMR titration experiments. Only the chemical shift resonances of NLRP12 PYD that exhibited the most significant change upon titration with FAF-11–57/99 were used for this calculation. CSPs were fitted to a one site saturation binding equation using Sigmaplot 11.0 (Systat Software Inc.).
Site-directed Mutagenesis
NLRPP12 PYD mutants W45A, W45E, W45R, W45F and W45I were generated using the QuickChange Mutagenesis kit (Agilent). All mutated DNA constructs were sequence verified (Beckman Coulter).
Supplementary Material
Acknowledgments
The authors thank Dr. Rebecca Page for discussion and careful reading of the manuscript. This work was supported by MCEXT-033534 to R.S., FWF-W1213 to C.E. and NIH R01NS056128 to W.P. 800 MHz NMR data was recorded on the 800 MHz spectrometer at Brandeis University funded by NIH S10-RR017269.
Abbreviations
- NMR
nuclear magnetic resonance
- HSQC
heteronuclear single-quantum coherence
- NOE
nuclear Overhauser effect
- RMSD
root-mean-square deviation
- NLR
nucleotide-binding domain and leucine-rich repeat containing proteins
- LRR
leucine-rich repeats
- DD
death domain
- PYD
pyrin domain
- CARD
caspase activation and recruitment domain
- ASC
apoptosis-associated speck-like protein containing a CARD
- FAF-1
fas-associated factor 1
Footnotes
Accession Numbers. Chemical shift assignments of NLRP12 PYD were deposited in the Biological Magnetic Resonance Data Bank under accession number 17305, and the atomic coordinates were submitted to the Protein Data Bank under accession code 2L6A.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–7. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 2.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–59. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 4.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Faustin B, Reed JC. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008;18:4–8. doi: 10.1016/j.tcb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–82. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 7.Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and autoimmunity. Trends Mol Med. 2011;17:57–64. doi: 10.1016/j.molmed.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proell M, Riedl SJ, Fritz JH, Rojas AM, Schwarzenbacher R. The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One. 2008;3:e2119. doi: 10.1371/journal.pone.0002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–7. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 11.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–22. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino L, Stehlik C, Oliveira V, Ariza ME, Godzik A, Reed JC. A novel PAAD-containing protein that modulates NF-kappa B induction by cytokines tumor necrosis factor-alpha and interleukin-1beta. J Biol Chem. 2002;277:35333–40. doi: 10.1074/jbc.M200446200. [DOI] [PubMed] [Google Scholar]
- 13.Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–60. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- 14.Ting JP, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327:286–90. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams J. Cutting Edge: A novel lab-on-a-tube for multimodality neuromonitoring of patients with traumatic brain injury (TBI) Lab Chip. 2009;9:1987. doi: 10.1039/b906110j. [DOI] [PubMed] [Google Scholar]
- 16.Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, McElreavey K, Sarkisian T, Grateau G, Alnemri ES, Amselem S. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105:1614–9. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macaluso F, Nothnagel M, Parwez Q, Petrasch-Parwez E, Bechara FG, Epplen JT, Hoffjan S. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp Dermatol. 2007;16:692–8. doi: 10.1111/j.1600-0625.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 18.Arthur JC, Lich JD, Aziz RK, Kotb M, Ting JP. Heat shock protein 90 associates with monarch-1 and regulates its ability to promote degradation of NF-kappaB-inducing kinase. J Immunol. 2007;179:6291–6. doi: 10.4049/jimmunol.179.9.6291. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita T, Kondoh C, Hasegawa M, Imamura R, Suda T. Fas-associated factor 1 is a negative regulator of PYRIN-containing Apaf-1-like protein 1. Int Immunol. 2006;18:1701–6. doi: 10.1093/intimm/dxl104. [DOI] [PubMed] [Google Scholar]
- 20.Park MY, Moon JH, Lee KS, Choi HI, Chung J, Hong HJ, Kim E. FAF1 suppresses IkappaB kinase (IKK) activation by disrupting the IKK complex assembly. J Biol Chem. 2007;282:27572–7. doi: 10.1074/jbc.C700106200. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Park JK, Lee JJ, Choi YS, Ryu KS, Kim JH, Kim E, Lee KJ, Jeon YH, Kim EE. Structure and interaction of ubiquitin-associated domain of human Fas-associated factor 1. Protein Sci. 2009;18:2265–76. doi: 10.1002/pro.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–9. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 23.de Sa Pinheiro A, Ehart A, Ebner N, Proell M, Schwarzenbacher R, Peti W. Backbone and sidechain (1)H, (15)N and (13)C assignments of the NLRP7 pyrin domain. Biomol NMR Assign. 2009;3:207–9. doi: 10.1007/s12104-009-9176-2. [DOI] [PubMed] [Google Scholar]
- 24.Pinheiro AS, Proell M, Eibl C, Page R, Schwarzenbacher R, Peti W. Three-dimensional structure of the NLRP7 pyrin domain: insight into pyrin-pyrin-mediated effector domain signaling in innate immunity. J Biol Chem. 2010;285:27402–10. doi: 10.1074/jbc.M110.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Alba E. Structure and interdomain dynamics of apoptosis-associated speck-like protein containing a CARD (ASC) J Biol Chem. 2009;284:32932–41. doi: 10.1074/jbc.M109.024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiller S, Kohl A, Fiorito F, Herrmann T, Wider G, Tschopp J, Grutter MG, Wuthrich K. NMR structure of the apoptosis- and inflammation-related NALP1 pyrin domain. Structure. 2003;11:1199–205. doi: 10.1016/j.str.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Natarajan A, Ghose R, Hill JM. Structure and dynamics of ASC2, a pyrin domain-only protein that regulates inflammatory signaling. J Biol Chem. 2006;281:31863–75. doi: 10.1074/jbc.M605458200. [DOI] [PubMed] [Google Scholar]
- 28.Srimathi T, Robbins SL, Dubas RL, Chang H, Cheng H, Roder H, Park YC. Mapping of POP1-binding site on pyrin domain of ASC. J Biol Chem. 2008;283:15390–8. doi: 10.1074/jbc.M801589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liepinsh E, Barbals R, Dahl E, Sharipo A, Staub E, Otting G. The death-domain fold of the ASC PYRIN domain, presenting a basis for PYRIN/PYRIN recognition. J Mol Biol. 2003;332:1155–63. doi: 10.1016/j.jmb.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Güntert P. Automated NMR Structure Calculation With CYANA. Methods Mol Biol. 2004;278:353–78. doi: 10.1385/1-59259-809-9:353. [DOI] [PubMed] [Google Scholar]
- 31.Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54 (Pt 5):905–21. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 32.Richter S, Wenzel A, Stein M, Gabdoulline RR, Wade RC. webPIPSA: a web server for the comparison of protein interaction properties. Nucleic Acids Res. 2008;36:W276–80. doi: 10.1093/nar/gkn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature. 1999;399:549–57. doi: 10.1038/21124. [DOI] [PubMed] [Google Scholar]
- 34.Esposito D, Sankar A, Morgner N, Robinson CV, Rittinger K, Driscoll PC. Solution NMR investigation of the CD95/FADD homotypic death domain complex suggests lack of engagement of the CD95 C terminus. Structure. 2010;18:1378–90. doi: 10.1016/j.str.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Park HH, Lo YC, Lin SC, Wang L, Yang JK, Wu H. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu Rev Immunol. 2007;25:561–86. doi: 10.1146/annurev.immunol.25.022106.141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed JC, Doctor KS, Godzik A. The domains of apoptosis: a genomics perspective. Sci STKE. 2004:re9. doi: 10.1126/stke.2392004re9. [DOI] [PubMed] [Google Scholar]
- 37.Espejo F, Patarroyo ME. Determining the 3D structure of human ASC2 protein involved in apoptosis and inflammation. Biochem Biophys Res Commun. 2006;340:860–4. doi: 10.1016/j.bbrc.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 38.Martinon F, Hofmann K, Tschopp J. The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr Biol. 2001;11:R118–20. doi: 10.1016/s0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 39.Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, Hornung V. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68:765–83. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontalba A, Gutierrez O, Fernandez-Luna JL. NLRP2, an inhibitor of the NF-kappaB pathway, is transcriptionally activated by NF-kappaB and exhibits a nonfunctional allelic variant. J Immunol. 2007;179:8519–24. doi: 10.4049/jimmunol.179.12.8519. [DOI] [PubMed] [Google Scholar]
- 41.Sheffield P, Garrard S, Derewenda Z. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr Purif. 1999;15:34–9. doi: 10.1006/prep.1998.1003. [DOI] [PubMed] [Google Scholar]
- 42.Peti W, Page R. Strategies to maximize heterologous protein expression in Escherichia coli with minimal cost. Protein Expr Purif. 2007;51:1–10. doi: 10.1016/j.pep.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 43.Herrmann T, Guntert P, Wuthrich K. Protein NMR structure determination with automated NOE-identification in the NOESY spectra using the new software ATNOS. J Biomol NMR. 2002;24:171–89. doi: 10.1023/a:1021614115432. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann T, Güntert P, Wüthrich K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J Mol Biol. 2002;319:209–27. doi: 10.1016/s0022-2836(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 45.Güntert P. Automated structure determination from NMR spectra. Eur Biophys J. 2009;38:129–43. doi: 10.1007/s00249-008-0367-z. [DOI] [PubMed] [Google Scholar]
- 46.Nederveen AJ, Doreleijers JF, Vranken W, Miller Z, Spronk CA, Nabuurs SB, Güntert P, Livny M, Markley JL, Nilges M, Ulrich EL, Kaptein R, Bonvin AM. RECOORD: a recalculated coordinate database of 500+ proteins from the PDB using restraints from the BioMagResBank. Proteins. 2005;59:662–72. doi: 10.1002/prot.20408. [DOI] [PubMed] [Google Scholar]
- 47.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–93. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 48.Johnson BA. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol Biol. 2004;278:313–52. doi: 10.1385/1-59259-809-9:313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.