Abstract
Purpose
We investigated the effect of circulating factors and protein kinase Cβ (PKCβ) on blood-brain barrier permeability and edema during hyperglycemic stroke.
Methods
Male Wistar rats that were hyperglycemic by streptozotocin (50 mg/kg) for 5–6 days underwent middle cerebral artery occlusion (MCAO) for 2 hours with 2 hours of reperfusion. Blood-brain barrier permeability was measured in MCAs that were ischemic (MCAO) or nonischemic (CTL) and perfused with plasma (20% in buffer) from MCAO or CTL animals. A separate set of MCAO vessels was perfused with the PKCβ inhibitor CGP53353 (0.5 μmol/L) and permeability measured. Lastly, hyperglycemic rats were treated i.v. with CGP53353 (10 or 100 μg/kg or vehicle 15 minutes prior to reperfusion and edema formation measured by wet:dry weights (n=6/group).
Results
MCAO vessels had increased permeability compared to controls, regardless of the plasma perfusate. Permeability (water flux, μm3 × 108) of CTL vessel/CTL plasma (n=8), CTL vessel/MCAO plasma (n=7), MCAO vessel/CTL plasma (n=6) and MCAO vessel/MCAO plasma (n=6) was 0.98±0.11, 1.13±0.07, 1.36±0.02, and 1.34±0.06; p<0.01). Inhibition of PKCβ in MCAO vessels (n=6) reversed the increase in permeability (0.92±0.1; p<0.01). In vivo, hyperglycemia increased edema vs. normoglycemia after MCAO (water content = 78.84±0.11% vs. 81.38±0.21%; p<0.01). Inhibition of PKCβ with 10 or 100 μg/kg CGP53353 during reperfusion prevented the increased edema in hyperglycemic animals (water content = 79.54±0.56% and 79.99±0.43%; p<0.01 vs. vehicle).
Conclusions
These results suggest that the pronounced vasogenic edema that occurs during hyperglycemic stroke is mediated in large part by activation of PKCβ.
Keywords: Protein kinase Cβ, vasogenic edema, hyperglycemia, reperfusion injury, blood-brain barrier
Hyperglycemia is common in acute stroke.1,2 Thirty to sixty percent of stroke patients have high glucose levels, regardless of preexisting diabetes, due to a generalized stress reaction and increased levels of glucocorticoids (for review see [3,4]). Hyperglycemia during acute stroke is associated with significantly worsened outcome, including larger infarction, edema formation and a higher risk of mortality.2,5–7 Both diabetic and non-diabetic patients are adversely affected by hyperglycemia, suggesting it is elevated glucose and not diabetic complications that increase stroke damage.2,7
The development of brain edema is one of the most detrimental consequences of stroke and is greatly augmented in the presence of hyperglycemia.6,8,9 Increased blood-brain barrier (BBB) permeability occurs during hyperglycemic stroke and is essential for development of cerebral edema.8–10 The BBB is therefore an important therapeutic target to limit edema formation that can be fatal during hyperglycemic stroke.6,9 While several mechanism are thought to contribute to enhanced edema during hyperglycemic stroke, activation of protein kinase C (PKC) in the cerebral endothelium is likely a central mediator of the BBB changes that occur. PKC activity is rapidly increased in endothelium in response to hyperglycemia due to de novo synthesis of diacylglycerol, the primary activator of PKC.11,12 PKC activation can directly affect BBB permeability through its ability to phosphorylate zona occluden-1(ZO-1) and disrupt tight junctions13,14 as well as promote calcium/calmodulin-dependent endothelial cell contraction.15 Further, other agents that induce BBB permeability including bradykinin, histamine and thrombin produce these effects through PKC-dependent mechanisms (for review see [9]).
Ischemic stroke is also associated with a systemic inflammatory response and release of circulating factors that could increase BBB permeability independent of the effects of either hyperglycemia or ischemia/reperfusion (I/R).16,17 Although a cascade of inflammatory events occur during I/R, release of pro-inflammatory cytokines could impact BBB integrity and exacerbate edema formation.17 Tumor necrosis factor-α, interferon gamma and interleukin-6 are increased in plasma from stroke patients and in experiment models within 4–6 hours of reperfusion.16,17 In addition to pro-inflammatory cytokines, other circulating factors are released during I/R that could increase BBB permeability and promote edema formation, including vascular endothelial growth factor (VEGF), histamine and thrombin.9
The present study had three goals. First, we determined the contribution of peripheral circulating factors vs. a direct effect of I/R to increased BBB permeability during hyperglycemic stroke. This was accomplished by measuring BBB permeability in nonischemic and ischemic vessels perfused with plasma from hyperglycemic animals that underwent 2 hours of ischemia and 2 hours of reperfusion or plasma from nonischemic controls. We found that the direct effect of I/R on BBB permeability during hyperglycemic stroke was greater than that of plasma. Thus, a second goal of this study was to determine if the direct effect of I/R on BBB permeability during hyperglycemic stroke could be prevented by inhibition of PKCβ. This isoform of PKC was chosen because it is preferentially elevated in the vasculature by hyperglycemia11,12 and hypoxia.13 Thus, inhibition of PKCβ during hyperglycemic stroke may be an important target to limit the detrimental effects of both hyperglycemia and I/R on BBB permeability. The third goal of this study was then to determine if inhibition of PKCβ activation during postischemic reperfusion in hyperglycemic animals could prevent enhanced edema formation compared to normoglycemic stroke.
Materials and Methods
Animal model of transient focal ischemia
All procedures were approved by the Institutional Animal Care and Use Committee and complied with the NIH guidelines for the care and use of laboratory animals. Male Wistar rats (~300 g) were used for all experiments. Temporary filament occlusion of the middle cerebral artery (MCA) was used to induce I/R in both normoglycemic and hyperglycemic animals, as previously described.18 Animals were anesthetized with inhaled isoflurane (1.5% in air). I/R were confirmed using laser Doppler and any animal that had <50% drop in cerebral blood flow was excluded from study. Ischemic animals were exposed to 2 hours of ischemia and 2 hours of reperfusion by suture removal. Sham control animals (CTL) underwent anesthesia and a midline incision without filament occlusion. Animals were made hyperglycemic by a single intraperitoneal injection of streptozotocin (STZ, 50 mg/kg) 5–6 days prior to middle cerebral artery occlusion, also as previously described.18 Glucose was measured on the day of the surgery by a commercially available glucose monitor (Freestyle Lite, Abbott, Abbott Park, IL).
The middle cerebral artery occlusion (MCAO) model was used in hyperglycemic rats to both obtain plasma and distal MCAs exposed to I/R for measurement of BBB permeability, described below. Plasma from hyperglycemic animals was obtained from trunk blood and collected into vacutainer tubes containing heparin. Blood was centrifuged at 1400–1600 g, the plasma removed, aliquoted, and the pooled samples frozen at −80 °C until experimentation.
Measurement of BBB permeability in vitro in response to plasma, I/R and PKCβ inhibition
BBB permeability was measured in isolated and pressurized MCAs obtained from animals that underwent MCAO or sham control (CTL) surgery. MCAs were taken between the M2 and M3 region to eliminate any potential damage from the filament to the BBB during the MCAO procedure. Arteries were dissected from the ischemic side of the brain (MCAO arteries) or the right side for control (CTL arteries). The arteries were mounted on a glass cannula in an arteriograph chamber and perfused with plasma (20% in buffer) obtained from CTL or MCAO animals, as previously described.19 For these experiments, all animals were hyperglycemic (described above) and the perfusate was matched to the level of glucose of the animals from which MCAs were taken. Thus, four groups of vessels were compared in order to assess the contribution of circulating factors vs. direct effects of I/R on BBB permeability: Control vessel + Control plasma (CTLv/CTLp; n=8); Control vessel + MCAO plasma (CTLv/MCAOp; n=7); MCAO vessel + Control plasma (MCAOv/CTLp; n=6); MCAO vessel + MCAO plasma (MCAOv/MCAOp; n=6).
BBB permeability of isolated and pressurized MCAs perfused with different plasmas was accomplished as previously described with modifications.19,20 Briefly, arteries were perfused with plasma and mounted within an arteriograph chamber that was superfused with physiological saline solution (PSS) at pH 7.4±0.05 and kept at 37 °C. The proximal cannula of the arteriograph chamber was connected to an in-line pressure transducer and servo system that allowed for measurement and adjustment of intravascular pressure. The vessels were only mounted on one cannula and tied off at the other end to prevent leaks not due to filtration. MCAs were equilibrated at an intravascular pressure of 60 mmHg for 3 hours. Intravascular pressure was then increased to 80 mmHg and the servo controlling pressure was disconnected from the pressure transducer. This allowed measurement of the pressure drop without compensation by the servo system. The measured pressure drop due to filtration of water through the vessel wall in response to hydrostatic pressure was used as a measure of BBB permeability (please see Supplemental Figures S1A and S1B at http://stroke.ahajournals.org), as previously described.20 The drop in pressure was then converted to flux through the vascular wall as a measure of permeability using a conversion curve that relates the volume of water coming out of the cannula per mmHg (please see Supplemental Figure S2 at http://stroke.ahajournals.org). The MCA was used for these experiments because these vessels have BBB properties,9,21 can be exposed to I/R and plasma, and are kept in their physiological, pressurized state. This method of measuring BBB permeability has been used successfully in previous studies.19,20
A separate set of MCAs from hyperglycemic animals that were either CTL or MCAO vessels were perfused with CTL plasma plus 0.5μmol/L of the PKCβ inhibitor CGP53353 (n=6) to determine if inhibition of PKCβ reversed the increase in BBB permeability in MCAO vessels. According to the manufacturer, CGP53353 is a selective inhibitor of PKCβII but does inhibit PKCβI at 10-fold higher concentrations (IC50 values are 0.41 μmol/L for PKCβII and 3.8 μmol/L for PKCβI). We therefore chose to use a concentration that was relatively selective for PKCβII over PKCβI. The selectivity of CGP53353 for PKCβII inhibition was tested previously.12 The efficacy of CGP53353 for PKC-induced permeability was determined in a separate set of experiments in which permeability was measured in response to 0.05 μmol/L indolactam-V, a non-selective activator of PKC, in the absence and presence of 0.5 μmol/L CGP53353. Supplemental Figure 4 shows that this concentration of CGP53353 prevented the increase in permeability due to PKC activation with indolactam-V (please see Figure S3 at http://stroke.ahajournals.org).
Inhibition of PKCβ during MCAO and measurement of brain water content
Separate sets of animals underwent middle cerebral artery occlusion for measurement of vasogenic edema using wet and dry weights. All animals underwent 2 hours of ischemia and 2 hours of reperfusion. Ten minutes prior to reperfusion, hyperglycemic animals were treated intravenously with either 10 μg/kg or 100 μg/kg CGP53353 to inhibit PKCβ during reperfusion. These doses were chosen based on estimates of IC50 values for inhibition of PKCβI and PKCβII. Treated hyperglycemic animals were compared to both hyperglycemic and normoglycemic animals that were infused with vehicle (sterile saline). Blood gases and pH were maintained within normal ranges (Supplemental Table S1 at http://stroke.ahajournals.org). At the end of the reperfusion period, the animals were decapitated and the brain removed for measurement of water content, as previously described.18 Thus, four groups of animals underwent middle cerebral artery occlusion and were compared to determine the effect of hyperglycemia and PKCβ inhibition on vasogenic edema: normoglycemic vehicle-treated (n=6); hyperglycemic vehicle-treated (n=6), hyperglycemic + 10 μg/kg CGP 53353 (n=6), and hyperglycemic + 100μg/kg CGP53353.
Measurement of PKCβ activation in the cerebral circulation during hyperglycemia
In order to determine if hyperglycemia increased PKCβ activation in the cerebral circulation in our model, we examined phosphorlyation of PKCβ using immunoblotting. Cerebral vessels were isolated from brain tissue and processed for Western analysis, as described in Supplemental materials (see http://stroke.ahajournals.org).
Drugs and solutions
In vitro BBB permeability experiments were conducted in a bicarbonate-based PSS, the ionic composition was (mmol/L): NaCl 119.0, NaHCO3 24.0, KCl 4.7, KH2PO4 1.18, MgSO4•7H2O 1.17, CaCl2 1.6, EDTA 0.026, and glucose 5.5. PSS was made each week and stored without glucose at 4 °C; glucose was added to the PSS prior to each experiment. PSS was aerated with 5% CO2, 10% O2 and 85% N2 to maintain pH. CGP53353 was purchased from Tocris (Ellisville, MO).
Data calculations and statistical analysis
BBB permeability was compared as water flux over time between the different plasmas and vessel combinations using two-way analysis of variance (ANOVA) with 4 treatment groups and a posthoc Student-Newman-Keuls test for multiple comparisons. To determine the effect of PKCβ inhibition on BBB permeability, the flux at each time point was compared between CTL, MCAO and MCAO + CGP53353 all perfused with CTL plasma using one-way ANOVA with 3 treatment groups and a posthoc Student-Newman-Keuls test for multiple comparisons. Percent water content was compared between ipsilateral and contralateral sides of the brain using paired t-test. Percent water content was compared between groups for both contralateral and ipsilateral sides of the brain separately using ANOVA with 4 treatment groups and a posthoc Student-Newman-Keuls test for multiple comparisons.
Results
Role of circulating factors and I/R in BBB permeability during hyperglycemic stroke
Circulating factors can increase BBB permeability independent of I/R.19 We therefore compared BBB permeability in nonischemic MCAs perfused with plasma from CTL (nonischemic) hyperglycemic animals or hyperglycemic animals that underwent MCAO (Figure 1). MCAO plasma tended to increase BBB permeability in CTL vessels but this was not statistically significant. In contrast, MCAO arteries that were exposed to I/R were significantly more permeable compared to CTLv/CTLp vessels, regardless of the plasma perfusate. Further, the combination of MCAO plasma to the MCAO vessels did not have an additive effect on BBB permeability. Thus, there was no significant interaction between plasma and vessel type.
Figure 1. Effect of plasma and I/R on BBB permeability during hyperglycemic stroke.
The effect of circulating factors vs. a direct effect of I/R on BBB permeability was assessed in MCA from animals that were nonischemic controls and perfused with plasma from nonischemic animals (control vessel/control plasma; CTLv/CTLp, closed circles) or control vessels perfused with plasma from MCAO animals (CTL vessel/MCAO plasma; CTLv/MCAOp, open circles). The effect of I/R on BBB permeability was assessed in vessels exposed to MCAO for 2 hours with 2 hours of reperfusion and perfused with plasma from control animals (MCAO vessel/CTL plasma, MCAOv/CTLp, closed down triangles) or MCAO vessels perfused with plasma from MCAO animals (MCAO vessel/MCAO plasma, MCAOv/MCAOp, open up triangles). I/R significantly increased vascular permeability, as measured by the flux of water in response to hydrostatic pressure, regardless of the plasma perfusate. **p<0.01 vs. CTLv/CTLp.
Role of PKCβ in enhancing BBB permeability during hyperglycemic stroke
Because plasma did not have a significant effect on BBB permeability, we chose to focus on other mechanisms that might account for increased permeability following I/R. Thus, we used vessels perfused with CTL plasma only for subsequent studies. Figure 2 shows that BBB permeability was significantly increased in MCAO vessels compared to CTL vessels. Perfusion of MCAO vessels with the PKCβ inhibitor CGP53353 reversed the increase in BBB permeability. In fact, permeability of the MCAO vessel in which PKCβ was inhibited had similar permeability as CTL vessels.
Figure 2. Effect of PKCβ inhibition on BBB permeability during hyperglycemic stroke.
Perfusion of ischemic hyperglycemic MCAs with 0.5 μmol/L of the PKCβ inhibitor CGP53353 (MCAO + GCP53353, closed up triangles) reversed the increase in BBB permeability that was similar to nonischemic controls (CTL, closed circles) but significantly different from ischemic MCAs (MCAO, closed down triangles). **p<0.01 vs. sham/sham plasma and MCAO/sham plasma.
Role of PKCβ in enhancing vasogenic edema during hyperglycemic stroke
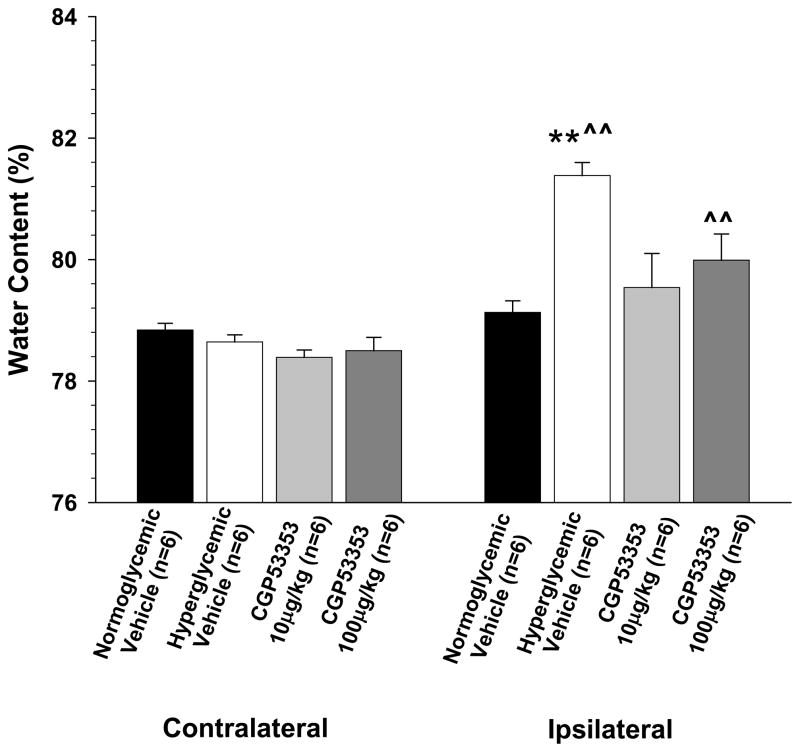
The in vitro studies above suggested there was a direct effect of I/R on the BBB during hyperglycemic stroke to increase permeability that was reversed by inhibition of PKCβ. As increased BBB permeability is a primary contributor to vasogenic edema formation,9 we next wanted to determine if in vivo treatment with the PKCβ inhibitor CGP53353 could prevent increased edema formation during hyperglycemic stroke. Figure 3 shows the percent water content of contralateral and ipsilateral cerebral cortex after middle cerebral artery occlusion in normoglycemic and hyperglycemic animals. Notice that hyperglycemia significantly increased water content in the ipsilateral brain compared to normoglycemic vehicle-treated animals, demonstrating the significant effect of hyperglycemia on vasogenic edema during stroke. Treatment to inhibit PKCβ 10 minutes prior to reperfusion prevented the increase in edema formation with both doses of CGP53353 being effective. Thus, similar to the in vitro BBB measurements, inhibition of PKCβ during reperfusion prevented edema formation in hyperglycemic animals in vivo.
Figure 3. Effect of PKCβ inhibition on edema formation during hyperglycemic stroke.
Hyperglycemia significantly increased brain water content in the ipsilateral cortex of vehicle-treated animals compared to normoglycemic animals. The increase in brain water content was prevented by treatment with the PKCβ inhibitor CGP53353 15 minutes prior to reperfusion. Both concentrations of CGP53353 (10 μg/kg and 100 μg/kg) were effective at preventing edema formation. **p<0.01 vs. ipsilateral;; ^^p<0.01 vs. contralateral.
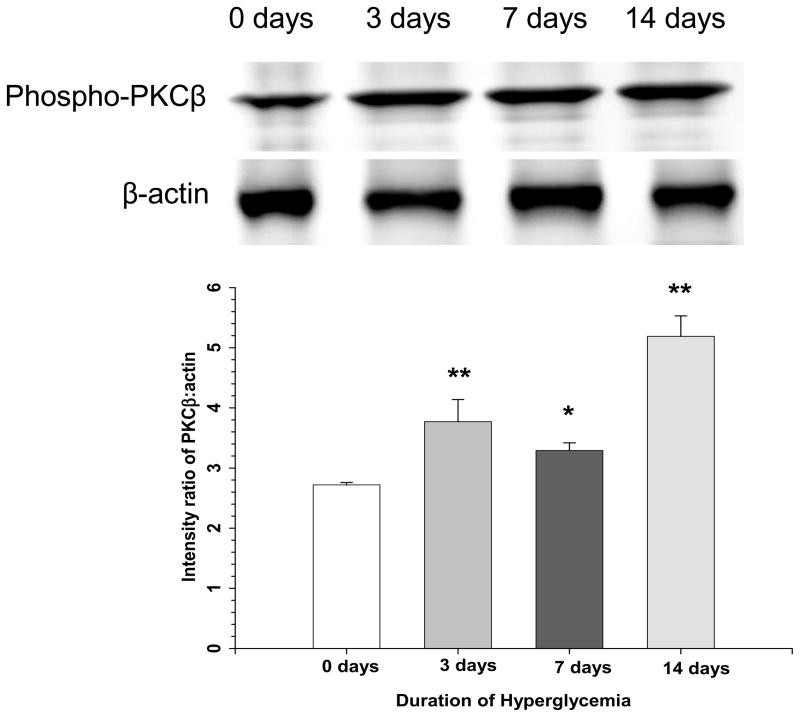
Hyperglycemia increases PKCβ activity in endothelium and is thought to underlie many of the vascular complications associated with diabetes.11,12 To further evalute involvement of PKCβ in mediating increased permeability and vasogenic edema in hyperglycemic animals, we measured levels of phosphorylated PKCβ in isolated cerebral vessels by Western blot. Figure 4 shows that phosphorylated PKCβ is significantly increased in the cerebral circulation at 3, 7 and 14 days of hyperglycemia compared to normoglycemic controls (0 days of hyperglycemia). Thus, PKCβ activation occurs during hyperglycemia in the cerebral circulation. These findings are consistent with our pharmacological and physiological data supporting the concept that PKCβ is a key contributor to increased permeability and vasogenic edema in hyperglycemic animals during I/R.
Figure 4. Effect of hyperglycemia on PKCβ activation in the cerebral circulation.
Western blot showing increased phosphorylated PKCβ in cerebral vessels isolated from brains of animals that were exposed to hyperglycemia for 3, 7, and 14 days compared to normoglycemic controls (0 days of hyperglycemia).
Discussion
There were several major findings of the present study. First, we investigated the effect of circulating factors present during hyperglycemic stroke on BBB permeability compared to direct effects of I/R by measuring permeability of MCAs that were either nonischemic or exposed to I/R and perfused with plasma from control vs. MCAO animals. We found that while MCAO plasma had little effect on permeability in nonischemic vessels, I/R significantly increased vascular permeability. Second, the effect of I/R on BBB permeability during hyperglycemic stroke was reversed by inhibition of PKCβ with CGP53353, suggesting that activation of PKCβ is an underlying mechanism by which permeability was increased. Activation of PKCβ in cerebral vessels during hyperglycemia was confirmed by Western blot of phosphorylated PKCβ. Lastly, treatment to inhibit PKCβ during postischemic reperfusion in hyperglycemic animals prevented increases in brain water content. Thus, it appears that PKCβ inhibition may be an important therapeutic target for prevention of fatal edema formation that is relatively common during hyperglycemic stroke.
The findings from this study demonstrate that inhibition of PKCβ reversed the increased permeability of hyperglycemic MCAs that were exposed to I/R. These results suggest that increased BBB permeability can be a rapid and reversible process involving PKCβ activation. For example, zona occludens-1 (ZO-1) has been shown to be a substrate for PKC and thus PKCβ activation could directly affect the integrity of the tight junction through phosphorylation.14 Kim et al., showed using a cell culture model that hypoxia-induced increased BBB permeability and disruption of occludin and ZO-1 was attenuated by PKCβ inhibition.13 Alternatively, PKCβ activation could affect endothelial calcium to enhance contractile properties, a primary means of increasing paracellular permeability.15 Although the regulation of endothelial cell calcium is not well-understood, activation of transient receptor potential V4 (TRPV4) channels is thought to be a primary contributor.22 These channels are expressed in cerebral endothelium and are activated by PKC.23 Lastly, PCKβ inhibition could protect the BBB through decreasing oxidative stress. PKC activation increases superoxide production through a direct effect on NADPH oxidase.24 In a similar study, three days of hyperglycemia prior to MCAO caused increased edema and BBB permeability that was prevented in a transgenic rat overexpressing superoxide dismutase, suggesting superoxide production is a primary means of enhancing stroke damage during hyperglycemia.10 It is worth noting that normoglycemic animals did not exhibit increased edema formation at this time period of I/R (Figure 3), demonstrating the significant effect of hyperglycemia on the BBB. These results support the concept that hyperglycemia-induced PKCβ activation has a greater role in edema formation during I/R than ischemia alone.
An inflammatory reaction occurs peripherally and centrally in response to stroke and includes release of pro-inflammatory cytokines that can affect BBB integrity.16,17,25 Circulating factors have been shown to have a significant effect on BBB properties that could enhance the detrimental effects of I/R.19 In the present study, we found that nonischemic arteries perfused with plasma from hyperglycemic MCAO animals did not significantly increase BBB permeability. However, MCAs were exposed to plasma for only 3 hours and it is possible that a longer exposure to the plasma would have had a greater effect on barrier properties. Another limitation of this approach was that plasma was diluted to 20% prior to perfusion because of limited quantities. Physiologically, normal plasma concentration in blood is ~55% and thus a more concentrated plasma may have a greater effect. However, a previous study found that 20% plasma from preeclamptic women significantly increased BBB permeability suggesting this concentration is sufficient to affect barrier properties under certain conditions.19
Summary and Conclusions
In the present study, we found that during MCAO, hyperglycemia significantly increased BBB permeability and edema formation compared to normoglycemic stroke. The effect on barrier properties appeared to be predominantely due to direct effects of I/R and not circulating factors. In addition, increased BBB permeability and edema formation during hyperglycemic stroke was reversed by inhibition of PKCβ, suggesting that this pathway may be an important therapeutic target for limiting edema formation.
Supplementary Material
Acknowledgments
Sources of Funding
We gratefully acknowledge the continued support of the NIH grants RO1 NS043316 and PO1 HL95488-01. We also gratefully acknowledge the Totman Medical Research Trust.
Footnotes
Disclosure/conflict of interest
None.
References
- 1.Allport L, Baird T, Butcher K, Macgregor L, Prosser J, Colman P, et al. Frequency and temporal profile of poststroke hyperglycemia using continuous glucose monitoring. Diabetes Care. 2006;29:1839–1844. doi: 10.2337/dc06-0204. [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 3.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 4.Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 5.Matchar DB, Divine GW, Heyman A, Feussner JR. The influence of hyperglycemia on outcome of cerebral infarction. Ann Intern Med. 1992;117:449–456. doi: 10.7326/0003-4819-117-6-449. [DOI] [PubMed] [Google Scholar]
- 6.de Courten-Myers G, Kleinholz M, Wagner KR, Myers RE. Fatal strokes in hyperglycemic cats. Stroke. 1989;20:1707–1715. doi: 10.1161/01.str.20.12.1707. [DOI] [PubMed] [Google Scholar]
- 7.Pulsinelli W, Levy DE, Sigsbee B. Increased damage after ischemic stroke in patients with hyperglycemia with or without established diabetes mellitus. Am J Med. 1983;74:540–544. doi: 10.1016/0002-9343(83)91007-0. [DOI] [PubMed] [Google Scholar]
- 8.Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2007;27:1573–82. doi: 10.1038/sj.jcbfm.9600454. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla MJ. Stroke and the Blood-Brain Interface. In: Dermietzel R, Spray D, Nedergaard M, editors. Blood-brain Barrier Interfaces. Weinheim, Germany: Wiley Press; 2006. pp. 631–634. [Google Scholar]
- 10.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee T-S, Saltsman K, Ohashi H, King G. Activation of protein kinase C by elevation of glucose concentration: Proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci USA. 1989;86:5141–5144. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 12.Kouroedov A, Eto M, Joch H, Volpe M, Lüscher TF, Cosentino F. Selective inhibition of protein kinase Cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–96. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- 13.Kim YA, Park SL, Kim MY, Lee SH, Baik EJ, Moon CH, et al. Role of PKCbetaII and PKCdelta in blood-brain barrier permeability during aglycemic hypoxia. Neurosci Lett. 2010;468:254–258. doi: 10.1016/j.neulet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Clarke H, Marano CW, Soler AP, Mullin JM. Modification of tight junction function by protein kinase C isoforms. Adv Drug Deliv Rev. 2000;41:283–301. doi: 10.1016/s0169-409x(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: modulation of basal and agonist-stimulated venular permeability. Am J Physiol. 1997;272:H1437–H1443. doi: 10.1152/ajpheart.1997.272.3.H1437. [DOI] [PubMed] [Google Scholar]
- 16.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad M, Graham SH. Inflammation after stroke: Mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1:74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cipolla MJ, Godfrey JA. Effect of hyperglycemia on brain penetrating arterioles and cerebral blood flow before and after ischemia/reperfusion. Transl Stroke Res. 2010;1:127–134. doi: 10.1007/s12975-010-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amburgey O, Abbie C, Chapman, May Victor, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: Role of VEGF signaling. Hypertension. 2010;56:1003–1008. doi: 10.1161/HYPERTENSIONAHA.110.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts TM, Chapman AC, Cipolla MJ. PPARγ activation with rosiglitazone reverses increased hydraulic conductivity induced by chronic hypertension. Am J Physiol. 2009;297:H1347–H1353. doi: 10.1152/ajpheart.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt AM, Jones HC. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 1992;569:100–105. doi: 10.1016/0006-8993(92)90374-i. [DOI] [PubMed] [Google Scholar]
- 22.Marrelli SP, O’neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 23.Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 25.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–1396. doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.