Abstract
Humans exhibit a remarkable capacity for flexible thought and action. Despite changing internal needs and external context, individuals maintain stable goals and pursue purposeful action. Functional neuroimaging research examining the neural underpinnings of such behavioral flexibility has progressed within several distinct traditions, as evident in the largely separate literatures on “cognitive control” and on “decision making.” Both topics investigate the formulation of desires and intentions, the integration of knowledge and context, and the resolution of conflict and uncertainty. Additionally, each recognizes the fundamental role of the prefrontal cortex in supporting flexible selection of behavior. But despite this notable overlap, neuroimaging studies in cognitive control and decision making have exerted only limited influence on each other, in part due to differences in their theoretical and experimental groundings. Additionally, the precise organization of control processing within prefrontal cortex has remained unclear, fostering an acceptance of vague descriptions of decision making in terms of canonical cognitive control functions such as “inhibition” or “self-control.” We suggest that a potential unifying role for models of the hierarchical organization of action selection within prefrontal cortex. These models provide an important conceptual link between decision-making phenomena and cognitive-control processes, potentially facilitating cross-fertilization between these topics.
Keywords: Cognitive control, Decision making, Neuroeconomics, Prefrontal cortex
1. Introduction
Human thoughts and actions are notable for their flexibility. In the face of environmental uncertainty, we adaptively adjust our immediate behavior to reach our long-term goals. This flexibility carries significant computational costs: the brain must reconcile the shifting relationships among external stimuli, inhibit unimportant stimuli to focus on current goals, integrate past and present information, and project the consequences of an immediate action for future outcomes. Collectively, these psychological processes are referred to as “cognitive control” (Miller and Cohen, 2001).
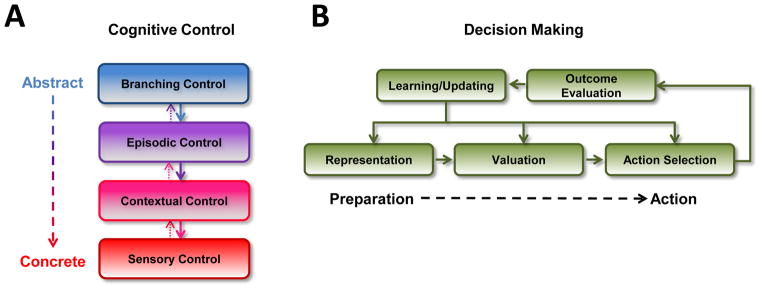
Research on the neural basis of cognitive control has focused on the prefrontal cortex (PFC), particularly its lateral (Brass et al., 2005; Koechlin and Summerfield, 2007; Petrides, 2005) and dorsomedial (Botvinick et al., 2004; Paus, 2001; Ridderinkhof et al., 2004a; Rushworth et al., 2004) aspects. Seminal early work described control processes in broad terms, based on deficits shown in animals and humans with prefrontal lesions (Franz, 1902; Milner, 1963; Shallice and Burgess, 1991; Spaet and Harlow, 1943). The advent of human functional neuroimaging, along with parallel advances in single-unit electrophysiology, has allowed researchers to parse cognitive control into a set of specific sub-processes associated with distinct parts of PFC (Carter et al., 2000; Johnston et al., 2007; Mansouri et al., 2007; Smith and Jonides, 1999). New models (Figure 1a) integrate psychological properties of cognitive control (e.g., organizing goals at multiple temporal scales) with functional properties of PFC (e.g., connectivity with other regions; Koechlin et al., 2003). Though many aspects of prefrontal organization remain unknown, the development of new models for behavioral control represents one the most active areas of research in cognitive neuroscience.
Figure 1. Models of cognitive control and of decision making.
(A) In recent models of cognitive control, such as the cascade model shown here, control processes are organized according to their level of abstraction: from low-level control over actions to high-level control over broad plans for behavior. Information transfer is asymmetric, with processing of abstract information exerting a stronger influence (solid arrows) over more concrete processing than the reverse (dotted arrows). (B) Models of decision making, like that of Rangel and colleagues (2008) shown here, represent processes in terms of their functional, sequential contributions to choice. Potential actions are integrated with contextual/motivational information, assigned a value, and then compared in order to execute a decision/select action. Feedback about outcomes alters computations at each stage of processing.
In parallel, research on the neural basis of decision making – often described as the emerging discipline of “neuroeconomics” or “decision neuroscience” – has emphasized similar sets of psychological concepts and brain substrates. As outlined in typical reviews of this field, successful decision making requires adaptive behavior in a range of contexts: identifying a set of choice options, inhibiting the temptation of immediate or certain outcomes, integrating different variables like probability and value, and projecting the consequences of a choice for our goals (Figure 1b; Camerer et al., 2005; Platt and Huettel, 2008; Rangel et al., 2008). Brain substrates within PFC have been found to play a critical role in flexible decision making (Koechlin and Hyafil, 2007; Ridderinkhof et al., 2004b), in part through modulatory influences on affective and reward systems (Beauregard et al., 2001; Hare et al., 2009; Knoch et al., 2006).
Despite this striking conceptual and mechanistic overlap, human neuroimaging research into decision making has proceeded in a surprisingly separate fashion from research examining cognitive control. There have been only limited attempts to incorporate models of cognitive control into decision neuroscience research (e.g., Daw et al., 2006), with most current work describing PFC function in broad terms of “self-control” or “inhibition” (Figner et al., 2010; Hare et al., 2009; Knoch et al., 2006). Cognitive control research, in turn, has yet to capitalize on the innovations in experimental design common to decision neuroscience, such as advanced modeling of decision behavior or reinforcement learning (Dayan and Daw, 2008; Hsu et al., 2009; Pine et al., 2009). Yet, recent developments in each area suggest that conditions may be ripe for the emergence of new connections between cognitive control and decision neuroscience research.
2. Contrasting Approaches to Flexible Behavior
Neuroimaging research in these two domains – cognitive control and decision making – shares a common goal of understanding the neural systems supporting flexible selection of behavior, particularly in the face of uncertainty. Yet, researchers in each area pursue this goal via independent research traditions, emphasize different sorts of psychological processes, and employ distinct experimental designs and protocols. These differences of method and tradition obscure a key similarity: these areas examine complimentary action-selection mechanisms at overlapping stages in the preparation-action-feedback cycle characteristic of goal-directed behavior.
Interest in the neural basis of cognitive control emerged relatively recently from the longstanding interest in psychological processes related to executive function (Fuster, 2008). Within the context of neuroscience research, cognitive control refers to the goal-directed biasing of neural processing, as when the PFC exerts a modulatory influence on other brain regions (Miller and Cohen, 2001). Commonly studied aspects of cognitive control include resolving conflict between competing action representations, switching of resources between concurrent tasks, and learning and implementing rules for behavior. To evoke these control processes, researchers often employ tasks which ask participants to act contrary to an overlearned tendency or to withhold a prepotent response, as in the Stroop (Egner and Hirsch, 2005), Simon (Peterson et al., 2002), and stop-signal tasks (Aron and Poldrack, 2006a). Other common paradigms require participants to learn and implement experimenter-provided rules for mapping stimuli to responses, often under conditions which require flexible switching between task rules or response strategies (Bunge, 2004a). These tasks tend to emphasize experimental control of behavior, requiring participants to pursue artificial goals or respond according to rules dictated by the experimenter.
Neuroscientific research into the mechanisms of decision making has been oriented toward understanding particular phenomena, rather than psychological processes. In large part, this reflects the tradition within behavioral economic research – and, to a lesser extent, within judgment and decision making research – to treat choices themselves as the explanatory targets for models (Bateman et al., 2004; Hensher and Bradley, 1993). Thus, research in decision neuroscience frequently adopts experimental paradigms that evoke interesting choice biases, such as ambiguity aversion (Huettel et al., 2006), loss aversion (Tom et al., 2007), framing effects (De Martino et al., 2006), or counterintuitive rejection of money in economic games (Sanfey et al., 2003). Most studies require participants to make repeated and meaningful decisions, typically by linking the choice outcomes to participants’ compensation. This aligns participants’ personal interests with the goals of the experimenter, allowing the choices in the experiments to be used as an index of preferences within choice functions (Grether and Plott, 1979; Sen, 1971). Accordingly, research has heretofore emphasized the study of how individuals assess the desirability of each decision option and compare those options within some common neural currency (Hare et al., 2010; Smith et al., 2010), or “valuation” and “value comparison”, respectively (Montague and Berns, 2002).
Three methodological differences may particularly underlie the divergence between cognitive control and decision neuroscience research. First, studies of cognitive control’s neural mechanisms typically target a particular brain region (i.e., the lateral PFC), whereas studies of decision making investigate particular phenomena (e.g., temporal discounting). Second, cognitive-control paradigms generally involve the learning and execution of experimenter-defined rules mapping stimuli to actions, while participants in decision-making paradigms are provided incentives to choose according to their own personal preferences. Third, paradigms in cognitive control often (but not always) seek to minimize interindividual heterogeneity in behavior, to ensure a common process across participants, whereas decision-making studies use interindividual variability to better understand brain function (Chiu and Yantis, 2009; McGuire and Botvinick, 2010; Venkatraman et al., 2009a).
Despite these methodological differences, both cognitive-control and decision-making experiments often target neural computations occurring within the action-selection cycle. A response inhibition task and a gambling task, for example, seem superficially dissimilar, and would typically be used to investigate different processes. These tasks share key similarities from an action-selection perspective, however: both require the representation of a controlling goal, the integration of past experience (from prior trials or individual preferences), the selection of the most goal-appropriate response, and the use of feedback to reinforce or modify future response tendencies. Accordingly, cognitive control and decision neuroscience studies address neural computations occurring throughout overlapping stages of the action-selection cycle, from goals through action to feedback. (The literatures do differ in the domain of control processes required, as when studying response conflict versus value comparisons.) Thus, although each literature approaches the problem of flexible action selection with different methods and traditions, there are fundamental similarities between the action-selection processes their tasks engage and between the neural computations supporting that processing.
3. Potential Integration: Specifying Control Processes
From seminal neurological observations to modern functional neuroimaging, substantial evidence implicates the lateral prefrontal cortex (lPFC) in goal-directed behavior. Behavioral deficits following brain lesions have consistently implicated the PFC in the exercise of cognitive control (Kimberg et al., 1997; Robbins, 1996), and prefrontal patients evince deficits in decision making, motivation, planning, and simulating actions, despite often largely intact perceptual abilities (Bechara et al., 1994; Eslinger and Damasio, 1985; Shallice, 1982). Such cases provided early clues to the control functions supported by the lPFC, and remain experimentally important today (Badre et al., 2009). In recent years, however, advances in functional neuroimaging have revolutionized our understanding of the mechanisms of prefrontal control. These tools allow neuroscientists to explore in detail the functional organization of cognitive control within the lPFC.
A key question within cognitive control research has been whether lateral prefrontal control processing might demonstrate some form of topographic organization. Findings from functional neuroimaging studies have provided converging evidence for a rostral-caudal axis of control processing (Badre and D’Esposito, 2007; Koechlin et al., 2003). Control over concrete stimulus-response associations is enacted by caudal regions, such as the dorsal premotor cortex. More abstract action representations, such as plans for simple sequences or rules for selecting action based on context, are processed in more rostral areas, such as the dorsolateral PFC. Finally, control over highly abstract plans, goals, and response strategies is exercised by the most anterior portion of PFC, the frontopolar cortex. Control processing along this rostral-caudal axis is hierarchical, such that increasingly abstract control engages additional cortical regions along the axis (Badre, 2008; Christoff and Gabrieli, 2000).
Influential examples of this rostral-caudal framework are the cascade model (Koechlin et al., 2003) and the policy abstraction model (Badre and D’Esposito, 2009). The cascade model argues that control processes are organized according to their degree of temporal abstraction away from the present. That is, most posterior regions of lPFC support control based on the current context (e.g., mapping actions onto stimuli as they are perceived). More anterior regions integrate current information with information carried forward from a prior context, including longer-term goals. The policy abstraction model describes an explicit hierarchy in which higher-order abstract rules manage lower-order, concrete rules for action selection. First-order policies allow for selection between multiple competing actions (least abstract), second-order policies enable selection between different first-order policies (more abstract), and so on up multiple levels of increasing abstraction. Though these two models conceptualize abstraction and control in somewhat different ways, they posit a nearly identical rostral-caudal axis supported by very similar neuroimaging results (Badre and D’Esposito, 2007; Koechlin et al., 2003) and by data from patients with PFC lesions (Badre et al., 2009) or schizophrenia (Chambon et al., 2008). Together, these findings present a compelling case for the organization of action selection within lPFC according to a principle of abstraction.
Such hierarchical models of action selection have been influential within cognitive control research, but they offer even greater potential advantages to researchers exploring the functional neuroanatomy of decision making. Lateral PFC activity is a common finding in decision neuroscience (Liu et al., 2010; Mohr et al., 2010), but functional interpretations of this processing remain rudimentary. This may be in part due to decision neuroscience’s focus on behavioral phenomena and individual differences, as well as the use of more open-ended designs to evoke preference-driven behavior. While advantages in many regards, these design conventions have their drawbacks: decision neuroscience research has identified lateral prefrontal contributions to a variety of flexible, goal-driven behaviors (e.g. Bach et al., 2009; Bhatt et al., 2010; Serences, 2008), yet failed to integrate these findings within a common explanatory framework.
Hierarchical models of action selection drawn from the cognitive control literature suggest a ready antidote to the lack of structure within the decision neuroscience literature. These models provide an explanatory framework that can be used to generate predictions regarding a variety of goal-directed behaviors, such as implementing complex or layered control, organizing sub-tasks, or optimizing behavior across response strategies. Under such conditions, lPFC activation at different levels of the rostral-caudal hierarchy should correspond to control exerted at characteristic levels of abstraction. This potential to segregate levels of control processing makes these models especially well-suited for application to decision neuroscience, where they could strengthen inferences regarding the control processes active throughout the lPFC during decision making. Researchers examining a few topics of shared interest between cognitive control and decision neuroscience are realizing these advantages, but this potential remains mostly untapped.
In the following sections, we consider two points of contact between the cognitive-control and decision-making literatures: strategy switching and self-control. We evaluate how well current models of prefrontal function permeate those research areas, and we identify key topics for future investigation.
4. Relational Integration and Strategy Switching
Rules and policies that lead to good outcomes in one circumstance may not provide the same benefits at other times. Thus, adaptive behavior often requires individuals to maintain multiple plans and to shift between them to reach a particular, constant goal. The concept of “strategy switching,” though derived from decision-making research, can be linked to behavioral and neurochemical findings from the cognitive-control literature suggesting that reward, emotion, and control mechanisms interact to balance stable and flexible responding (Braver and Cohen, 2000; Cools and Robbins, 2004; Doya, 2008; Dreisbach and Goschke, 2004; Müller et al., 2007). In particular, strategy switching in decision tasks could be subsumed within the most abstract level of cognitive control (see Section 3). Within the cascade model, for example, switching between two different response rules requires a process of branching control that holds one rule in reserve while another is engaged. This process activates rostral PFC, like other tasks that involve integrating multiple outcomes in pursuit of a higher goal (Ramnani and Owen, 2004).
Initiating a switch requires an evaluative component of control to detect circumstances justifying a change to behavior (Botvinick et al., 2001). This process reflects strategic regulation of control resources by a more abstract, superordinate evaluative system. Significant attempts have been made to integrate cognitive control and decision making accounts of the evaluative system; these suggest that PFC regions such as the dorsomedial PFC monitor for either conflicts in information processing or action outcome values (Botvinick, 2007; Rushworth and Behrens, 2008). These influential models have established a precedent within the literature that supports the integration of research from both cognitive control and decision making studies.
A canonical example of strategy switching can be seen in the shifts between exploratory and exploitative behavior. Organisms harvesting resources from a changing environment must balance an exploitative strategy that allows them to accumulate known rewards and an exploratory strategy that allows them to gather information regarding possibly more lucrative rewards (Cohen et al., 2007; Montague and King-Casas, 2007). Daw and colleagues (2006) used fMRI to study voluntary transitions between exploratory and exploitative strategies. Reinforcement learning models classified participants’ trial-by-trial choices as either exploitative or exploratory, with these classifications then used to identify neural correlates of these strategies. Exploitative choices were associated with activation in the ventromedial PFC, consistent with the calculation of action value (Wunderlich et al., 2009). Intriguingly, exploratory decisions were associated with increased activation in bilateral frontopolar cortex and medial intraparietal sulcus. Frontopolar activation in these cases may have reflected the higher-order decision making processes necessary to pursue a strategy of exploration, in the face of greater immediate rewards through exploitation. The authors suggested that bias signals from the frontopolar cortex exert inhibitory control over the current response strategy, in order to allow for the expression of the alternate exploratory strategy.
Further investigation by Boorman and colleagues (2009) has clarified the critical role played by frontopolar cortex in selecting between response strategies. In this study, participants made choices between two virtual slot machines. The payoff magnitude for each machine was varied randomly on every trial, so there was no advantage to tracking these amounts over time. By contrast, the probability of winning for each machine changed slowly over time, so participants could use that information to help them select which machine to play on each trial. Since the frontopolar cortex was known to be involved in switching between different response strategies, the authors suspected it might be involved in executing switches between the two slot machines. Their results supported this hypothesis, while also revealing a more specific mechanism: frontopolar cortex activation was found to encode the probability of winning on the unchosen slot machine, relative to the chosen machine. In other words, frontopolar cortex carried a signal about the benefits of the alternative choice strategy, relative to the current default choice strategy. Furthermore, both increased activation of frontopolar cortex and increased functional connectivity between the frontopolar cortex and parietal/premotor regions tended to predict a strategy switch on the subsequent trial. This evidence suggests that the frontopolar cortex plays both a general role in monitoring available response strategies and a specific role in implementing switches to the most beneficial strategy.
These examples from the decision-making literature support the idea that frontopolar cortex tracks the relative benefits of behavioral strategies and initiates strategic shifts based on new information. By integrating advanced behavioral learning models common within decision neuroscience and prior work in cognitive control, these investigators were able to derive tractable neural explanations for aspects of strategy switching, an important component of goal-directed decision making. It is probably not coincidental that this topic (strategy switching) and this brain region (frontopolar cortex) provide a nexus for integration between these two literatures. The frontopolar cortex is functionally both more specific and more selective than more posterior regions of PFC; that is, its activation tends to be reliably evoked by tasks that require some sort of relational integration (Christoff et al., 2001; Kroger et al., 2002) but not by most other sorts of executive processing (Banich et al., 2000; Ford et al., 2005; Garavan et al., 2002). Thus, the observation of activation in frontopolar cortex provides clearer insight into the likely underlying functional processes, as compared to activation in other parts of PFC (Poldrack, 2006).
5. Inhibitory Function and Self-Control
Successfully implementing our decisions often requires inhibition of some form of temptation (e.g., immediate outcomes, certain rewards). Anyone who has undertaken a diet, struggled with an addiction, or contemplated cheating realizes the powerful forces arrayed against our long-term goals. Accordingly, there has been substantial interest in the ability to hew to a plan and pursue a goal, particular in the face of superficially more appealing alternatives, or “self-control”. Self-control has been of particular interest to decision-making researchers because of the violations of standard theories associated with its failures (Bickel and Marsch, 2001; Thaler and Shefrin, 1981). But, its neural instantiation poses challenges. Unlike relational integration, self-control has not been unambiguously localized to a specific aspect of PFC (Cojan et al., 2009; Kuhn et al., 2009; Sharp et al., 2010). Yet, the integration of insights from the cognitive neuroscience literature holds promise for clarifying the neuroanatomical conception of self-control processes in decision making.
Understanding how people inhibit unwanted actions has been a longstanding area of research in cognitive psychology, and now in cognitive neuroscience (Aron, 2007; Miyake et al., 2000). Canonical tasks––such as the oddball, anti-saccade, or stop signal paradigms—involve the execution of a highly automatic or practiced motor response, accompanied by intermittent signals to withhold or change that response. Successfully overcoming such pre-potent responses requires the contribution of a network of brain regions including the lPFC, as shown by converging evidence from lesion (Aron et al., 2003), single-unit (Sakagami et al., 2001), and functional neuroimaging studies (Liddle et al., 2001). Regions such as the dorsolateral PFC, the anterior cingulate cortex, and the basal ganglia are often active in tasks involving self-control or response inhibition, with their engagement thought to reflect control processes related to rule or conflict monitoring and response halting (Aron et al., 2007b; Bunge, 2004b; Krug and Carter, 2010). Additionally, a region of lPFC, the right ventrolateral PFC, has now been implicated in self-control under a variety of tasks conditions, such as response inhibition, delay of gratification, and thought suppression (Cohen and Lieberman, 2010). Such research has been recently extended to establish a role for lPFC in the inhibition of a variety of automatic processes, such as in emotional regulation (Wager et al., 2008; Winecoff et al., 2010), as well as in conflict during goal-directed decision making (Hare et al., 2009).
Decision neuroscientists initially examined self-control in the context of scenarios contrasting patience with temptation. A common experimental task asks participants to choose between a small reward received immediately and a larger reward received after a time delay. Resisting the temptation to choose the immediate (but less valuable) option is hypothesized to require self-control. In a series of such experiments, McClure and colleagues (2004; 2007) identified a limbic-reward network activated by tempting options, and a prefrontal-executive network active during all decisions. Additionally, the relative level of activation between these two networks could predict participants’ choices of either the tempting or patient option. This suggests that a neural system associated with cognitive control contributes when valuation requires patience (see Kable and Glimcher, 2007 for an alternative perspective).
Expanding on this literature, decision neuroscientists have begun to examine compromises between conflicting goals and desires. Hare and colleagues (2009) explicitly examined self-control’s role in a familiar conflict: the decision to choose foods based on health or taste. While undergoing fMRI, hungry participants rated images of healthy snacks and junk foods for both taste and healthiness, and then chose between such food items. Participants were sorted into two groups based on their behavior: the self-control group balanced health and taste information when choosing foods, while non-self-controllers chose based on taste alone. Imaging results demonstrated that an area of the left dorsolateral PFC was activated when participants resisted the temptation to choose tasty but unhealthy foods. Furthermore, this region was negatively functionally coupled, via an intermediate lPFC region, to the ventromedial PFC area representing the net expected value from the food choice. These results suggest a self-control mechanism based in the lPFC may modulate value representations elsewhere in the brain. Furthermore, this modulatory influence may reflect the integration of abstract long-term goals, such as a desire for health, with more primary stimulus values, such as a food’s taste.
While these studies suggest a lateral prefrontal role in self-controlled decision making, neuroimaging evidence alone cannot support causal conclusions regarding brain-behavior relationships. Techniques which allow the manipulation of neural processing, such as transcranial magnetic stimulation (TMS), serve as an essential compliment to measurement-based techniques such as fMRI (Paus, 2005). Figner and colleagues (2010) applied low-frequency TMS to the dorsolateral PFC to study interactions between self-control and valuation. Research participants made choices between small immediate or larger delayed rewards, as in prior work (Kable and Glimcher, 2007; McClure et al., 2004), after TMS disruption of processing within either the left or right dorsolateral PFC. Disruption of left dorsolateral PFC caused participants to choose impatiently, foregoing larger later rewards for smaller immediate ones. By contrast, participants’ explicit valuations of these rewards were unaffected by TMS. These results suggest self-control processes instantiated in the left lPFC exert control over decisions independent of medial prefrontal/striatal valuation. Self-control of behavior may thus depend on a distinct lateral prefrontal process capable of arresting behaviors after the valuation stage of decision making (Luo et al., 2009).
While this evidence broadly implicates the lPFC in self-control (Fuster, 2008), important questions remain regarding the particular roles played by different lPFC regions. Dorsolateral PFC, for example, engages flexible cognitive control through interactions with the dorsomedial PFC and perceptual cortex (Egner and Hirsch, 2005; Kerns et al., 2004). Ventral and posterior lPFC, by contrast, support inhibitory control and response stopping via circuit interactions with the pre-supplementary motor area and subthalamic nucleus (Aron and Poldrack, 2006b; Nachev et al., 2007). An understanding of both the independence and interactions of such control circuits will be key to shedding light on the mechanisms underlying self-controlled action.
Given the variety of control processes supported by the lPFC, self-controlled behavior might be attained through a number of distinct strategies, each engaging characteristic lPFC control circuits. According to this perspective, self-control is not a single process, but a class of processes which similarly restrain behavior (Magen and Gross, 2010). Self-control processes could, for example, be organized according to levels of abstraction, in a manner consistent with the rostral-caudal hierarchical models of cognitive control described above (see Section 3). Rostrolateral PFC would support self- control implemented via highly abstract goals, intentions, and strategies, such as the creation of a binding precommitment to a non-tempting alternative (e.g., joining Weight Watchers) (Ariely and Wertenbroch, 2002; Gul and Pesendorfer, 2001). Dorsolateral PFC might support self-control in situations rather less removed from temptation, through mechanisms based on rules, plans, or the decision making context (e.g., re-routing to avoid passing the candy store). Finally, more caudal regions such as ventrolateral PFC might implement self-control in the face of temptation through inhibitory control over motor responses directly evoked by a tempting stimulus (e.g. stopping your hand in mid-reach for a candy bar). Such an integrated approach makes clear and falsifiable predictions regarding the contributions of distinct prefrontal control systems (Aron et al., 2004), and would anchor the various findings from imaging (Aron et al., 2007a; Diekhof and Gruber, 2010; Hare et al., 2009) and neurostimulation (Coxon et al., 2006; Fecteau et al., 2007; Figner et al., 2010; Knoch et al., 2006) experiments addressing different forms of self-control.
6. Conclusions and Future Directions
Cognitive control and decision making research thus address related questions: How is behavior adapted to fit changing circumstances? How are expected costs and benefits weighed to select actions? How are undesirable actions controlled or inhibited? In addressing such questions, cognitive-control models typically identify top-down, often prefrontal, executive processes which modulate target brain regions (Koechlin and Summerfield, 2007; Miller and Cohen, 2001). Decision-making models, by contrast, translate such questions into terms of choices and value, and seek out neural responses correlated with individual preferences revealed by these choices (Rangel et al., 2008; Samanez-Larkin et al., 2008). These approaches draw on distinct traditions and methods, but are clearly related, and, we argue, complementary. As the example of strategy switching illustrates, the integration of theory and technique from cognitive control and decision making can foster unique insights into the computational mechanisms supporting behavioral control. Other topics of shared interest, such as self-control, possess great potential — yet still await the benefits of an integrative approach.
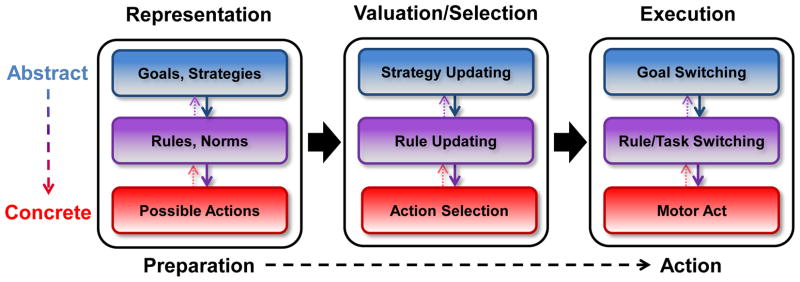
Figure 2 illustrates the complimentary contributions that decision neuroscience and cognitive control research can make to the understanding of action selection. Models of decision making emphasize sequential stages in the action-selection cycle, and thus provide organization along a temporal dimension. Cognitive control models contribute an anatomical dimension, consistent with hierarchical control of action selection: abstract representations engage rostral lPFC, while more concrete action representations engage caudal lPFC. Thus, representations residing at characteristic levels of abstraction (e.g. goals and strategies, rules and plans, and possible motor acts) are able to influence hierarchically lower and anatomically more caudal action representations throughout the stages leading from preparation to action execution. Valuation, a hallmark of decision neuroscience, plays a central role in the comparison and selection of competing representations at each level of abstraction. Value, reward, and outcome signals, derived from regions such as the ventromedial and orbital PFC as well as the anterior cingulate cortex and striatum, are integrated via functional interactions with the lPFC, where this information can be used to guide action selection (Daw et al., 2006; Diekhof and Gruber, 2010; Hare et al., 2009; Savine and Braver, 2010). Importantly, the functional neuroanatomy of these interactions should specifically reflect the abstractness of the valued representation, be it a broad goal or strategy or a particular motor act. Such sensitivity to abstractness allows for flexible (e.g., strategically selected) and yet regulated (e.g., self-controlled) pursuit of goal-directed action.
Figure 2. A schematic, integrative model of goal-directed decision making.
Each stage of decision making requires control processes at multiple levels of abstraction. Competing actions, rules and strategies are selected via a value-comparison process, in which cognitive control coordinates the assignment of action values based on the representations of superordinate goals, objectives, and strategies.
Recently, cognitive control researchers have taken the initiative in exploring links with decision neuroscience. Emboldened by the successes of the rostral-caudal axis model of control, researchers are extending it to address processes typically associated with decision making. Koechlin and colleagues, for example, have described interactions between their cascade model hierarchy and a parallel hierarchy of medial incentive-based motivation (Charron and Koechlin, 2010; Kouneiher et al., 2009). Likewise, Badre, Kayser, and D’Esposito demonstrated the role of their policy abstraction hierarchy in supporting abstract rule acquisition during an open-ended reinforcement learning task (Badre et al., 2010). Despite their advantages, these models have not attracted the attention of decision neuroscience researchers, with some exceptions. As one example, comparison of distinct decision-making and cognitive-control tasks has demonstrated a medial prefrontal topography engaged by increasingly abstract response, decision, and strategy control (Venkatraman et al., 2009b). Measures of resting-state functional connectivity reveal hierarchically organized connections between this medial topography and the lPFC rostro-caudal axis (Taren et al., 2011), solidifying this model’s utility for understanding the prefrontal mechanism underlying control of goal-directed action.
With future adoption of these rostral-caudal models, decision neuroscience has much to contribute at its interface with cognitive control. Decision neuroscience’s experimental repertoire includes a range of advanced quantitative models of choice behavior and valuation (Behrens et al., 2008; Glascher et al., 2010; Kable and Glimcher, 2007). These techniques can deepen our understanding of the computational roles played by brain regions and networks—particularly when used in conjunction with analyses of functional connectivity (Friston et al., 2003; Roebroeck et al., 2005). Additionally, the emphasis within decision neuroscience on modeling both within-subject choices and between-subject individual differences provides a complimentary approach to the process-isolation common in cognitive control experiments. Modeling these sources of experimental variability and correlating them with phenomenon of interest should expand the explanatory power of control models, and foster the development of richer theories of flexible behavior. These advances in turn should allow researchers studying cognitive control and decision making to together establish a more rigorous theory of the prefrontal control of thought and action.
Acknowledgments
We thank Tobias Egner for discussion, and John Clithero, Vinod Venkatraman, and David V. Smith for comments on the manuscript. This research was supported by an Incubator Award from the Duke Institute for Brain Sciences (SAH), by NIMH RC1-088680 (SAH), and by NIDA P30-023026.
Abbreviations
- fMRI
functional Magnetic Resonance Imaging
- PFC
PreFrontal Cortex
- lPFC
lateral PreFrontal Cortex
- TMS
Transcranial Magnetic Stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher G. Coutlee, Email: christopher.coutlee@duke.edu.
Scott A. Huettel, Email: scott.huettel@duke.edu.
References
- Ariely D, Wertenbroch K. Procrastination, deadlines, and performance: Self-control by precommitment. Psychological Science. 2002;13:219–224. doi: 10.1111/1467-9280.00441. [DOI] [PubMed] [Google Scholar]
- Aron A, Fletcher P, Bullmore T, Sahakian B, Robbins T. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006a;26:2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. The Journal of Neuroscience. 2006b;26:2424. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience. 2007a;27:3743. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. The Journal of Neuroscience. 2007b;27:11860. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Seymour B, Dolan RJ. Neural activity associated with the passive prediction of ambiguity and risk for aversive events. The Journal of Neuroscience. 2009;29:1648. doi: 10.1523/JNEUROSCI.4578-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–99. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in cognitive sciences. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–69. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Hoffman J, Cooney JW, D’Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat Neurosci. 2009;12:515–22. doi: 10.1038/nn.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Kayser AS, D’Esposito M. Frontal cortex and the discovery of abstract action rules. Neuron. 2010;66:315–26. doi: 10.1016/j.neuron.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich M, Milham M, Atchley R, Cohen N, Webb A, Wszalek T, Kramer A, Liang Z, Wright A, Shenker J. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Bateman I, Carson R, Day B, Hanemann M, Hanley N, Hett T, Jones-Lee M, Loomes G, Mourato S, Ozdemiroglu E. Economic valuation with stated preference techniques: a manual. Citeseer 2004 [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008;456:245–9. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt MA, Lohrenz T, Camerer CF, Montague PR. Neural signatures of strategic types in a two-person bargaining game. Proceedings of the National Academy of Sciences. 2010;107:19720. doi: 10.1073/pnas.1009625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W, Marsch L. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Boorman E, Behrens T, Woolrich M, Rushworth M. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological review. 2001;108:624. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–6. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. Control of cognitive processes: Attention and performance. 2000;XVIII:713–737. [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004a;4:564–79. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cognitive, Affective, & Behavioral Neuroscience. 2004b;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Camerer C, Loewenstein G, Prelec D. Neuroeconomics: How neuroscience can inform economics. Journal of economic Literature. 2005;43:9–64. [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon V, Franck N, Koechlin E, Fakra E, Ciuperca G, Azorin J, Farrer C. The architecture of cognitive control in schizophrenia. Brain. 2008;131:962. doi: 10.1093/brain/awn032. [DOI] [PubMed] [Google Scholar]
- Charron S, Koechlin E. Divided representation of concurrent goals in the human frontal lobes. Science. 2010;328:360–3. doi: 10.1126/science.1183614. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Yantis S. A domain-independent source of cognitive control for task sets: shifting spatial attention and switching categorization rules. J Neurosci. 2009;29:3930–8. doi: 10.1523/JNEUROSCI.5737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli J. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–49. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Cohen J, McClure S, Yu A. Should I stay or should I go? How the human brain manages the trade-off between exploitation and exploration. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:933. doi: 10.1098/rstb.2007.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Lieberman MD. The common neural basis of exerting self-control in multiple domains. Self-control in society, mind, and brain. 2010:141–160. [Google Scholar]
- Cojan Y, Waber L, Schwartz S, Rossier L, Forster A, Vuilleumier P. The brain under self-control: modulation of inhibitory and monitoring cortical networks during hypnotic paralysis. Neuron. 2009;62:862–75. doi: 10.1016/j.neuron.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philosophical Transactions of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences. 2004;362:2871. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. Journal of Neurophysiology. 2006;95:3371. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–9. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Daw ND. Decision theory, reinforcement learning, and the brain. Cogn Affect Behav Neurosci. 2008;8:429–53. doi: 10.3758/CABN.8.4.429. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–7. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Gruber O. When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. Journal of Neuroscience. 2010;30:1488. doi: 10.1523/JNEUROSCI.4690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nature Neuroscience. 2008;11:410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: Reduced perseveration at the cost of increased distractibility. Learning, Memory. 2004;30:343–353. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–90. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;27:12500–5. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–9. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Ford K, Goltz H, Brown M, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. Journal of Neurophysiology. 2005;94:429. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Franz S. ON THE FUNCTIONS OF THE CEREBRUM: I.--THE FRONTAL LOBES IN RELATION TO THE PRODUCTION AND RETENTION OF SIMPLE SENSORY-MOTOR HABITS. American Journal of Physiology. 1902;8:1. [Google Scholar]
- Friston K, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex. Academic Pr; 2008. [Google Scholar]
- Garavan H, Ross T, Murphy K, Roche R, Stein E. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Glascher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66:585–95. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether D, Plott C. Economic theory of choice and the preference reversal phenomenon. The American Economic Review. 1979;69:623–638. [Google Scholar]
- Gul F, Pesendorfer W. Temptation and self-control. Econometrica. 2001;69:1403–1435. [Google Scholar]
- Hare T, Camerer C, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci. 2010;30:583–90. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensher D, Bradley M. Using stated response choice data to enrich revealed preference discrete choice models. Marketing Letters. 1993;4:139–151. [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer CF. Neural response to reward anticipation under risk is nonlinear in probabilities. J Neurosci. 2009;29:2231–7. doi: 10.1523/JNEUROSCI.5296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–75. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–62. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kable J, Glimcher P. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kimberg D, D’Esposito M, Farah M. Cognitive functions in the prefrontal cortex: working memory and executive control. Current Directions in Psychological Science. 1997;6:185–192. [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–8. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–35. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–45. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–85. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Krug MK, Carter CS. Anterior Cingulate Cortex Contributions to Cognitive and Emotional Processing: A General Purpose Mechanism for Cognitive Control and Self-Control. Self Control in Society, Mind, and Brain. 2010;1:3–27. [Google Scholar]
- Kuhn S, Haggard P, Brass M. Intentional inhibition: how the “veto-area” exerts control. Hum Brain Mapp. 2009;30:2834–43. doi: 10.1002/hbm.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle P, Kiehl K, Smith A. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2010 doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Giragosian L, Monterosso JR. Behavioral and neural evidence of incentive bias for immediate rewards relative to preference-matched delayed rewards. J Neurosci. 2009;29:14820–7. doi: 10.1523/JNEUROSCI.4261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen E, Gross JJ. Getting Our Act Together: Toward a General Model of Self-Control. Self Control in Society, Mind, and Brain. 2010;1:335–354. [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–90. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- McClure S, Laibson D, Loewenstein G, Cohen J. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McClure S, Ericson K, Laibson D, Loewenstein G, Cohen J. Time discounting for primary rewards. Journal of Neuroscience. 2007;27:5796. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci U S A. 2010;107:7922–6. doi: 10.1073/pnas.0910662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting: The role of the frontal lobes. Archives of Neurology. 1963;9:90. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR. Neural processing of risk. Journal of Neuroscience. 2010;30:6613. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–84. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Montague PR, King-Casas B. Efficient statistics, common currencies and the problem of reward-harvesting. Trends in cognitive sciences. 2007;11:514–519. doi: 10.1016/j.tics.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Müller J, Dreisbach G, Goschke T, Hensch T, Lesch KP, Brocke B. Dopamine and cognitive control: the prospect of monetary gains influences the balance between flexibility and stability in a set shifting paradigm. European Journal of Neuroscience. 2007;26:3661–3668. doi: 10.1111/j.1460-9568.2007.05949.x. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O’Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images: a perturbation approach. Philosophical Transactions B. 2005;360:1109. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13:427–40. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–95. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, Dolan RJ. Encoding of marginal utility across time in the human brain. J Neurosci. 2009;29:9575–81. doi: 10.1523/JNEUROSCI.1126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nat Neurosci. 2008;11:398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R. Can cognitive processes be inferred from neuroimaging data? Trends in cognitive sciences. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen A. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004a;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004b;56:129–40. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1463–70. doi: 10.1098/rstb.1996.0131. discussion 1470–1. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–7. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O. A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. J Neurosci. 2001;21:4801–8. doi: 10.1523/JNEUROSCI.21-13-04801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19:320–3. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: Reward incentives modulate preparatory neural activity during task-switching. The Journal of Neuroscience. 2010;30:10294. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sen A. Choice functions and revealed preference. The Review of Economic Studies. 1971:307–317. [Google Scholar]
- Serences JT. Value-based modulations in human visual cortex. Neuron. 2008;60:1169–1181. doi: 10.1016/j.neuron.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–11. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490–5. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Spaet T, Harlow H. Problem solution by monkeys following bilateral removal of the prefrontal areas. II. Delayed reaction problems involving use of the matching-from-sample method. Journal of Experimental Psychology. 1943;32:424–434. doi: 10.1037/h0054430. [DOI] [PubMed] [Google Scholar]
- Taren AA, Venkatraman V, Huettel SA. A Parallel Functional Topography between Medial and Lateral Prefrontal Cortex: Evidence and Implications for Cognitive Control. The Journal of Neuroscience. 2011;31:5026. doi: 10.1523/JNEUROSCI.5762-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler R, Shefrin H. An economic theory of self-control. The Journal of Political Economy. 1981:392–406. [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–8. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 2009a;62:593–602. doi: 10.1016/j.neuron.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Rosati AG, Taren AA, Huettel SA. Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. J Neurosci. 2009b;29:13158–64. doi: 10.1523/JNEUROSCI.2708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Davidson M, Hughes B, Lindquist M, Ochsner K. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, LaBar K, Madden D, Cabeza R, Huettel S. Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich K, Rangel A, O’Doherty J. Neural computations underlying action-based decision making in the human brain. Proceedings of the National Academy of Sciences. 2009;106:17199. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]