Abstract
Purpose
To develop a consistent and reproducible method in an animal model for studies of radiofrequency ablation of primary HCC.
Materials and Methods
Fifteen woodchucks were inoculated with woodchuck hepatitis virus to establish chronic infections. When serum gamma glutamyltranspeptidase levels became elevated, the animals were evaluated with ultrasound and in most cases, a pre-operative magnetic resonance imaging to confirm tumor development. Ultimately, radiofrequency ablation of tumors was performed using a 1-cm probe with the animal submerged in a water bath for grounding. Ablation effectiveness was evaluated with contrast-enhanced MRI and gross and histopathologic analysis.
Results
Radiofrequency ablation was performed in 15 woodchucks. Modifications were made to the initial study design to adapt methodology for the woodchuck. The last ten of these animals were treated with standardized protocol using a 1 cm probe that produced a consistent area of tumor necrosis (mean size of ablation of 10.2mm × 13.1mm) and that led to no complications.
Conclusions
We have developed a safe, reliable and consistent method to study radiofrequency ablation of spontaneous primary hepatocellular carcinoma using chronically woodchuck hepatitis virus-infected woodchucks, an animal model of Hepatits B virus-induced hepatocellular carcinoma.
Introduction
Since the first reports of using radiofrequency ablation (RFA) in ex-vivo studies, RFA has been extensively evaluated in both animal and clinical investigations (1,2). Animal models have included a variety of species, with swine being one of the most common. The advantages of using swine are the low cost and availability of the animal and the size of the animal, which translates well to clinical work with humans. Though an excellent model for evaluating the size of the ablation in normal liver, it does not incorporate the effects of the presence of tumor and background liver disease since there are no spontaneously developing hepatic neoplasms in this model. On the other hand, the woodchuck (Marmota monax) is a well recognized animal model of virally-induced hepatocellular carcinoma (3). Chronic infection with woodchuck hepatitis virus (WHV), a member of the hepadnavirus family which includes hepatitis B virus, leads to a very high risk of hepatocellular carcinoma (HCC) development within 2-4 years following infection (3). The animals are considerably larger than conventional laboratory rodents weighing 2-5kg and develop HCCs that reach nearly 100cm3 in size, making them amenable to studies using conventional probes and other instrumentation (4). Therefore, we report our early experience with the use of the woodchuck (Marmota monax) as an animal model for radiofrequency ablation of primary HCC with magnetic resonance imaging (MRI) and pathologic correlation.
Materials and Methods
This study was performed as part of a larger study sponsored by the National Institute of Health (NIH) comparing traditional ultrasound guidance to a more sophisticated guidance system with 3-D display. This study was overseen and approved by the Institutional Animal Care and Use Committees (IACUC) of the two participating institutions.
Animals
Twenty woodchucks were purchased from a commercial supplier (Northeastern Wildlife, Harrison, ID 83833) as captive-born animals approximately 8 months of age. The animals were injected at birth with a pooled WHV inoculum to establish infection. Animals were tested at approximately 6 months of age for serum WHV polymerase and woodchuck hepatitis virus core antigen (WHcAg) to establish that they were chronically infected.
The animals were housed in Laboratory Animal Resources facilities at North Carolina State University. They were kept individually in stainless steel cages, fed Agway rabbit pellets (Richmond, IN) ad libidum and given water ad libidum. They were maintained in a 12-hour light-and-dark cycle and cared for by personnel trained in the care of woodchucks under the conditions specified by the IACUC guidelines.
Blood samples were obtained every 3-4 months to assess levels of WHV DNA levels, to verify that the animals remained virus carriers, as well as levels of serum gamma glutamyl transpeptidase (GGT), a serum marker for the presence of hepatocellular tumors (5). Animals with GGT levels greater than 50 IU/dl were evaluated by ultrasound to search for hepatic tumors and evaluate their size and position. When hepatic tumors of at least 1cm in diameter were identified by ultrasound, the RFA procedure was scheduled within approximately 1 week.
RFA Procedure and Post Care
The RFA procedure was performed by an experienced Interventional Radiologist (CTB). Animals were sedated with 2-5mg/kg ketamine (100 mg/ml; Fort Dodge Laboratories, Fort Dodge, IA) and 0.1-0.2mg/kg of acepromazine maleate (10mg/ml, Fort Dodge Laboratories, Fort Dodge, IA) intramuscularly. When possible, an endotracheal tube was placed, and a nasogastric tube was passed for gastric decompression. General anesthesia was performed using isoflurane (1-5%) administered by endotracheal tube (or face mask in cases when intubation was unsuccessful.
The abdomen was shaved and prepped with iodine and alcohol scrub and draped with sterile towels. Initially, the animal was placed on a heating pad; pediatric grounding pads (Neonatal Patient Return Electrode, Valleylab, Boulder, CO) were placed on the caudal back and rear legs. Later, the protocol was modified such that the woodchuck was placed in a metal pan connected to electrodes that were then attached to the grounding wires of the RFA generator. The metal pan was filled with warm saline (approximately 38° C) and the caudal half of the body was submerged (Figure 1). Heating pads were placed beneath the pan in order to maintain the temperature of the water bath and the animal’s body temperature was monitored.Using ultrasound imaging, a 1-cm probe (Soloist, LeVeen, Boston Scientific, Natick, MA) was placed into the center of the lesion and activated.. Because of the size of the woodchuck, a low energy protocol was used. The generator was started at 10W and left on for 30 seconds or until roll-off. If roll-off was not achieved, the energy was increased to 20W and ablation was performed for another 30 seconds or until roll off. If roll off was not achieved after the higher energy was used, the ablation was terminated.
Figure 1.
Schematic representation of RFA ablation on woodchuck in saline bath. In order to prevent skin injury associated with grounding pad burns, the woodchucks were submerged in a saline bath within a metal pan. The pan was connected to the generator and served as grounding for the ablation procedure.
Following the RFA, the woodchucks were dried and warmed with heated air (Bair Hugger, Augustine Medical, Eden Prairie, MN) to re-establish body temperature, and recovered in the recovery area. All animals were given 0.05-0.1mg/kg buprenorphine subcutaneously for post-operative pain control. The woodchucks were monitored over the next 7 days for signs of collateral injury. After approximately 1 week, the animals underwent a follow-up MRI exam. Following the MRI, the woodchucks were euthanized with an overdose of pentobarbital (90mg/kg) and the liver was collected for analysis.
MRI
All MRIs were performed on a 3T scanner (Allegra, Siemens, Erlangen, Germany) using the standard CP head coil. The animals were pre-sedated with a mixture of acepromazine and ketamine administered intramuscularly, as above. During imaging, the animals were anesthetized with isoflurane (1-5%) administered via face mask.
The full imaging protocol may be found online.
Each scan was evaluated for evidence of ablation, based primarily on lack of enhancement on post-contrast T1 sequences, and size of ablation measured in two dimensions. If there was residual tumor, an estimate of maximum residual tumor thickness was recorded.
Pathological Assessment
Gross pathological and histological examination of the liver was performed by an experienced veterinary pathologist. Tumors were evaluated using standard rodent classification schemes (6). Following euthanasia, the liver was removed, photographed and the treated tumor was measured. Serial sections, approximately 4 mm thick, were taken through the tumors and the adjacent normal parenchyma. Sections were stained with hematoxylin and eosin. The dimensions of the liver tumors and the diameters of RFA-induced necrosis were recorded.
Results
Of the initial 20 woodchucks, 5 were not used in the study. Of these, two recovered from their WHV infection and did not develop tumors before the study concluded, one died from hemorrhage due to aortic dissection and another died from unknown causes. The fifth woodchuck died prior to treatment and the tumor was discovered on necropsy.
Hepatocellular carcinoma was treated by RFA in 15 woodchucks. Using a conventional human protocol for the first 2 animals, we discovered that the human grounding pads that had been trimmed to conform to the woodchuck’s body became excessively heated and produced significant burns to the abdominal wall and site of attachment. These two animals were euthanized because of the skin injury. The addition of ice packs at the site of the grounding pads was not sufficient to prevent thermal skin injury.
Subsequently, a total of 13 woodchucks were treated. The grounding protocol was altered to increase the surface area involved in grounding the animal. To do this, we immersed the caudal aspect of the woodchuck into warmed saline (Figure 1) during treatment. This modification eliminated all cutaneous injury associated with grounding. RFA was performed using the 1-cm LeVeen RFA probe. Eleven of the 13 animals had post-ablation MRI (Figure 2). Two of the woodchucks did not undergo MRI: one woodchuck became sluggish, refused to eat in the days following the procedure, and was euthanized before it could undergo MRI. Postmortem evaluation revealed that thermal injury from the RFA had damaged the gastric wall. The second woodchuck had thermal injury to soft tissue adjacent to the tumor and died during transport to be imaged.
Figure 2.


MRI appearance of treated HCC in woodchuck model. (a) precontrast T1 image shows a spherical area of hyperintense signal at the site of ablation (arrow). (b) Following intravenous gadolinium administration, the lesion shows minimal peripheral enhancement with subtle areas of nodularity (arrow).
In all 11 woodchucks that underwent MRI after RFA, there was evidence of ablation (Figure 3). Using the modified protocol, the burn areas were consistent, each approximately 1 cm in diameter (mean 10.2mm × 13.1mm, range [7-14]mm × [9-23]mm). Since all HCC treated were larger than 1 cm in diameter, there was evidence of residual tumor in all treated lesions with peripheral nodular enhancement (Figure 4).
Figure 3.
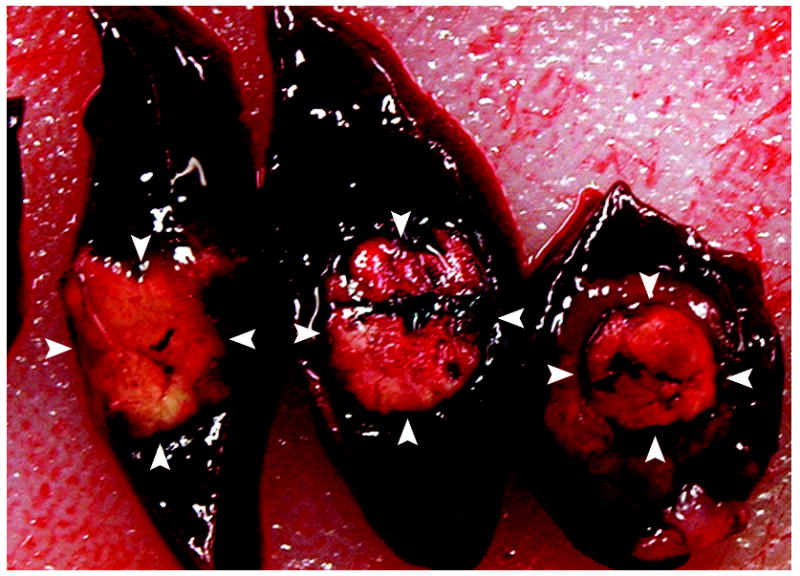
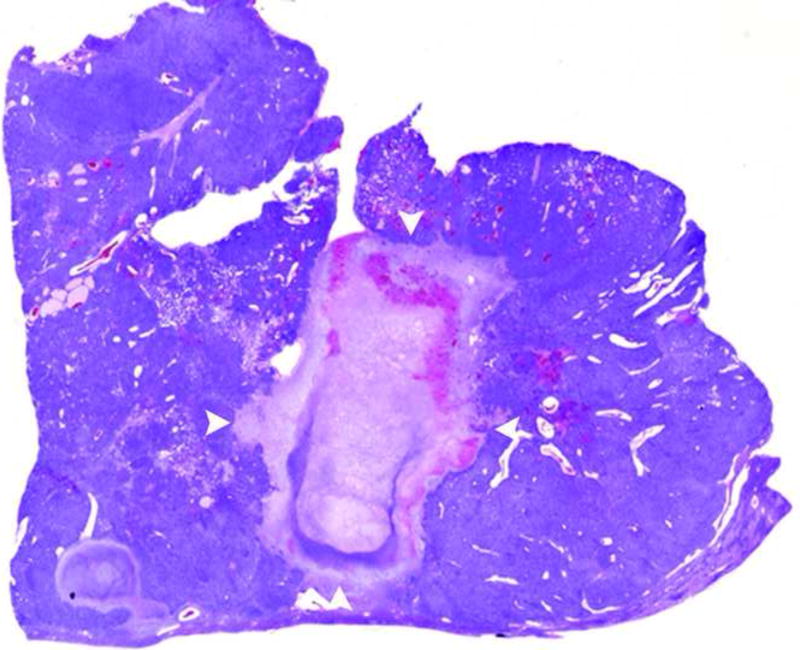
a and b. Gross (a) and histopathological (b) sections through treated woodchuck liver tumors reveal a central ablation zone within the tumor (arrowheads).
Figure 4.

(a) T1 post-gadolinium MR image through the level of ablation shows nodular, peripheral enhancement (curved arrow) along the lateral edge of the ablation site (arrowheads) consistent with residual HCC . (b) Histological section through this lesion shows the central treated area (arrows) surrounded by residual basophilic HCC (arrowheads).
All tumors were confirmed to be hepatocellular carcinomas based on typical histological appearance. Viable tumor tissue was readily discerned from treated tissue. Approximately one week following RFA, the treated volume was roughly spherical and showing complete loss of viable tissue and replacement with eosinophilic tissue debris and a circumferential rim of edema and early granulation tissue, characterized by an ingrowth of reactive fibroblasts and small caliber blood vessels. A light infiltration of neutrophils and smaller numbers of lymphocytes was present in the majority of cases. In the two cases that were euthanized or died within a day of RFA, the treated areas consisted of a clearly defined area of coagulative necrosis rimmed with a mild infiltrate of neutrophils.
Discussion
Woodchucks are naturally susceptible to infection with WHV, which is closely related to the hepatitis B virus (7). Nearly 100% of chronically WHV- infected woodchucks will develop primary hepatocellular carcinoma within 1 to 4 years of age with tumors measuring nearly 100cm3 in volume (4,5,7). These tumors are primary liver cancer and possess normal tumor vascularization, differentiating the woodchuck from other animal models which rely on tumor cells that are implanted and grow in the liver.
Thus far, many of the animal models used in studying radiofrequency ablation of the liver do not incorporate tumor in the evaluation (8-12). Thus, much of the work evaluating the effectiveness of RFA on tumor coagulation relies on explant or pathologic reports. At least one animal study has been reported that does incorporate liver tumors in an animal model with RFA: Ahmed et al. developed a large animal model by inoculating immunosuppressed dogs with venereal sarcomas (transmissible venereal tumor) in order to induce tumor development (12). This model was used to compare the use of RFA alone with the use of a combination of RFA and acetic acid injection.
The use of the woodchuck in studying a variety of HCC therapies is not new. The association between the WHV and HCC was initially described in 1978, and the woodchuck has served as a laboratory model to study HCC and a variety of treatments since 1980 (13). However, most of the work toward treatment has focused on systemic therapies, such as vaccines, antiviral agents, and immunotherapy. Nevertheless, there is at least one report describing the use of the woodchuck model for percutaneous therapy. Gouillat et al. treated seven animals with percutaneous alcohol ablation and one animal with laser photocoagulation (7). Three of the woodchucks treated with alcohol ablation died in the early postoperative period, and the remaining four underwent tumorectomy one month later. The single woodchuck treated with photocoagulation was sacrificed one month after the procedure. The authors concede that this model is limited by the high perioperative mortality of the animals. They also describe greater fragility of the animals around the hibernation period. However, the woodchuck is now a well-established animal model for virally induced HCC (3).
Our work required modification of procedures and protocols to adapt to the smaller size of the woodchucks, which are significantly smaller than even most pediatric patients. Early on, we attempted to perform the RFA procedure with algorithms similar to those used in larger animal models and humans. However, this resulted in significant injury to the animals and the protocol had to be modified. Even neonatal grounding pads proved to be a challenge due to the small size of the animal’s caudal extremities. Also, in order to use the pads effectively, a large portion of the animal had to be shaved, resulting in considerable heat loss post-operatively. Nevertheless, skin burns at the grounding pad sites remained a problem despite the use of large quantities of tape and ice packs. For this reason, we devised a metal pan that was modified to connect to the electrode return sockets of the RFA generator. By submerging the caudal half of the animal in saline, sufficient electrical grounding could be achieved without the need for shaving the animal and without the risk of grounding pad skin injury.
The second challenge encountered was the development of collateral damage during the ablation. The initial use of higher energy with larger electrodes resulted in a larger volume of ablation than predicted based on the manufacturer’s IFU. Initially, we opted to reduce the power while continuing to use the larger ablation probe, with the intent of being able to treat larger tumors completely. Unfortunately, this did not fully correct the problem. The feedback system of the LeVeen generator is through impedance and when using the lower energy, we did not see the normal increase in impedance; thus, there was no way to determine when sufficient ablation was achieved. Without this feedback, the ablation was carried out for 15 minutes, as this is the typical time required for complete ablation in humans. This resulted in a very large area of thermal injury to the abdominal cavity. From this point onward we switched our protocol to using a smaller probe with short ablation times of low energy. Using this new target, a consistent one centimeter ablation could be achieved with little risk of collateral injury.
The primary limitation of this study was the restricted size of lesions that could be fully treated. Since woodchucks’ spontaneous development of tumors in a variety of sizes and locations is seen as the primary advantage to this model, the inability to treat larger lesions is a limitation that should be addressed in the future. We experienced significant injuries to the animals when attempting to fully ablate larger lesions. We postulate that the increased current transmitted between probe and ground when using larger probes and higher energies caused many of the complications encountered when more aggressive ablations were attempted. It remains to be seen whether larger lesions can be fully ablated using a different technology, such as bipolar radiofrequency probes that restrict the current to the site of treatment. Alternatively, another energy delivery method, such as microwave or interstitial laser, may allow for safe ablation of larger tumors. Further investigation is needed to answer this question. Nevertheless, the fact that these animals develop tumors in a variety of sizes and locations potentially makes this an attractive model for comparing different treatment strategies. Since it has been shown that HCC also has a dominant arterial blood supply in animals as in humans, it is possible that this model may also be used for evaluating transarterial therapies in addition to ablative therapies (14).
In conclusion, the woodchuck does provide an animal model with naturally occurring HCC that can be used to evaluate the efficacy of percutaneous ablation with MRI and pathologic evaluation of efficacy of treatment. We have developed a protocol that enables us to create consistently a predictable, controlled RFA-induced burn in primary HCC. However, this early work is limited and further work with evolving technology is needed to develop a model that can be used for the treatment and complete ablation of larger tumors. With this, it may be possible in the future to use the woodchuck as a model to compare new minimally invasive therapies or combinations of therapies.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (1 R01 CA101186-01A2).
Appendix (for electronic publication only)
MRI Protocol
Imaging sequences included T2 HASTE (200mm × 200mm FOV, 1mm × 1mm × 5mm voxels, 2000ms TR, 65ms TE, 1 Average, 1:02 TA), T2 TSE with Flow Compensation (169mm × 200mm FOV, 0.9mm × 0.8mm × 5mm voxels, 5880ms TR, 84ms TE, 3 Averages, 5:25 TA), T1 GRE (200mm × 200 mm FOV, 1.3mm × 1.0mm × 4mm voxels, 2000ms TR, 2.83ms TE, 1 Average, 7:14 TA), DTI with 21 directions (224mm × 275mm FOV, 2.1mm × 2.1mm × 2.5mm voxels, 5100ms TR, 64ms TE, 2 Averages, 3:50 TA), T1 TSE with Flow Compensation (150mm × 160mm FOV, 0.6mm × 0.6mm × 5mm voxels, 600ms TR, 12ms TE, 3 Averages, 4:47 TA), VIBE (200mm × 200mm FOV, 1mm × 0.8mm × 2mm voxels, 5ms TR, 1.69ms TE, 1 Average, 0:39 TA) pre and post contrast injection (IV bolus injection, gadodiamide 0.1mmol/kg), T1 GRE FS 10 minutes post contrast (250mm × 219 mm FOV, 1.6mm × 1.0mm × 4mm voxels, 196 ms TR, 2.46ms TE, 1 Average, 0:28 TA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery in the animal model. Invest Radiol. 1990;25:267–70. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Fornari F, Pathies C, Buscarini L. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori. 1990;76:54–7. doi: 10.1177/030089169007600114. [DOI] [PubMed] [Google Scholar]
- 3.Tennant BC, Toshkov IA, Peek SF, et al. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology. 2004 Nov;127(5 Suppl 1):S283–93. doi: 10.1053/j.gastro.2004.09.043. Review. [DOI] [PubMed] [Google Scholar]
- 4.Jacob J, Sterczer A, Toshkov I, et al. Integration of woodchuck hepatitis and N-myc rearrangement determine size and histologic grade of hepatic tumors. Hepatology. 2004;39:1008–16. doi: 10.1002/hep.20106. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie E, Jackson M, Sun J, Volotovskyy V, Gruwel M. Monitoring the development of hepatocellular carcinoma in woodchucks using 31P-MRS. MAGMA. 2005;18:201–5. doi: 10.1007/s10334-005-0120-x. [DOI] [PubMed] [Google Scholar]
- 6.Maronpot R, Montgomery C, Jr, Boorman G, McConnell E. National toxicology program nomenclature for hepatoproliferative lesions of rats. Toxicol Pathol. 1986;14:263–73. doi: 10.1177/019262338601400217. [DOI] [PubMed] [Google Scholar]
- 7.Gouillat C, Manganas D, Zoulim F, et al. Woodchuck hepatitis virus-induced carcinoma as a relevant natural model for therapy of human hepatoma. J Hepatol. 1997;26:1324–30. doi: 10.1016/s0168-8278(97)80468-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee F, Jr, Haemmerich D, Wright A, Mahvi D, Sampson L, Webster J. Multiple probe radiofrequency ablation: Pilot study in an animal model. J Vasc Interv Radiol. 2003;14:1437–42. doi: 10.1097/01.rvi.0000096771.74047.c8. [DOI] [PubMed] [Google Scholar]
- 9.Denys A, De Baere T, Mahe C, et al. Radio-frequency tissue ablation of the liver: effects of vascular occlusion on lesion diameter and biliary and portal damages in a pig model. Eur Radiol. 2001;11:2102–8. doi: 10.1007/s003300100973. [DOI] [PubMed] [Google Scholar]
- 10.Marchal F, Elias D, Rauch P, et al. Biliary lesions during radiofrequency ablation in liver. Study on the pig. Eur Surg Res. 2004;36:88–94. doi: 10.1159/000076648. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Lim HK, Ryu JA, et al. Radiofrequency ablation of rabbit liver in vivo: effect of the pringle maneuver on pathologic changes in liver surrounding the ablation zone. Korean J Radiol. 2004;5:240–9. doi: 10.3348/kjr.2004.5.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed M, Weinstein J, Liu Z, et al. Image-guided percutaneous chemical and radiofrequency tumor ablation in an animal model. J Vasc Interv Radiol. 2003;14:1045–52. doi: 10.1097/01.rvi.0000083254.29749.e1. [DOI] [PubMed] [Google Scholar]
- 13.Menne S, Cote P. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol. 2007;13:104–24. doi: 10.3748/wjg.v13.i1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerolami R, Cardoso J, Bralet MP, et al. Enhanced in vivo adenovirus-mediated gene transfer to rat heaptocarcinomas by selective administration into the hepatic artery. Gene Ther. 1998;5(7):896–904. doi: 10.1038/sj.gt.3300664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.