Abstract
Free swimming zebrafish larvae depend mainly on their sense of vision to evade predation and to catch prey. Hence there is strong selective pressure on the fast maturation of visual function and indeed the visual system already supports a number of visually-driven behaviors in the newly hatched larvae. The ability to exploit the genetic and embryonic accessibility of the zebrafish in combination with a behavioral assessment of visual system function has made the zebrafish a popular model to study vision and its diseases. Here, we review the anatomy, physiology and development of the zebrafish eye as the basis to relate the contributions of the zebrafish to our understanding of human ocular diseases.
Keywords: zebrafish, eye, anterior segment, retina, lens, photoreceptor, glaucoma, myopia, coloboma, holoprosencephaly
A. The cornea and lens
Together, the transparent cornea and lens make up the refractive unit of the eye responsible for focusing light onto the retina, which collectively have been termed the refracton (Piatigorsky, 2001). In addition, the cornea serves as a protective barrier between the eye and the external environment. The relative refracting power of either the cornea or lens varies between species. For zebrafish and other aquatic animals, the lens provides relatively more focusing power than the lens of terrestrial animals. This is because the refractive index of water, as compared to air, is better matched to that of the corneal cells and less refracting power is needed by the cornea (Greiling and Clark, 2008). While the zebrafish lens provides the majority of light refraction, unlike some other teleosts, the zebrafish lens does not significantly change shape with focal point accommodation (Easter and Nicola, 1996). In the following sections, we describe the anatomy and physiology of the zebrafish cornea and lens.
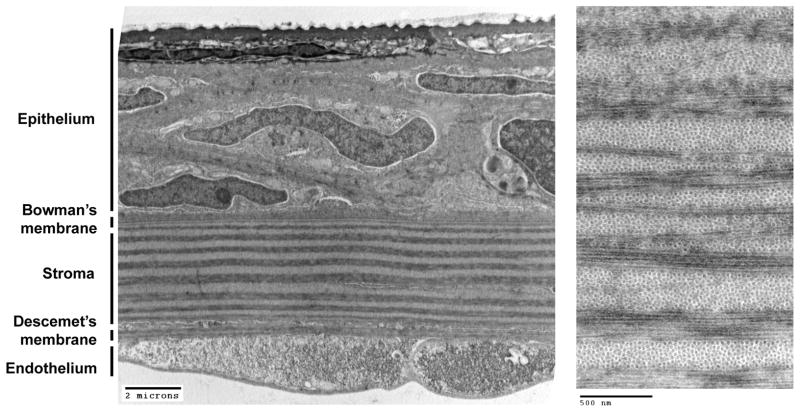
The vertebrate cornea is the transparent tissue covering the front of the eye. It is continuous with the opaque sclera that forms the outer shell of the eye globe. Five basic layers make up the cornea: the epithelium, Bowman’s layer, the stroma, Descemet’s membrane and the endothelium (Figure 1). Like other vertebrates, the zebrafish cornea is avascular. The adult zebrafish cornea has been characterized histologically and ultrastructurally (Swamynathan et al., 2003; Soules and Link, 2005; Zhao et al., 2006; Akhtar et al., 2008). When mature, it is approximately 20 microns thick. The most superficial layer, the epithelium, is in direct contact with the aqueous environment. It is approximately 3–6 cell bodies thick. These stratified squamous cells make up over half of the thickness of the zebrafish cornea – a larger proportion than that of the mammalian cornea. Zebrafish corneal epithelial cells show interdigitations and are connected with numerous intercellular junctions. The external layer of corneal epithelial cells show abundant microplicae and reticulations that form “microridges” (Collin and Collin, 2000). However, unlike most mammals, birds and reptiles, microvilli are absent from bony fishes. En face, the microridges give the surface of these hexagonal cells a “fingerprint-like” appearance (Zhao et al., 2006). At the periphery of the cornea, mucus-secreting goblet cells are frequently interspersed within the epithelium (Soules and Link, 2005). The microplicae of the superficial epithelial cells are thought to stabilize the glycocalyx produced by the goblet cells, as well as increase surface area to aid in the exchange of nutrients and metabolites. In other vertebrates studied, epithelial cells continually renew, being shed at the central region and generated in the peripheral limbal zone (Davies and Di Girolamo, 2010). Corneal epithelial cell renewal and wound healing, however, has not been investigated in zebrafish. Bowman’s layer, a thin extracellular deposition separates the epithelial cells from the stromal layer. The stromal layer is also primarily made up of extracellular matrix. The most prominent feature of the corneal stroma is the exquisite collagen organization (Figure 1, right panel). Collagen fibrils are bundled into tightly packed units that run in parallel within a sublamina, but are orthogonal to those above and below. This organization facilitates transparency of the tissue and must be actively maintained (Benedek, 1971). Spaces between the collagen bundles are enriched for proteoglycans and interspersed with flat keratocytes which synthesize collagen (Akhtar et al., 2008). As a proportion of corneal thickness, the zebrafish stromal layer is relatively thin as compared to mammals. The stroma overlies a basal lamina termed Descemet’s membrane. The innermost layer, the endothelium, is comprised of a single layer of junctionally-connected, polygonal cells. The corneal endothelium is critical for metabolic homeostasis and maintenance of stromal collagen organization, and therefore transparency.
Figure 1. Layers of the zebrafish cornea.
Transmission electron microscopy reveals the five principal layers of the adult zebrafish cornea (Left panel). Higher magnification of the orthogonally-arrayed collagen bundles (Right panel).
The adult zebrafish lens shows nearly all the morphological hallmarks that characterize lenses of other vertebrates (Soules and Link, 2005; Dahm et al., 2007; Greiling and Clark, 2008; Vihtelic, 2008). The fish lens is more spherical than the typical vertebrate, but is comprised of an outer epithelial layer covering elongated fiber cells. Most lens epithelial cells are quiescent, except for a band encircling the marginal equator. These marginal zone lens epithelial cells proliferate and give rise to additional epithelial cells as well as differentiating fiber cells. The region of lens fiber cell differentiation is also called the Bow region or transition zone. Within this area, elongating fiber cells loose their internal organelles, which aids in transparency. The process of organelle disassembly seems to happen more abruptly in zebrafish than mammals (Dahm et al., 2007). The ongoing growth of the lens results in a concentric shelled organization, where the inner fiber cells are older and more differentiated than the outer fiber cells. Electron microscopy highlighted the onion-like nature of the lens and revealed interdigitating lateral protrusions, or ball-and-socket joints, between the fiber cells (Soules and Link, 2005; Dahm et al., 2007; Vihtelic, 2008).
Development of the zebrafish cornea and lens has been characterized by a variety of approaches including morphology, time-lapse microscopy and by gene mutation analyses. Both structures develop from ectodermal cells of the lens placode. Thickening of the lens placode is visible by ~16–18 hours post fertilization (hpf) (Schmitt and Dowling, 1994; Li et al., 2000; Soules and Link, 2005; Zhao et al., 2006; Dahm et al., 2007; Vihtelic, 2008; Greiling et al., 2010). Shortly after the ectodermal cells become more columnar, the lens anlage invaginates as a mass of cells. The zebrafish lens does not go through a hollowed vesicle stage. As the lens anlage completely delaminates from the epidermal surface, the remaining cells become the prospective corneal epithelium. Within the nascent lens, primary fiber cells begin to elongate and differentiate at the posterior of the cellular mass, while those in the anterior region differentiate into epithelial cells (Greiling et al., 2010). This entire process takes approximately 10 hours and is coordinated with initiation of optic cup morphogenesis (Martinez-Morales and Wittbrodt, 2009). Between 24–36 hpf, periocular mesenchymal cells of neural crest and head mesoderm origin migrate into the newly formed anterior chamber. Sub-sets of these cells contribute to the corneal endothelium, as has been shown for other vertebrates. However, it should be noted that definitive fate mapping studies have not been done for the differentiated zebrafish anterior segment structures. Following immigration of corneal endothelial cells, differentiation continues and a rudimentary stromal layer is apparent shortly after the endothelial cells form a monolayer at ~48 hpf (Zhao et al., 2006). Ongoing differentiation continues until 1 month, when the cornea reaches the adult form (Soules and Link, 2005; Akhtar et al., 2008). In contrast, adult lens morphology is reached at earlier stages, although fiber cell compaction continues until the 1-month stage. The simplicity of anatomy and compartmentalization of zebrafish lens development has facilitated its use for general insight into cell biological and gene regulation processes (Harding et al., 2008; Imai et al., 2010; Tittle et al., 2010).
B The iridocorneal angle
The iridocorneal angle, the region of the ocular anterior chamber where the cornea meets the iris, hosts cells specialized in maintaining intraocular pressure. Intraocular pressure is balanced by aqueous humor production and drainage. In most mammals, tissues that mediate aqueous humor production and clearance run symmetrically around the iridocorneal angle (Gabelt and Kaufman, 1997; Goel et al., 2010). For example, the ciliary epithelia cells that produce aqueous humor are organized circumferentially just posterior to the iris. Aqueous humor flows past the lens and through the pupil where it filters through trabecular meshwork cells. Aqueous humor is ultimately collected through endothelial-lined collector channels of Schlemm’s canal. Anatomical characterization of the anterior segment of zebrafish, however, suggests differences in tissue organization and a higher degree of dorso-vental specialization in fish (Soules and Link, 2005). Aqueous humor tracer studies indicate that the ciliary epithelium of zebrafish, although lacking folds and processes, is the site of aqueous humor production (Gray et al., 2009). There appears to be enhanced production with the dorsal quadrant of the ciliary epithelium. In contrast, the outflow pathway is exquisitely localized to the ventral part of the angle ((Soules and Link, 2005; Gray et al., 2009); Figure 2). At this region, an endothelial-lined canalicular network can be seen in histology or by electron microscopy. Interestingly, within this region aqueous humor can enter at both the iridocorneal angle and behind the iris through a break in the ventral ciliary epithelium. The canalicular network leads to a relatively large sinus cavity that is continuous with an episcleral venous plexus.
Figure 2. Iridocorneal angle of the zebrafish eye.
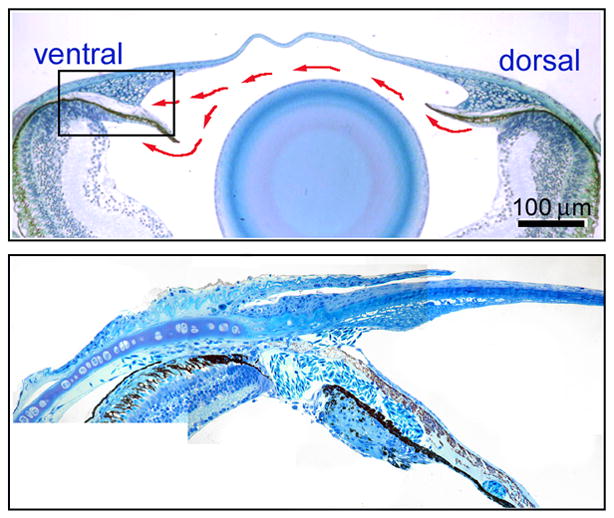
Histology showing low magnification of the adult zebrafish anterior segment (Upper panel). Red arrows show the general flow of aqueous humor. Higher magnification shows the openings of the ventral canalicular outflow pathway (Lower panel).
Another difference between the zebrafish iridocorneal angle and that of mammals is the presence of the annular ligament. This tissue has a hypertrophied appearance and seems to have a structural role in giving shape to the cornea and preventing collapse in the angle region. The annular ligament, while circumferential, does show differences in thickness in a nasal (shallow) to temporal (deep) manner (Yoshikawa et al., 2007). Although the overall tissue organization and presence of the annular ligament of the zebrafish iridocorneal angle is strikingly different from mammals, at the cellular and ultrastructural levels, the ciliary epithelium and cells of outflow pathway show a high-degree of conservation (Soules and Link, 2005; Gray et al., 2009).
Analysis of anterior segment development in the zebrafish also suggests conservation with other vertebrates. Like other species studied, zebrafish periocular cells of neural crest and mesodermal origin migrate into the eye and contribute to the specialized structures of the anterior eye (Soules and Link, 2005; Yoshikawa et al., 2007; Langenberg et al., 2008). Furthermore, when genes known to regulate anterior segment development in other vertebrates are disrupted in zebrafish, dysgenesis also occurs (Gestri et al., 2009; McMahon et al., 2009; Skarie and Link, 2009; Verbruggen et al., 2010). Specific fate maps and lineage analyses of periocular cell contribution to each defined structure of the anterior segment has not been carried out. In principal, however, the zebrafish provides many experimental advantages for such studies.
C The neural retina
1. Development and anatomy
The zebrafish possesses a canonical vertebrate retina composed of one glial and six neural cell types that are arranged in three nuclear layers, separated by two synaptic (plexiform) layers (Figure 3). This distinct layering is already apparent at 3 dpf (days post fertilization).
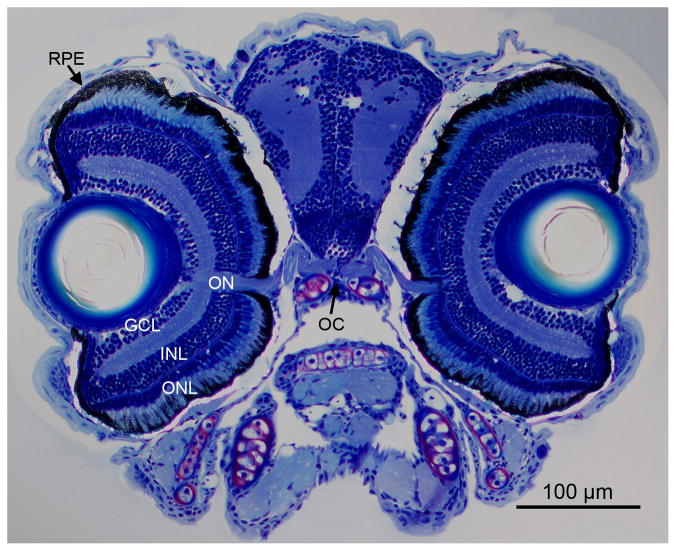
Figure 3.
Retina morphology of a 6-day-old larva. Histology shows the vertebrate typical layering of the larval zebrafish retina with the optic nerve and chiasm. ONL, outer nuclear layer; INL, inner nuclear layer; GLC, ganglion cell layer; ON, optic nerve; RPE, retinal pigment epithelium; OC, optic chiasm.
The outer most cell layer contains the photoreceptors that ultimately convert the physical light stimulus into a biological signal. The outer zebrafish retina contains one rod photoreceptor cell type and the full vertebrate complement of four cone photoreceptors cell types. These are classified by their absorption spectra and morphology. Red- and green sensitive cones are fused at the level of the inner segment and form double cones, while the ultraviolet (UV) and blue sensitive cones are separate and function as short and long single cones, respectively. Therefore zebrafish are, in contrast to humans, tetrachromats that are sensitive to light in the ultraviolet range.
In the inner nuclear layer the somata of bipolar, horizontal and amacrine interneurons are located, as are the cell bodies of the Müller glia cells. Synaptic contacts between photoreceptors and the inner retina are formed in the outer plexiform layer. Closest to the lens is the ganglion cell layer, containing the cell bodies of displaced amacrine cells and ganglion cells. The long axons of the latter constitute the optic nerve and after crossing the midline form the optic tract. At larval stages most of these axons project to the optic tectum, a dorsal midbrain structure homologous to the superior colliculus of mammals, but also to nine additional arborization fields (Burrill and Easter, 1994). The proportionally thick inner plexiform layer reflects the complexity of synaptic contacts between ganglion and amacrine cells and cells of the inner nuclear layer.
The development of the zebrafish retina is extraordinarily rapid facilitating numerous studies addressing retinal development and its genetic control (reviewed in (Tsujikawa and Malicki, 2004b; Fadool and Dowling, 2008)).
The first ganglion cells differentiate around 32 hpf, followed by cells of the inner nuclear layer (Schmitt and Dowling, 1994; Hu and Easter, 1999; Schmitt and Dowling, 1999). Rod and cone photoreceptor outer segments become apparent around 55 dpf (Schmitt and Dowling, 1999). Synaptic structures indicative of functional maturation (ribbon triads) arise within photoreceptor synaptic terminals at around 65 hpf, followed by bipolar cell ribbon synapses approximately at 70 hpf. Signal transmission from photoreceptors to second order neurons starts around 84 hpf and becomes fully functional at 5 dpf (Biehlmaier et al., 2003). This extraordinary fast maturation of the visual system is mirrored by electrophysiological properties and the wealth of visually guided behaviors that can be evoked at these early larval stages (Neuhauss, 2010). In some respects the zebrafish retina never stops developing, since the mature adult retina continues to proliferate. All cell types of the retina are constantly generated in the circumferential germinal zone at the ciliary margin (see Cerveny, Varga & Wilson, this issue). Additionally, rod photoreceptors originating from rod precursor cells of the inner retina are added throughout life (Raymond et al., 2006).
The zebrafish retina has remarkable regenerative capacities. Müller glia cells of the inner nuclear layer are able to produce all retinal cell types in response to injury (Bernardos et al., 2007; Fimbel et al., 2007).
2. Physiology and function
The simple scheme described above does not do justice to the diversity and complexity of retinal cell types. Already the outer retina contains four cone and one rod photoreceptor cell type, distinguished by their morphology and absorption spectra. These two photoreceptor types are adapted to different illumination levels with the cones mediating vision at bright light levels, while rods being functional at low light conditions. These two systems develop asynchronously in the zebrafish. Larval vision is largely dominated by cones, while rod function starts to impact vision only at later stages starting at around 15 dpf (Bilotta et al., 2001). Intriguingly, zebrafish larvae turn off their visual system at night, as evidenced by loss of visual responsiveness, a near absence of electrical responses to light and the disassembly of presynaptic structures used in neurotransmitter release during the subjective night (Emran and Dowling, 2010; Emran et al., 2010).
The complexity of five different photoreceptor cell types is more than matched by the perplexing variety of inner retinal cell types.
There are multiple subtypes of horizontal, bipolar and amacrine cells that can be distinguished by morphology, neurochemistry and physiology. The description of inner retinal cells of the zebrafish is certainly not complete and in particular the functional characterization of the various subtypes is in its infancy.
Recent advances in transgenic technology has allowed labeling of specific cell types, either by using cell types specific promoters or by fortuitous transgenic labeling. Subsequent morphological and electrophysiological analysis of labeled cells will help in the characterization of unique subpopulations of retinal cells (e.g. (Cederlund et al., 2010)). Similar experiments will likely yield a much more complete functional wiring diagram in the near future.
Comparable to other teleosts, the zebrafish contains four distinguishable horizontal cell types, as identified by a number of labeling methods (Connaughton and Dowling, 1998; Yazulla and Studholme, 2001; Connaughton et al., 2004; Song et al., 2008; Li et al., 2009). One large field horizontal cell is rod-specific(, while the two small field horizontal cells (H1, H2) connect solely to cones, just as a large field horizontal cell (H3), which responds best to UV light (Li et al., 2009; Connaughton and Nelson, 2010).
At least 17 bipolar cell types have been recognized by morphological criteria. These can be classified into three groups according to their dendritic termination pattern. These three groups containing cells of ON, OFF, and multistratified ON- and OFF type reflect their functional properties as well (Connaughton and Nelson, 2000; Connaughton et al., 2004).
ON bipolar cells hyperpolarize in response to glutamate released by photoreceptors. The mechanism of hyperpolarization by the archetypical excitatory neurotransmitter glutamate is largely undisputed in mammals. In mammalian species bipolar cells hyperpolarization is thought to be mediated by the activation of a metabotropic glutamate receptor (mGluR6) that ultimately leads to the closure of a transient receptor potential like (TRP) channel (Masu et al., 1995; Shen et al., 2009).
At least in the teleost retina, there is growing evidence for an additional mechanism involved in the cone ON-response. Members of the excitatory amino acid transporter (EAAT) family mediate a chloride conductance activated by glutamate that likely underlies the cone ON response (Wong et al., 2005b; Wong et al., 2005a; Wong and Dowling, 2005). In teleosts and other vertebrates there is now mounting evidence for the parallel existence of both mechanisms. However, firm evidence for an EAAT mediated hyperpolarization of mammalian ON bipolar cells is missing. Intriguingly, mammals have lost two of the full vertebrate complement of seven EAAT family members (Gesemann et al., 2010), which might explain the small or absent contribution of this transporter mediated mechanism in the mammalian retina.
As for most central nervous system synapses, the action of glutamate on depolarizing OFF-type bipolar cells is mediated by ionotropic glutamate receptors of the AMPA and kainate type (Dowling, 1987).
We are only beginning to cope with the diversity of amacrine cell types in the teleost retina with up to 70 morphological and neurochemically distinct cell types (Wagner and Wagner, 1988). In the larval zebrafish retina 28 morphological subtypes have already been distinguished by virtue of an elegant transgenic labeling approach (Jusuf and Harris, 2009). Similar experiments with different transgenic reporters at more mature stages will likely result in the identification of additional subtypes.
Little work has been done on the functional characterization of amacrine cells in the zebrafish retina, with the exception of interplexiform cells. These dopaminergic cells extends processes into both plexiform layers and are involved in feedback signaling from the inner to the outer retina. Due to the position of their cell bodies they can arguably be referred to as amacrine cells. These cells receive input from the olfactory bulb (Zucker and Dowling, 1987) and likely play a crucial role in network adaptation of the retina to light (Witkovsky, 2004). Functional disruption of these cells or of their afferent olfactoretinal centrifugal pathway leads to defects in visual sensitivity (Li and Dowling, 2000a, b).
The larval retina likely contains most of the functionally specialized amacrine cell types known from studies of the mammalian retina. For instance, evidence for the presence of motion sensitive amacrine cells of the Starburst type was gained by the analysis of a metabolic zebrafish mutant (Maurer et al., 2010).
The zebrafish ganglion cell layer contains at least 11 morphologically distinct ganglion cells types (Mangrum et al., 2002; Ott et al., 2007). This number likely underestimates the true diversity of ganglion cells, since the identified morphological types overlap not completely with ganglion cell types identified in similar studies of the rabbit retina (Rockhill et al., 2002; Ott et al., 2007). The physiological characterization of ganglion cell types is just beginning. Six classes can be distinguished by their response to full field stimulation, yielding similar ON-OFF, sustained and transient characteristics as has been reported for other vertebrates (Emran et al., 2007).
In contrast to the puzzling cellular diversity of the five neuronal cell types of the retina, there is only one major glial cell type intrinsic to the retina, the Müller glia cells. Their morphological uniformity is more than matched by the multiple diverse functions they play in retinal physiology. Among these essential functions are the maintenance of ionic homeostasis, metabolic support of neurons, uptake and recycling of neurotransmitters from synapses (for a general reviewed see (Bringmann et al., 2006)).
Müller glia cells have been implicated in visual pigment regeneration of all-trans retinal back to 11-cis retinal following photoisomerization after photon capture. This recycling of visual pigment is crucial for continuous vision and takes place in a series of biochemical reactions variably called the visual or retinoid cycle (extensively reviewed in (Lamb and Pugh, 2004)). Reactions of the canonical visual cycle take place in photoreceptors and the retinal pigment epithelium (RPE). Recently Müller glia cells have been implicated in an alternative visual cycle, likely exclusively serving cone photoreceptors (reviewed in (Wang and Kefalov, 2011)). Studies in the zebrafish have shown a function for a Müller glia cell specific retinoid binding protein in recycling of cone visual pigments, proving a role for a Müller glia cell based recycling pathway involved in cone vision. Cone visual pigment is regenerated by both the canonical and the alternative pathway, while rod visual pigment only has access to the canonical pathway (Fleisch et al., 2008; Fleisch and Neuhauss, 2010).
Müller glia cells in the zebrafish have become a focus of research due to their aforementioned ability to dedifferentiate to a pluripotent state and reconstitute all retinal cell types after injury (Bernardos et al., 2007; Fimbel et al., 2007). Understanding and adapting the cellular mechanism of regenerating injured retinal cells to the human retina has the potential to revolutionize our treatment options for degenerative retinal disorders.
The function of the neural retina hinges on the interaction with the retinal pigment epithelium (RPE) (reviewed in (Strauss, 2005)). These cells form a single layer of cells directly abutting the outer retinal photoreceptors. Apart from their eponymous pigmentation, these cells are characterized by long cellular protrusions. These microvilli interdigitate the photoreceptor’s outer segments. Such an intimate proximity to the photoreceptor’s outer segments enable RPE cells to phagocytose and digest the (potentially damaged) tips of the outer segments, thereby maintaining photoreceptor integrity. RPE cells separate the retina from the blood vessels of the choriocapillaris and are thus ideally situated to regulate the transport of nutrients and oxygen from the blood to the retina and move metabolic waste products back to the blood circulation. Among other physiological functions, key steps of the canonical visual cycle take place in RPE cells.
D The sclera
The sclera is the outermost layer of the eye and is rich in extracellular matrix, particularly collagen, elastin and proteoglycans. This tough, fibrous tissue gives the eye shape and serves a protective role. The sclera is also the site of attachment and insertion of the extraocular muscles. In most vertebrates there are three principle layers of the sclera: the episclera, the stroma and the lamina fusca (reviewed in (Watson and Young, 2004)). The outermost episcleral layer is thin, but more cellularized than the other layers. The episcleral surface is decorated with microplicae which helps form a glycocalyx that lubricates the eye. The stroma is made of interwoven groups of collagen bundles, interspersed with flattened scleral fibroblasts. Unlike the corneal stroma that efficiently transmits light, the scleral stroma scatters light, giving it an opaque appearance. The innermost layer of the sclera is composed of various types of pigmented cells and is rich in elastin. In addition, many lower vertebrates, including zebrafish and some other teleosts, have a scleral ring or scleral ossicle (Walls, 1942). This cartilaginous structure encircles the anterior part of the eye and is embedded within the sclera between the pigmented layer and the stroma, where it is thought to reinforce the sclera and provide further protection to the eye. Detailed anatomical characterization of the zebrafish sclera has not been carried out. However, light and electron microscopic analysis of peripheral nerve processes in the sclera and choroid has been done (Chapman et al., 2009). This study not only revealed that nerve bundles traverse both the choroid and sclera to innervate vascular smooth muscle of the choroid, but also showed general features of the peripheral sclera of zebrafish.
E Immigrant glial cells of the eye
Multiple types of glia are found within the eye or in association with the optic nerve. In addition to the “intrinsic” Muller glial cells that develop from retinal neuroepithelia, there are several “non-native” glial cell types that are generated outside of the eye, but establish an ocular residence. Retinal glia, like glia throughout the nervous system, function in homeostasis processes and respond to injury. Retinal immigrant glia include microglia/macrophages and reticular astrocytes that are found within the eye, as well as oligodendrocytes that myelinate and associate with the optic nerve. Unlike “neuroglia” such as astrocytes and oligodendrocytes, microglia are derived from mesodermal, hematopoietic stem cells that migrate into the developing nervous system during early embryonic stages (Streit and Xue, 2009). At this point, they are broadly termed macrophages. Later, once the cells have taken up residence in the nervous system, they are typically called microglia. In zebrafish, microglia originate from anterior part of lateral mesoderm, differentiate in the yolk sac before the onset of blood circulation and invade the cephalic mesenchyme starting at 22 hpf (Herbomel et al., 1999). With regard to the eyes, the first microglia migrate through the ventral fissure and into the vitreous space by 25 hpf (Herbomel et al., 2001). As retinal lamination progresses, the embryonic microglia become enriched in the synaptic layers, although some can be found within the inner cell body layers. Direct observation of the microglia was facilitated by injecting Neutral Red, which accumulates in the phagocytic cells. Time-lapse observation showed that microglia continually patrol throughout the neural retina – most often migrating within the plexiform layers (Herbomel et al., 2001). More recently, transgenic zebrafish lines have been generated using either apoE or mpeg-1 regulatory sequence to drive GFP expression (Peri and Nusslein-Volhard, 2008; Ellett et al., 2011). These lines should make detailed analysis of microglia/macrophage behaviors more convenient, particularly for adult studies or within diseased or mutant conditions. Generally, microglia function in immune surveillance and neuronal homeostasis (Graeber and Streit, 2010). They clear debris via phagocytosis and can coordinate reactivity and functions of other glia. Microglia also modulate synaptic connections by responding to cues of the complement pathway(Fourgeaud and Boulanger, 2007).
Another class of immigrant glial cells are the reticular astrocytes that derive at least in part from Pax2-positive optic stalk neuroepithelial cells (Macdonald et al., 1997). In zebrafish, differentiated reticular astrocytes populate the optic nerve just posterior to the exit point from the eye (Maggs and Scholes, 1990; Macdonald et al., 1997). These cells form a thin monolayer situated just beneath the basal lamina and thus ensheathe the optic nerve. Processes from the reticular astrocytes appear to extend into the nerve bundle and contact nodes of Ranvier – regions devoid of axon wrapping by the oligodendrocytes. In addition to the optic nerve, reticular astrocytes are found at the inner limiting membrane of the neural retina, with a particular high density at the optic nerve head. In contrast to mammals, zebrafish reticular astrocytes do not express Glial fibrillary acidic protein (GFAP) and instead are marked by cytokeratin-18 immunoreactivity. Like mammals, however, the zebrafish retinal reticular astocytes are often found in association with the nerve fiber layer and blood vessels (Koke et al., 2010). The function of reticular astrocytes in zebrafish has not been directly assessed. However, in mammals these cells are thought to mediate several diverse processes. During development, reticular astrocytes help pattern the retinal vasculature (Fruttiger, 2007). Within the mature retina, reticular astrocytes can influence vascular tone through their interactions with endothelial cells, but also provide metabolic support to neurons (Attwell et al., 2010; Allaman et al., 2011).
Oligodendrocytes represent a third class of glial cells generated outside of the eye. Oligodendrocytes wrap the axons of the optic nerve and provide the components that make the myelin sheath. Oligodendrocytes of the CNS in general derive from ventricular zone neuroepithelial cells (Rowitch and Kriegstein, 2010). These cells first give rise to neurons and later to oligodendrocyte precursor cells (OPCs). In zebrafish, like other vertebrates studied, these OPCs are Olig2- and Sox10-positive (Takada et al., 2010). Oligodendrocyte precursor cells migrate away from the ventricular zone to differentiate in association with the axons they will wrap. The exact ventricular zone location which gives rise to optic nerve oligodendrocytes has not been described in zebrafish or other vertebrates. However, as development progresses, mammalian optic nerve OPCs reside centrally within the fascicles of the optic nerve (Jennings et al., 2002). Currently, the only established function of oligodendrocyctes is to provide insulation to retinal ganglion cell axons and facilitate saltatory propagation of action potentials (Baumann and Pham-Dinh, 2001).
F Development of the vasculature of the eye
The process of intraocular vascularization is initiated by a population of mesodermal and neural crest cells that have migrated around the optic cup to enter the eye through the choroid fissure to form the periocular mesenchyme (POM). This mesenchyme gives rise to the first hyaloid vessels. Rapidly, the vessels organize in a hemispherical basket at the back of the lens forming the hyaloid vasculature, which is composed entirely of arterial vessels (Gariano, 2003; Kitambi et al., 2009).
This embryonic hyaloid vasculature is eventually replaced by the mature retinal vasculature that is tightly associated with the retina ganglion cell (RGC) layer. In humans, the hyaloid vasculature is a transient structure that regresses and is replaced by the mature retinal vasculature. In zebrafish, this transition is less dramatic and occurs without replacement of the hyaloid vasculature (Alvarez et al., 2007). Failure of regression of the hyaloid vasculature in humans is associated with a developmental disorder known as retinopathy of prematurity (ROP). The vasculature of the mature eye consists of independent retinal and choroidal vessels. The retinal vasculature is less extensive in zebrafish than humans; the vessels associate with the RGC layer, but do not branch to form subretinal plexi within the inner and outer plexiform layer as in the human retina. Despite the difference in the distribution of this vascular network, ultrastructural analysis shows that the zebrafish vessels share similar features to mammalian retinal vasculature including a muscular coat containing vascular smooth muscle cells (Alvarez et al., 2007). In mammals, retinal vascular development is controlled by interactions between sensors of hypoxic stress including retinal ganglion cells, glial cells (retinal astrocytes and Muller glia) and endothelial cells (Dorrell and Friedlander, 2006; Fruttiger, 2007; Sapieha et al., 2008). The cell populations involved in stimulating retinal vasculogenesis in fish are still to be determined (Maggs and Scholes, 1990; Alvarez et al., 2007; Koke et al.).
The choroidal vasculature is a second circulatory system that surrounds the retina, is a dense capillaries network closely associated with the RPE and supplies oxygen and nutrients to the photoreceptor cell layer. As for the hyaloid vessels, it is believed that endothelial cells originate from the mesodermal component of the POM while stromal cells, melanocytes and pericytes are derived from neural crest cells. Although the molecular mechanisms are largely unstudied, the development of the choroidal vasculature appears to depend on the presence of differentiated RPE (Saint-Geniez and D’Amore, 2004). Defects in this set of vessels are associated with Age-Related-Macular Degeneration (AMD).
In addition to these two canonical vasculature systems, most fish have an extra vasculature system located behind the retina, the choroid rete mirabile. This is a counter current capillary exchange system responsible at least in part for the maintenance of a high partial pressure of oxygen in the retina (Barnett, 1951; Wittenberg and Wittenberg, 1974).
The final step in the development of a functional vasculature is the formation of the blood retinal barrier (BRB) which has an endothelial component formed by vascular endothelial cells lining the retinal vessels and an epithelial component formed by the RPE. Increased vascular permeability and breakdown of BRB is associated with loss of vision and can be induced by hypoxic conditions. The recent development of a transgenic fish line that visualizes the BRB (Xie et al., 2010) will facilitate studies aimed at understanding the formation and maintenance of this important barrier in both health and disease.
II. Ocular Disease Models
A thorough understanding of the development of the visual system forms the basis to understand ocular diseases. Congenital ocular deficits arising from abnormal developmental processes are common in the human population, since they are mostly compatible with life and reproduction. Indeed, the eye is involved in almost one-quarter of all the phenotypes listed in “Online Mendelian Inheritance in Man” and many of these disorders are congenital (Freund et al., 1996; Chang et al., 2006).
A. Ocular Diseases Linked to Early Eye Development
In this section of the review, we discuss how errors in the development of the eye can lead to a variety of ocular abnormalities. We use illustrative examples of deficits arising from abnormal early specification and/or evagination of the optic vesicles, from morphogenetic errors in choroid fissure closure and from altered vascular development. In each case, we indicate how research using zebrafish can help us to understand the various conditions.
1. From specification of the eye field to optic vesicle evagination
Although the first overt sign of eye development is the evagination of the optic vesicle from the lateral wall of the forebrain, the initial step in eye formation is the specification of a coherent domain of anterior neural plate cells destined to form the optic vesicles (Chuang and Raymond, 2002). This eye field is the anlage of the neural retinas, retinal pigmented epithelia and optic nerves. The eye field is defined by the overlapping expression of about ten eye field transcription factors (EFTFs). EFTF encoding genes are highly conserved across the animal kingdom and form a cross-regulatory network essential for eye development (Chow and Lang, 2001; Zuber et al., 2003; Agathocleous and Harris, 2009).
Signaling pathways that spatially regulate the expression of the EFTFs profoundly affect eye formation. For instance, Wnt/ßcatenin signaling suppresses expression of various EFTFs (Heisenberg et al., 2001; Houart et al., 2002; Lagutin et al., 2003; Cavodeassi et al., 2005) and consequently it is essential to antagonize this pathway within the eye field (Wilson and Houart, 2004). Failure to suppress Wnt/ßcatenin signaling leads to a fate conversion of both the eye field and prospective telencephalon to a caudal diencephalic identity (Heisenberg et al., 2001; Houart et al., 2002) (Kim et al., 2000). Similarly, levels of Bmp activity are low within eye field and suppression of Bmp signaling in the anterior neural plate and bordering ectodermal cells can lead to expansion of the eye field (Houart et al., 2002; Hammerschmidt et al., 2003; Gestri et al., 2005) FGF, IGF and non-canonical Wnt pathways most likely promote eye field formation indirectly through antagonism of Wnt/ßcatenin signaling (Richard-Parpaillon et al., 2002; Kudoh et al., 2004; Cavodeassi et al., 2005). Surprisingly, purinergic signaling also promotes eye formation although the mechanisms of action are not known (Masse et al., 2007).
Once specified, the eye field splits in two domains that evaginate laterally to give rise to the left and right optic vesicles. Nodal and hedgehog signals from axial tissues are required for the formation of bilaterally paired eyes (Echelard et al., 1993; Gritsman et al., 1999; Varga et al., 2001) and disruption of these signals leads to various degrees of synophthalmia/cyclopia and holoprosencephaly (HPE). However, these pathways actually affect regionalization of the evaginating optic vesicles into proximal optic stalk and distal retinal domains (Ekker et al., 1995; Macdonald et al., 1995; Masai et al., 2000), rather than splitting of the eye field and/or evagination per se (Macdonald et al., 1995; Varga et al., 2001; England et al., 2006). As yet, we know very little about the molecular pathways that directly underlie the complex morphogenetic processes that accompany early steps of eye development. Given that the evagination behavior of eye field cells is very different from surrounding anterior neural plate cells (see Cavodeassi and Houart, this issue), it seems likely that the EFTFs influence optic vesicle morphogenesis as well as tissue specification. In support of this, evagination largely fails to occur in the absence of EFTF Rx3 function (Loosli et al., 2001; Loosli et al., 2003; Kennedy et al., 2004; Rojas-Munoz et al., 2005; Rembold et al., 2006; Stigloher et al., 2006). How Rx3 regulates cell behavior is largely unclear although recent studies suggest that Nlcam is a downstream effector required for normal eye formation (Brown et al., 2010).
The development of new tools to label cells, advances in microscopy and advent of image analysis techniques to track cells continue to improve our ability to analyze the complex cell behaviors that accompany eye formation. To date, there are very few studies of eye morphogenesis. Early publications showing differences in the convergence and migratory behaviors of eye field cells compared to surrounding forebrain cells (England et al., 2006; Rembold et al., 2006) need to be re-visited using the more sophisticated imaging approaches now available. Among the most promising recent developments are light-sheet based microscopy techniques that, when combined with sophisticated image analysis, enable the tracking of many thousands of cells over extended periods of time (Reynaud et al., 2008; Keller et al., 2010).
2. Modeling holoprosencephaly in zebrafish
Defects in eye field specification or optic vesicle evagination underlie developmental disorders such as holoprosencephaly (HPE), cyclopia, microphthalmia and anophthalmia. HPE, the incomplete separation of the cerebral hemispheres, is the most common human forebrain malformation (Geng and Oliver, 2009). The causes of HPE are heterogeneous and the phenotype is highly variable with the most severe forms associated with cyclopia. Mutations in about 10 genes have been identified in patients with HPE and among these are the EFTF, Six3 and the midline signaling protein Sonic hedgehog (Shh) (Roessler and Muenke, 2010).
Although it is relatively straightforward to link human HPE to genetic loci and even to identify specific mutant alleles, it is more challenging to determine the function of the mutated alleles. Elegant studies in zebrafish have shown that it is possible to develop robust screening methods to assess both the function of mutant human alleles and the nature of the pathways they affect (Domene et al., 2008; Geng et al., 2008). Heterozygous mutations in Six3 are frequently detected in human HPE and 46 of these mutations have been functionally tested in zebrafish (Domene et al., 2008). Six3 activity antagonises Wnt/ßcatenin (Lagutin et al., 2003) and Bmp signaling (Gestri et al., 2005) pathways and through interaction with members of the Groucho family of transcriptional co-repressors also helps to maintain a balance between growth and differentiation (Kobayashi et al., 2001; Zhu et al., 2002; Lopez-Rios et al., 2003). Reflecting its role in local antagonism of Wnt signaling, conditional elimination of Six3 activity leads to progressive caudalization of the forebrain in both mice and zebrafish (Lagutin et al., 2003; Lavado et al., 2008).
Human SIX3 alleles were injected in zebrafish embryos in order to test the activity of the resulting proteins in in vivo assays (Domene et al., 2008). For instance, various mutant forms were tested for their ability to suppress Wnt/ßcatenin signaling and restore eye development in embryos with hyperactivation of this pathway in the anterior neural plate (due either to loss of activity of Tcf7l (Kim et al., 2000) or of Six3ba and Six7 (Inbal et al., 2007)). A subsequent assay was employed to test the ability of the SIX3 disease associated alleles to dorsalize embryos through suppression of Bmp signaling. These in vivo assays indicated that HPE-associated SIX3 mutations are mostly variable loss of function alleles. Furthermore, the majority of mutations affect the ability of SIX protein to repress Bmp activity. These studies show that well-designed assays in fish can be efficiently used to functionally annotate human mutant alleles.
Differences in allele activity can only partially explain the wide variability of human HPE phenotypes. Only about 28% of HPE cases are caused by mutations in the known HPE genes, indicating that other genetic and/or environmental factors contribute to this condition. Moreover given that the penetrance and expressivity of HPE is highly variable - even in families carrying the same mutation - it is clear that HPE is not a simple monogenic condition, but can arise due to the effects of two or more genetic and/or environmental factors. In support of this, loss of one copy of Shh in the presence of one hypomorphic Six3 mutation in mouse dramatically increases the percentage of embryos with HPE (Geng et al., 2008). Future zebrafish studies are well positioned to further contribute to such analyses of multi-gene effects, by simply exploiting the logistic advantage of their large clutch size.
3. Late eye morphogenesis and choroid fissure fusion
Once the eye field is established, forebrain neuroepithelial cells evaginate to form the optic vesicles. The distal cells of the optic vesicle contact the overlying lens-forming ectoderm and invaginate to form the optic cup while the most proximal region of the vesicle constricts to form the optic stalk that connects the eye to the brain before formation of the optic nerve. Throughout this process, the choroid fissure is present along the ventral retina and optic stalk (Figure 4A). Through this transient opening blood vessels enter and retinal axons leave the developing eye. Once the periocular mesenchyme (POM) that will give rise to the retinal vasculature has entered, and the retinal axons have exited the eye, the fissure lips fuse to permanently close the choroid fissure (Figure 4B). Failure of the choroid fissure to close results in ocular colobomas, a family of common ocular pathogeneses that can cause severe visual impairment (Figure 4C,D; (Chang et al., 2006)).
Figure 4.
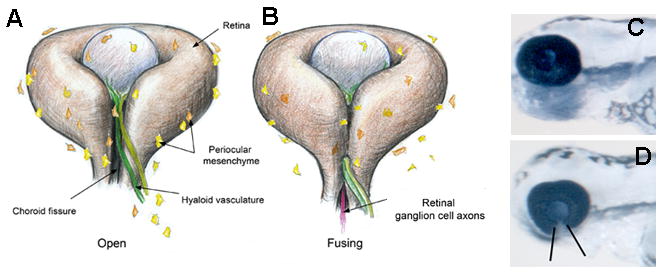
(A,B) Schematics of the forming eye showing the ventrally positioned choroid fissure before and during closure (drawing by Clarissa Scholes). (C,D) Wildtype (C) and colobomatous (D) zebrafish eyes
A recent high resolution imaging analysis of the cellular movements that accompany optic cup morphogenesis (Picker et al., 2009) has helped to reveal the origin of the ventro-nasal (VN) and ventro-temporal (VT) retinal tissues that eventually are apposed in the choroid fissure. Initially, the superficial layer of the optic cup (prospective neural retina) is entirely composed of prospective nasal retina. As development proceeds, prospective temporal retinal cells stream around the temporal rim of the optic vesicle/cup to form the temporal half of the neural retina. As a consequence, the definitive VT lip of the fissure is not present until the optic cup is well-formed. Thus, any genetic or environmental insult that disrupts these phases of optic cup formation (or specification of the cell groups involved) will inevitably result in coloboma due to a failure in formation of the definitive VT lip of the fissure. Such colobomas should be considered an indirect consequence of the initial lesion as the fissure never properly forms (and consequently the fissure cannot close). Colobomas of this type can be very severe and associated with microphthalmia and other severe ocular malformations. In other cases, VN and VT lips of the retina come into close apposition but fusion does not occur suggesting the genetic lesion more directly affects the fusion process.
Choroid fissure closure is radically different from other well-studied fusion events, such as neural tube closure, wound healing and dorsal closure of Drosophila embryos. In these cases, epithelial sheets have a leading edge of polarised cells with very active apical filopodial protrusions that guide the migration and fusion of the opposing epithelia (Martin and Parkhurst, 2004) (Morriss-Kay and Tuckett, 1985). Surprisingly, during choroid fissure closure, the cells of the VN and VT retina that undergo fusion face the fissure with their basal surfaces covered by basal lamina and no protrusion activity has been observed (Hero, 1989); GG unpublished data). Thus it is likely that degradation of the basal lamina lining the fissure is essential for fusion, since this would allow cells of the nasal and temporal retina to come into direct contact (Geeraets, 1976; Hero, 1989; Torres et al., 1996). Confirming this possibility and then defining the nature of the driving forces that bring the nasal and temporal retina together are priorities for future research.
The development of the eye is influenced by reciprocal signaling interactions with the neighboring surface ectoderm, ventral forebrain and POM (Chow and Lang, 2001). POM, comprising cells of both cranial neural crest and lateral plate mesodermal origin, surrounds the eye during its development and some of these cells subsequently enter the fissure before its closure (Gage et al., 2005). A potential role for the POM in eye morphogenesis and fusion of the choroid fissure has recently come to light. Abrogation of activity of genes encoding transcription factors required for POM development, such as lmx1b and tfap2a, lead to ventral eye morphogenesis defects and coloboma (Gestri et al., 2009; McMahon et al., 2009; Bassett et al., 2010). Similarly, neural crest-specific abrogation of Pitx2 or all three retinoic acid receptor genes causes abnormal ventral optic cup development and coloboma (Evans and Gage, 2005; Matt et al., 2008). Moreover a late deficiency in retinoic acid prevents pitx2 expression in the neural crest POM and leads to persistence of the basal lamina in the choroid fissure and coloboma (See and Clagett-Dame, 2009). POM ultimately contributes to numerous anterior segment and extraocular structures (Gage et al., 2005; Soules and Link, 2005) and some human patients show coloboma associated with anterior segment defects (Ozeki et al., 1999; Tang et al., 2006), again suggesting that coloboma can be linked to defective POM.
Despite these indications, it remains unclear how POM cells influence ventral eye morphogenesis and whether coloboma phenotypes are always an indirect consequence of a failure to apposition the ventral retinal lips, or whether POM may have a more direct role in the actual fusion event. Since a key role for the choroid fissure is to allow access of reticular astrocytes and cells that form retinal vasculature, it is tempting to speculate that fusion is triggered by these cells once they have entered the eye. Indeed, fusion tends to commence from the level of the optic disk, where the vasculature enters the eye (Geeraets, 1976; Hero, 1989); Fig. 4B). One possibility is that the forming hyaloid vessels are the source of matrix metalloproteineases (Lafleur et al., 2003) that assist in basal lamina dissolution around the fissure margins. It will be important to analyze choroid fissure development and basal lamina degradation when cephalic angiogenesis is disrupted genetically or pharmacologically. Zebrafish are particularly well-suited for such studies.
4. Using zebrafish to understand coloboma in humans
Although there is variable prevalence in different countries, coloboma is estimated to underlie as much as 10% of childhood blindness. Despite this, the genetic basis of this condition remains largely unknown and relatively few genes (including those encoding the transcription factors PAX2, PAX6, PITX2, CHX10, AP2 and the signalling protein SHH; OMIM 120200) are implicated in the pathogenesis of ocular coloboma. Both sporadic as well as Mendelian (autosomal dominant, autosomal recessive and X-linked recessive) inheritance patterns have been reported (Chang et al., 2006) and, as described above, there is huge variability in the severity of coloboma and associated phenotypes. As with the other conditions we have discussed, some of the variability in coloboma phenotypes is likely due to the combined effects of two or more mutations affecting the same signaling pathway or developmental process. Such interactions are difficult to assess in humans but are simple to test in fish as there are techniques that allow the simultaneous disruption of two or more genes.
Analysis of the coloboma associated gene tfap2 in zebrafish confirmed that synthetic enhanced phenotypes could arise following abrogation of Tfap2 together with other genes implicated in eye formation (Gestri et al., 2009). Mutations in human TFAP2 are linked to branchio-oral-facial-syndrome (Milunsky et al., 2008) with ocular phenotypes ranging from anophthalmia to coloboma (Gestri et al., 2009). To explore whether this phenotypic variation could be due to additional mutations affecting eye formation, Tfap2 function was partially abrogated in embryos carrying mutations affecting either Bmp or Wnt signaling. These experiments showed that partial abrogation of Tfap2 in wildtype embryos had no obvious effect on eye development. In contrast, similar reduction in Tfap2 led to anophthalmia in embryos with enhanced Wnt signaling and coloboma in embryos with reduced Bmp signaling (Gestri et al., 2009). These studies demonstrate that complex genetic and signaling interactions contribute to inheritance of human developmental eye anomalies. Information on these interactions can be very helpful in determining genetic predisposition (risk) to eye problems that individuals or their children may face. Ongoing Genome Wide Association Studies (GWAS) in humans will undoubtedly reveal many candidate interacting loci. Studies in zebrafish, as well as other model systems, will enable scientists to move beyond correlation and directly assess the genetic interactions of candidate genes arising from these screens.
Complementing the identification of candidate loci through human GWAS, genetic screens in model systems will help to functionally annotate the vertebrate genome. Through a variety of large-scale community projects, null mutations will probably be generated in most protein coding genes in zebrafish in the next few years. Consequently, functional annotation of the genome has the potential to proceed much more quickly and cheaply than in humans or mammalian models. Indeed animal studies already have identified several new genes that are candidates for loci implicated in coloboma in humans (Huh et al., 1999; Brown et al., 2009; McMahon et al., 2009). Perhaps top of the list at present are the Vax1 and Vax2 transcription factors that, when mutated, lead to coloboma in both fish and mice (Barbieri et al., 1999; Bertuzzi et al., 1999; Take-uchi et al., 2003). Indeed it is surprising that, as yet, no human mutations in these genes have been linked to coloboma.
The potential of using zebrafish for large scale small molecule screen for drug discovery is reviewed elsewhere (Rihel and Schier reviews, this issue), but the following are examples where pharmacological intervention has been used to reveal insights on the mechanisms of coloboma. Coloboma in blowout zebrafish mutants can be suppressed by pharmacologically inhibiting the Hedgehog pathway between eye field to optic vesicle stage, revealing not only that enhanced Hh signalling underlies the phenotype, but also the stage at which the pathway acts (Lee et al., 2008). Similarly timed use of Retinoic Acid agonists and Wnt inhibitors in adenomatous polyposis coli (apc) mutant fish has enabled dissection of the discrete contributions of these two pathways to the complex ocular phenotypes of apc mutants and also revealed the timing of their actions during eye formation (Nadauld et al., 2006). Finally, rather surprisingly, two aminoglycoside drugs, gentamicin and paromomycin, appear to be able to rescue the coloboma phenotype in pax2 and lamb1 zebrafish models for ocular coloboma (Moosajee et al., 2008), most likely by promoting “non-sense readthrough” by skipping mutant-induced termination codons during protein translation.
Information from studies such as these undoubtedly aid in defining clinical treatments for ocular diseases.
B. Photoreceptor degeneration
Heritable blindness in humans is caused by numerous different mutations, many directly affecting the neural retina with by far the largest fraction compromising the survival of photoreceptors. This situation is mirrored in the zebrafish where a wealth of mutant strains with degenerating photoreceptors have been isolated. Consequently the zebrafish has established itself as an important model system for human outer retinal dystrophies. In the following we briefly discuss some of the contributions that the zebrafish model system has provided to our understanding of human outer retinal dystrophies and refer the interested reader to a number of recently published review articles and book chapters for a more in depth treatment of the subject (Fadool and Dowling, 2008; Gross and Perkins, 2008; Brockerhoff and Fadool, 2010; Samardzija et al., 2010).
1. Primary photoreceptor defects
Genetic screens for zebrafish vision mutants have isolated a large number of strains with outer retinal dystrophies directly affecting photoreceptors. The molecular nature of a growing number of these mutants have been identified. Similar to human retinal diseases, genes coding for components of the visual transduction cascade are among the identified genes (reviewed in (Berger et al., 2010; Brockerhoff and Fadool, 2010)).
The analysis of zebrafish visual mutants contributed particularly importantly to our understanding of the role of intraflagellar transport (IFT) for photoreceptor integrity (Insinna and Besharse, 2008). Defects in IFT is now recognized as a major contributor to human ciliopathies, including Bardet–Biedl and Senior-Loken syndrome, characterized by pronephric cysts and photoreceptor degeneration. The phenotype of a number of zebrafish mutants (oval, elipsa, fleer) closely resembled these human ciliopathies, including defects in photoreceptor outer segment formation (Doerre and Malicki, 2002; Bahadori et al., 2003). Positional cloning of the oval locus identified a mutation in the zebrafish ortholog of the intraflagellar transport protein 88 (IFT88) of Chlamydomonas (Tsujikawa and Malicki, 2004a). This protein is a component of the IFT complex, which is involved in the generation and maintenance of ciliated structures. Analysis of the IFT88 mutant and morphants of other IFT proteins, revealed that members of the IFT complex B (IFT88, IFT52 and IFT57) play an essential role in the maintenance, but not in the initial assembly of sensory cell cilia. Functional loss of IFT complex A members (IFT140) produced only mild phenotypes (Tsujikawa and Malicki, 2004a). The analysis of two retroviral insertion mutants in genes coding for IFT complex B genes (IFT57 and IFT172) yielded similar conclusions (Gross et al., 2005). Detailed biochemical studies showed that IFT88 is indeed required for outer segment formation, while IFT57 is vital for efficient intraflagellar transport. IFT57 likely mediates the association of IFT20, another IFT complex A component, with the IFT particle (Krock and Perkins, 2008). These studies have contributed importantly to our understanding of IFT complex B function in photoreceptor outer segment maintenance.
Recently, a requirement of tubulin polyglutamylation for ciliogenesis has been uncovered, by demonstrating that the ciliopathy of the zebrafish fleer mutant is caused by a mutation in a tubulin polyglutamylation modulator protein. Similar defects are observed by a direct knockdown of a tubulin polyglutamylase (Pathak et al., 2007). Finally, through a series of studies in zebrafish, several microtubular motors have been implicated in transport of IFT complexes during photoreceptor outer segment development (Krock et al., 2007; Insinna et al., 2008; Krock and Perkins, 2008; Insinna et al., 2009; Insinna et al., 2010).
2. RPE defects
Given the close cooperation between photoreceptors and RPE it is hardly surprising that genetic defects of RPE function often lead to subsequent photoreceptor degeneration. Indeed a number of zebrafish mutants that have been isolated due to their visual function deficit turned out to be primarily affected in the RPE (Neuhauss et al., 1999).
For some of these mutants, the underlying molecular defect has been identified and confirme an important role of intracellular transport for maintaining cellular integrity. One such mutant is the leberknödel (lbk) mutant, which was initially isolated due to hypopigmentation of skin melanocytes and the RPE. Subsequent analysis showed defects in vision, likely caused by reduced photoreceptor outer segments, internal organs and macrophages. These multicellular defects are caused by mutations in the vam6/vps39 gene coding for a component of the HOPS complex, which is essential for vesicle fusion and tethering (Schonthaler et al., 2008). Similarly, mutants defective in a Rab escort protein (REP1), which is involved in multiple aspects of intracellular transport, cause choroideremia, a form of retinal degeneration. Elegant transplantation experiments showed that the retinal defect is caused by the lack of REP1 function in the RPE, although the protein is expressed, among many other cell types, in both photoreceptors and the RPE (Krock et al., 2007). These results make it likely that other mutants with similar phenotypes are also affected in some aspect of RPE intracellular trafficking. One case in point is the fading vision (fad) mutant, which shows photoreceptor cell loss subsequent to pigmentation defect in the RPE (Bahadori et al., 2006). Interestingly, these mutants also show defects in blood clotting and hence model Hermansky-Pudlak syndrome, a rare human disease characterized by oculocutaneous albinism, bleeding and lysosome-related organelle defects (Wei, 2006).
More enigmatic are the findings that mutants with defects restricted to melanosome biogenesis can affect RPE metabolism and therefore the visual cycle, such that photoreceptors are secondarily damaged (Schonthaler et al., 2005).
3. Late onset retinal degeneration
A large number of zebrafish mutants with progressive outer retinal dystrophy have been identified. Although these mutants are valuable models for the corresponding human disease, they typically affect the larval retina, whereas the human degeneration is usually age-related. In the most extreme case of age-related macular degeneration (AMD) overt photoreceptor damage may occur as late as in the 8th decade of life. The scarcity of reports on adult onset retinal degeneration in zebrafish may be due to a combination of the limitations of recessive larval screens and underlying biological mechanisms. Only recently a few mutants which affect the survival of photoreceptors in the adult retina have been identified (Li and Dowling, 2000b; Stenkamp et al., 2008). All of these are dominant mutants displaying the adult phenotype in the heterozygous condition, while homozygous animals are embryonic lethal. For instance, heterozygous mutants for shh show a progressive late onset of cone photoreceptor loss, suggesting a role for sonic hedgehog signaling in the maintenance of adult photoreceptors (Stenkamp et al., 2008). There is now burgeoning interest in the analysis of adult vision phenotypes with efforts to adapt visual performance assays to adult fish (Li and Dowling, 1998; Mueller and Neuhauss, 2010) and to perform screens on adult fish (Li and Dowling, 2000b; Tschopp et al., 2010). This strategy will likely yield a number of adult onset degeneration mutants in the future, although the logistics and the genetic analysis of such mutants is daunting. The list of available mutants will be complemented by mutant strains generated by nascent gene knockout technologies developed in the zebrafish (reviewed in (Amacher, 2008; Moens et al., 2008)).
A promising alternative approach is to use targeted transgene expression to elicit degeneration. There are already a number of promoters available that drive gene expression specifically in rod (Fadool, 2003) and cone (Luo et al., 2004; Kennedy et al., 2007; Takechi et al., 2008) photoreceptors.
For example, expression of a membrane-targeted cyan fluorescent protein in rod photoreceptors was found to fortuitously induce apoptosis starting at larval stages and continuing at adult stages. In contrast to the human retina, rod apoptosis did not cause secondary cell death of cone photoreceptors (Morris et al., 2005). The absence of this bystander effect can possibly be explained by the difference in relative densities of rods in the human and fish retina, where cone densities are much higher in the fish retina (Punzo et al., 2009).
Photoreceptor type specific transgenic lines can now be used to specifically ablate expressing cells by a number of physical and biochemical means. The list of available promoters will likely grow significantly in the next few years and will be supplemented by strains with inducible transgene expression.
C Glaucoma
The glaucomas encompass a relatively diverse group of blinding diseases characterized by specific visual field defects and optic nerve damage (Ritch et al., 1996; Morrison and Pollack, 2003). In glaucoma, retinal ganglion cells, whose axons project out of the eye and make up the optic nerve, die progressively and typically in a focal manner. Most commonly, the visual field is first impaired in the periphery followed by progressive defects in central vision. The most common form of glaucoma is Primary Open Angle Glaucoma (POAG). This sub-type of glaucoma is contrasted with Closed Angle Glaucoma, where the iris physically blocks the flow of aqueous humor through the outflow channels at the iridocorneal angle, resulting in raised intraocular pressure. Additional sub-types of glaucoma have also been classified and include pigmentary glaucoma, developmental glaucoma and steroid-induced glaucoma. Raised intraocular pressure is a major risk factor for all forms of glaucoma. Other risk factors have also been established for POAGs. These include family history and myopia. Developmental glaucomas are the least common form of glaucoma, but have been the most approachable from a genetic standpoint. The developmental glaucomas encompass conditions associated with visible structural abnormalities in the anterior segment that are usually detected early in life. The age of onset for optic nerve damage varies from early childhood to adult and this form of glaucoma is generally more severe. Developmental glaucomas, which are most readily studied in zebrafish, were once believed to be ‘simpler’ Mendelian forms of glaucoma. However, the developmental glaucomas are now known to exhibit similar complexity to adult forms of glaucoma in terms of phenotype variability and etiology (Gould and John, 2002). In fact, heterogeneity is found with each type of glaucoma and variability includes the age of onset, severity of the disease, mode of inheritance and penetrance within affected families. These observations coupled with direct genetic linkage studies have demonstrated that the glaucomas are complex: multiple loci, as well as non-genetic factors, interact to cause the disease and affect its progression (Libby et al., 2005).
The complexity of glaucoma has made modeling this disease difficult in animals. Specific gene function has largely been explored in cell culture systems. Experimental elevation of intraocular pressure or optic nerve crush paradigms in rodents have historically been the favored approaches for studying the mechanisms of glaucoma pathogenesis. However, neither of these methods adequately account for the chronic and progressive damage associated with the disease. More recently, the DBA2J mouse model has been used as a genetic model for a pigmentary glaucoma and this mouse mutant does model the chronic nature of the diesease (John et al., 1998; Anderson et al., 2002). In addition, reverse engineering is being applied in mice to establish other models of glaucoma based on human disease associated genes (Johnson and Tomarev, 2010).
Zebrafish provide excellent opportunities for testing specific hypotheses related to glaucoma, as well as identifying glaucoma gene interactions by screening for mutations that result in glaucoma pathology or disease risk phenotypes. For example, zebrafish have been used to study the cellular etiology and gene-interactions of foxC1-mediated glaucoma and ocular dysgenesis (Tamimi et al., 2006; Berry et al., 2008; Skarie and Link, 2009). These studies demonstrated a key role for the transcription factor FoxC1 in regulating factors that mediate responses to oxidative stress and suppression of apoptosis in cells that regulate aqueous humor dynamics. As another example, zebrafish were used to determine the normal nucleolar function of Wdr36, one of the few genes known to be associated with human glaucoma (Skarie and Link, 2008). The role of Wdr36 in ribosomal RNA processing has been subsequently confirmed in other species (Footz et al., 2009; Gallenberger et al., 2011), emphasizing the relevance of zebrafish for rapidly identifying the in vivo functions of disease-associated proteins. Forward genetic analyses has also been fruitful in identifying zebrafish mutants that show risk phenotypes associated with glaucoma. For example the brass mutation was shown to exhibit elevated intraocular pressure (Link et al., 2004). However, it should be noted that brass mutants do not show retinal ganglion cell loss and the pressure elevation is relatively mild. More recently, another mutant has been identified that shows more dramatic elevated intraocular pressure and also displays progressive optic nerve damage (Veth et al., 2011). This mutant, called bugeye, disrupts the low density lipoprotein receptor-related protein 2, lrp2, gene, is adult viable and has visibly enlarged eyes that results in retinal stretch. Retinal ganglion cells show signs of stress as early as 2 months of age, but do not show elevated apoptosis until approximately 1 year of age. The bugeye mutant therefore provides another model for studying the relationships between chronic elevated intraocular pressure, prolonged mechanical (stretch) stress and ganglion cell health.
One of the caveats with using zebrafish to study a disease affecting ganglion cell axons and ganglion cell survival, is that zebrafish show a remarkable capacity for retinal cell regeneration – including the ganglion cells (Hitchcock and Raymond, 2004; Sherpa et al., 2008). However, this trait can be used to identify genes and pathways responsible for sensing and responding to axon damage. As an example, using a transgenic zebrafish line that expresses GFP upon optic nerve injury, the Goldman lab has identified several genes that are essential for ganglion cell stress response and regeneration (Veldman et al., 2007; Fausett et al., 2008; Ramachandran et al., 2010; Veldman et al., 2010).
D. Vascular disease of the eye
The retina is one of the most metabolically active tissues with a higher rate of oxygen consumption than the brain. Discordance between vascular supply and tissue demand for oxygen and nutrients results in a hypoxic retina and pathological neovascularization. Both over- and under-production of blood vessels leads to severe forms of human eye disease, including diabetic retinopathy, AMD, optic nerve head ischemia and ROP (Adamis et al., 1999). Significantly, these diseases are a leading cause of vision impairment and blindness collectively accounting for about 2/3 of blindness in developed countries. The common denominator of all these diseases is hypoxia. In the hypoxic state, the retina produces transcription and growth factors such as HIF and VEGF, as well as metabolites such as succinate that influence vessel integrity and promote abnormal vessel growth (Gariano and Gardner, 2005; Arjamaa and Nikinmaa, 2006; Sapieha et al., 2008).
Although there is an infant mouse model for hypoxia-induced retinal angiogenesis (Smith et al., 1994), until recently there was no adult animal model for such conditions. However, Cao and colleagues recently reported the fist adult model for hypoxia-induced retinal angiogenesis in zebrafish (Cao et al., 2010). Using a transgenic line to visualize the vasculature in vivo, they showed that fish exposed to hypoxic conditions developed severe retinal neovascularization after 3 to 15 days of incubation. In order to demonstrate the clinical relevancy of this model, they blocked hypoxia-induced neovascularization using drugs that block VEGF signaling. This demonstrated that VEGF plays a pivotal role in both zebrafish and human disorders characterized by retinal neovascularization.
Zebrafish vhl mutants are another excellent in vivo model for pathological angiogenesis and vascular retinopathies (van Rooijen et al., 2010). von Hippel-Lindau (VHL) syndrome is a rare disease, characterized by abnormal growth of blood vessels and tumors, in which the activation of hypoxia-induced transcription factor (HIF) plays a significant causative role. VHL patients can develop retinal neovascularization similar to diabetic retinopathy and age-related macular degeneration (Chew, 2005). As in humans, zebrafish vhl mutants develop severe neovascularization in the brain, eye, and trunk. Moreover, the retinal vasculature of vhl mutant zebrafish is leaky, with severe macular edema and retinal detachment, comparable to VHL associated diabetic retinopathy and AMD. As in humans and mice, VEGF signaling is enhanced by loss of VHL, and this is one of the key effectors of the vhl mutant angiogenic phenotype. Supporting this, VEGF receptor inhibitors can block pathological angiogenesis in mutant fish. Vascular defects are also present in VHL mutant mice but the condition is embryonic lethal (Gnarra et al., 1997) limiting the utility of the model. Consequently, in addition to their utility in studying VHL disease, zebrafish vhl mutants are a unique and clinically relevant model of pathological angiogenesis. Importantly, pathogenesis in the zebrafish model can be studied non-invasively in vivo and under normoxic conditions.
Furthermore, both of these zebrafish models can potentially be used for screening of anti-angiogenic drugs, for evaluation of therapeutic efficacy of existing anti-angiogenic agents and to study vascular patterning and remodeling of pathological angiogenesis. Such drug screening has already proved to be efficacious in wildtype zebrafish (Kitambi et al., 2009) providing the proof of principle to extend the screens to disease models.
E Diseases of cornea and lens
Corneal dystrophies and cataracts are vision-obscuring conditions that affect the transparency of the cornea and lens, respectively. The corneal dystrophies are a collection of disorders classified by the corneal layer that is affected (Krachmer and Palay, 1991). They are non-inflammatory, inherited, and result in opacity. Similarly, cataract is defined as clouding of the lens (Vavvas et al., 2002). A cataract is typically focal, but can encompass the entire lens. The genetics and mechanisms of pathology for these diseases of the refractive components of the eye are just beginning to be understood. Examples of both corneal and lens defects have been reported for zebrafish, but in general the phenotypes that have been described thus far are typically more severe than the human disease conditions. For example mutations in genes that encode proteins associated with apicobasal cell polarity and apical junction complex integrity result in corneal epithelial and/or stromal dysgenesis (Beyer et al., 2010). With regard to lens abnormalities, mutations in basement membrane components have been found to result in lens degeneration. For example, mutations to components of Laminin-1 each cause significant lens abnormalities during development, which result in rapid lens degeneration (Semina et al., 2006; Lee and Gross, 2007). Additionally, mutations in zebrafish collagen4a5 result in later onset lens degeneration (Xiao and Baier, 2007). Of relevance to human disease, mutations in human COL4A5 cause Alport syndrome in which patients can display a spectrum of CNS disorders along with gross ocular anomalies including lens degeneration (Hudson et al., 2003). Human mutations of the transcription factors PITX3 and FOXE3 cause specific lens defects ranging from cataract to aphakia (Semina et al., 1998; Semina et al., 2001). Morpholino knock-down of these genes in zebrafish also show such phenotypes (Shi et al., 2005; Shi et al., 2006). Recently, a new zebrafish mutant has been described, bumper, that shows lens epithelial hyperplasia following and fiber cell disorganization, resulting in opacity and ultimately lens degeneration (Schonthaler et al., 2010). Although the causative gene for this mutation has not been identified, it maps to a locus suggesting that it encodes a novel lens maintenance factor for vertebrates.
F Myopia
Myopia, or nearsightedness, is the result of abnormal eye growth that causes refractive error. It is the most common human ocular disorder worldwide and its incidence is increasing (Bloom et al., 2010). Although myopia is heritable, it is genetically complex and development of the disorder can be highly impacted by the visual environment (Young, 2009). Accordingly, myopia is heterogeneous with variability in phenotypes and inheritance. In a process known as emmetropization, the axial length of the eye globe is matched to the refractive properties of the cornea and lens, such that the focal point of light entering the eye can be precisely positioned on the photoreceptor layer. Experimentally, myopia can be induced by blurring light entering the eye or simulating blur in a manipulated visual environment (Sivak, 2008). Most often, blur is accomplished through application of defocusing lenses placed over an eye. Such experiments in young chicks have been successful in providing the basic framework for the neural circuits and signaling factors responsible for emmetropization.
In general, visual blur is detected by the photoreceptors and processed by inner retinal neurons, likely depending on amacrine cells, but not ganglion cells (Wallman and Winawer, 2004). Blur detection within the retina then results in signals that are transferred from the retina, through the retinal pigment epithelium, to the choroid and sclera – tissues which manifest changes in morphology. The early responses during emmetropization occur in the choroid, where thickness of this layer is modulated by vascular tone and changes in blood flow (Wallman et al., 1995). More long-term changes, however, occur in the sclera, which undergoes remodeling that facilitates axial length changes. Remodeling is principally carried out in the scleral stroma by modification to collagen bundling and cross-linking (McBrien et al., 2009). One of the advantages of using young chicks is that their eyes are still growing and therefore primed for showing measurable changes in eye shape and growth during experimentation. Zebrafish maintain this experimental advantage throughout their life, as teleost eyes are constantly growing. As discussed below, zebrafish may in fact serve as a valuable model for addressing mechanism that underlie myopia.
Despite successes is determining a general progression of events, fundamental questions in understanding emmetropization and myopia remain. For example, how blur is computed within the eye is unknown, as is the precise wiring of the neural circuit(s) that trigger signals for choroid and scleral modification. Similarly, identification of the specific signaling molecules that mediate emmetropization is lacking. From a disease perspective, there is a significant need to identify and define gene networks and loci involved in myopia. Answers to some of these questions can potentially come from studies using zebrafish. Significantly, work from the Sivak lab has shown that teleosts are susceptible to form-deprivation induced myopia (Shen et al., 2005; Shen and Sivak, 2007). In addition, previous studies with teleosts showed that their eyes will grow to compensate for defocus related to chromatic aberration (Kroger and Wagner, 1996). Furthermore, heritable models of myopia have been established in goldfish that were originally bred for their uniquely large and bulging eyes (Easter and Hitchcock, 1986; Raymond et al., 1988). Recently, a similar zebrafish model, bugeye/lrp2 mutants, has been established, as previously discussed above (Veth et al., 2011). Zebrafish with lrp2 mutations show measurable refractive errors as early as 1 month of age and visibly enlarged eyes by 3 months of age (Figure 5). Lrp2 is large transmembrane receptor know to mediate trafficking and clearance of numerous bioactive proteins (Christensen and Birn, 2002). Within the eye, Lrp2 is expressed on RPE cells and potentially facilitates movement and/or activity of factors that mediate emmetropization. In humans, LRP2 mutations cause Donnai-Barrow Syndrome, which is characterized in part by severe myopia (Pober et al., 2009). Collectively, these observations from teleost studies provide strong rationale for using zebrafish to probe the genetic causes and mechanisms of myopia.
Figure 5. Dorsal view of a myopic bugeye mutant.
Dorsal views of wild-type (Left) and bugeye mutant (Right) adult zebrafish.
Overall, the continued development of new mutant zebrafish models coupled with the availability of ever more sophisticated transgenes to visualize specific cells, proteins, signaling pathways and cellular processes in vivo contribute to the expanded use of zebrafish for modeling, understanding and assisting in finding treatments for human ocular diseases.
Acknowledgments
The authors thanks Clarissa Scholes for the drawing in fig 4 and for discussion and Steve Wilson for critical reading of the manuscript. The work in our groups is supported by the MRC (GG), National Institutes of Health R01EY016060 (BAL), the European commission (RETICIRC, ZF-HEALTH) and the Swiss National Science Foundation (3100A0-117782) (SCFN).
References
- Adamis AP, Aiello LP, D’Amato RA. Angiogenesis and ophthalmic disease. Angiogenesis. 1999;3:9–14. doi: 10.1023/a:1009071601454. [DOI] [PubMed] [Google Scholar]
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Schonthaler HB, Bron AJ, Dahm R. Formation of stromal collagen fibrils and proteoglycans in the developing zebrafish cornea. Acta Ophthalmol. 2008;86:655–665. doi: 10.1111/j.1600-0420.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends in Neuroscience. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Cederlund ML, Cottell DC, Bill BR, Ekker SC, Torres-Vazquez J, Weinstein BM, Hyde DR, Vihtelic TS, Kennedy BN. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev Biol. 2007;7:114. doi: 10.1186/1471-213X-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amacher SL. Emerging gene knockout technology in zebrafish: zinc-finger nucleases. Brief Funct Genomic Proteomic. 2008;7:460–464. doi: 10.1093/bfgp/eln043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83:473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadori R, Huber M, Rinner O, Seeliger MW, Geiger-Rudolph S, Geisler R, Neuhauss SC. Retinal function and morphology in two zebrafish models of oculo-renal syndromes. Eur J Neurosci. 2003;18:1377–1386. doi: 10.1046/j.1460-9568.2003.02863.x. [DOI] [PubMed] [Google Scholar]
- Bahadori R, Rinner O, Schonthaler HB, Biehlmaier O, Makhankov YV, Rao P, Jagadeeswaran P, Neuhauss SC. The Zebrafish fade out mutant: a novel genetic model for Hermansky-Pudlak syndrome. Invest Ophthalmol Vis Sci. 2006;47:4523–4531. doi: 10.1167/iovs.05-1596. [DOI] [PubMed] [Google Scholar]
- Barbieri AM, Lupo G, Bulfone A, Andreazzoli M, Mariani M, Fougerousse F, Consalez GG, Borsani G, Beckmann JS, Barsacchi G, Ballabio A, Banfi S. A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci U S A. 1999;96:10729–10734. doi: 10.1073/pnas.96.19.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett CH. The structure and function of the choroidal gland of teleostean fish. J Anat. 1951;85:113–119. [PMC free article] [PubMed] [Google Scholar]
- Bassett EA, Williams T, Zacharias AL, Gage PJ, Fuhrmann S, West-Mays JA. AP-2alpha knockout mice exhibit optic cup patterning defects and failure of optic stalk morphogenesis. Hum Mol Genet. 2010;19:1791–1804. doi: 10.1093/hmg/ddq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res. 2010;29:335–375. doi: 10.1016/j.preteyeres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry FB, Skarie JM, Mirzayans F, Fortin Y, Hudson TJ, Raymond V, Link BA, Walter MA. FOXC1 is required for cell viability and resistance to oxidative stress in the eye through the transcriptional regulation of FOXO1A. Hum Mol Genet. 2008;17:490–505. doi: 10.1093/hmg/ddm326. [DOI] [PubMed] [Google Scholar]
- Bertuzzi S, Hindges R, Mui SH, O’Leary DD, Lemke G. The homeodomain protein vax1 is required for axon guidance and major tract formation in the developing forebrain. Genes Dev. 1999;13:3092–3105. doi: 10.1101/gad.13.23.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer J, Zhao XC, Yee R, Khaliq S, McMahon TT, Ying H, Yue BY, Malicki JJ. The role of crumbs genes in the vertebrate cornea. Invest Ophthalmol Vis Sci. 2010;51:4549–4556. doi: 10.1167/iovs.09-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehlmaier O, Neuhauss SC, Kohler K. Synaptic plasticity and functionality at the cone terminal of the developing zebrafish retina. J Neurobiol. 2003;56:222–236. doi: 10.1002/neu.10243. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Saszik S, Sutherland SE. Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Dev Dyn. 2001;222:564–570. doi: 10.1002/dvdy.1188. [DOI] [PubMed] [Google Scholar]
- Bloom RI, Friedman IB, Chuck RS. Increasing rates of myopia: the long view. Curr Opin Ophthalmol. 2010;21:247–248. doi: 10.1097/ICU.0b013e328339f1dd. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Brockerhoff SE, Fadool JM. Genetics of photoreceptor degeneration and regeneration in zebrafish. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Dutta S, Bharti K, Bonner RF, Munson PJ, Dawid IB, Akhtar AL, Onojafe IF, Alur RP, Gross JM, Hejtmancik JF, Jiao X, Chan WY, Brooks BP. Expression profiling during ocular development identifies 2 Nlz genes with a critical role in optic fissure closure. Proc Natl Acad Sci U S A. 2009;106:1462–1467. doi: 10.1073/pnas.0812017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KE, Keller PJ, Ramialison M, Rembold M, Stelzer EH, Loosli F, Wittbrodt J. Nlcam modulates midline convergence during anterior neural plate morphogenesis. Dev Biol. 2010;339:14–25. doi: 10.1016/j.ydbio.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Easter SS., Jr Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio) J Comp Neurol. 1994;346:583–600. doi: 10.1002/cne.903460410. [DOI] [PubMed] [Google Scholar]
- Cao Z, Jensen LD, Rouhi P, Hosaka K, Lanne T, Steffensen JF, Wahlberg E, Cao Y. Hypoxia-induced retinopathy model in adult zebrafish. Nat Protoc. 2010;5:1903–1910. doi: 10.1038/nprot.2010.149. [DOI] [PubMed] [Google Scholar]
- Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, Tada M, Wilson SW. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederlund ML, Morrissey ME, Baden T, Scholz D, Vendrell V, Lagnado L, Connaughton VP, Kennedy BN. ZEBRAFISH Tg(7.2mab21l2:EGFP)ucd2 TRANSGENICS REVEAL A UNIQUE POPULATION OF RETINAL AMACRINE CELLS. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Blain D, Bertuzzi S, Brooks BP. Uveal coloboma: clinical and basic science update. Curr Opin Ophthalmol. 2006;17:447–470. doi: 10.1097/01.icu.0000243020.82380.f6. [DOI] [PubMed] [Google Scholar]
- Chapman GB, Tarboush R, Eagles DA, Connaughton VP. A light and transmission electron microscope study of the distribution and ultrastructural features of peripheral nerve processes in the extra-retinal layers of the zebrafish eye. Tissue Cell. 2009;41:286–298. doi: 10.1016/j.tice.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Chew EY. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495–511. [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Raymond PA. Embryonic origin of the eyes in teleost fish. Bioessays. 2002;24:519–529. doi: 10.1002/bies.10097. [DOI] [PubMed] [Google Scholar]
- Collin HB, Collin SP. The corneal surface of aquatic vertebrates: microstructures with optical and nutritional function? Philos Trans R Soc Lond B Biol Sci. 2000;355:1171–1176. doi: 10.1098/rstb.2000.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaughton VP, Dowling JE. Comparative morphology of distal neurons in larval and adult zebrafish retinas. Vision Res. 1998;38:13–18. doi: 10.1016/s0042-6989(97)00146-6. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Axonal stratification patterns and glutamate-gated conductance mechanisms in zebrafish retinal bipolar cells. J Physiol. 2000;524(Pt 1):135–146. doi: 10.1111/j.1469-7793.2000.t01-1-00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Spectral Responses in Zebrafish Horizontal Cells Include a Tetraphasic Response and a Novel UV-Dominated Triphasic Response. J Neurophysiol. 2010;104:2407–2422. doi: 10.1152/jn.00644.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaughton VP, Graham D, Nelson R. Identification and morphological classification of horizontal, bipolar, and amacrine cells within the zebrafish retina. J Comp Neurol. 2004;477:371–385. doi: 10.1002/cne.20261. [DOI] [PubMed] [Google Scholar]
- Dahm R, Schonthaler HB, Soehn AS, van Marle J, Vrensen GF. Development and adult morphology of the eye lens in the zebrafish. Exp Eye Res. 2007;85:74–89. doi: 10.1016/j.exer.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Davies SB, Di Girolamo N. Corneal stem cells and their origins: significance in developmental biology. Stem Cells Dev. 2010;19:1651–1662. doi: 10.1089/scd.2010.0201. [DOI] [PubMed] [Google Scholar]
- Doerre G, Malicki J. Genetic analysis of photoreceptor cell development in the zebrafish retina. Mech Dev. 2002;110:125–138. doi: 10.1016/s0925-4773(01)00571-8. [DOI] [PubMed] [Google Scholar]
- Domene S, Roessler E, El-Jaick KB, Snir M, Brown JL, Velez JI, Bale S, Lacbawan F, Muenke M, Feldman B. Mutations in the human SIX3 gene in holoprosencephaly are loss of function. Hum Mol Genet. 2008;17:3919–3928. doi: 10.1093/hmg/ddn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25:277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The retina: an approachable part of the brain. Cambridge: Belknap Press; 1987. [Google Scholar]
- Easter SS, Jr, Hitchcock PF. The myopic eye of the Black Moor goldfish. Vision Res. 1986;26:1831–1833. doi: 10.1016/0042-6989(86)90135-5. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Nicola GN. The development of vision in the zebrafish (Danio rerio) Dev Biol. 1996;180:646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117:e49–56. doi: 10.1182/blood-2010-10-314120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Dowling JE. Larval zebrafish turn off their photoreceptors at night. Commun Integr Biol. 2010;3:430–432. doi: 10.4161/cib.3.5.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Adolph AR, Dowling JE. Zebrafish larvae lose vision at night. Proc Natl Acad Sci U S A. 2010;107:6034–6039. doi: 10.1073/pnas.0914718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Adolph AR, Wong KY, Kraves S, Dowling JE. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proc Natl Acad Sci U S A. 2007;104:19126–19131. doi: 10.1073/pnas.0709337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England SJ, Blanchard GB, Mahadevan L, Adams RJ. A dynamic fate map of the forebrain shows how vertebrate eyes form and explains two causes of cyclopia. Development. 2006;133:4613–4617. doi: 10.1242/dev.02678. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258:277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics. Prog Retin Eye Res. 2008;27:89–110. doi: 10.1016/j.preteyeres.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch VC, Neuhauss SC. Parallel visual cycles in the zebrafish retina. Prog Retin Eye Res. 2010;29:476–486. doi: 10.1016/j.preteyeres.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Fleisch VC, Schonthaler HB, von Lintig J, Neuhauss SC. Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J Neurosci. 2008;28:8208–8216. doi: 10.1523/JNEUROSCI.2367-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footz TK, Johnson JL, Dubois S, Boivin N, Raymond V, Walter MA. Glaucoma-associated WDR36 variants encode functional defects in a yeast model system. Hum Mol Genet. 2009;18:1276–1287. doi: 10.1093/hmg/ddp027. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Boulanger LM. Synapse remodeling, compliments of the complement system. Cell. 2007;131:1034–1036. doi: 10.1016/j.cell.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Freund C, Horsford DJ, McInnes RR. Transcription factor genes and the developing eye: a genetic perspective. Hum Mol Genet. 1996;5:1471–1488. doi: 10.1093/hmg/5.supplement_1.1471. [DOI] [PubMed] [Google Scholar]
- Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Aqueous humor hydrodynamics. 10. St. Louis, MO: Mosby; 1997. [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gallenberger M, Meinel DM, Kroeber M, Wegner M, Milkereit P, Bosl MR, Tamm ER. Lack of WDR36 leads to preimplantation embryonic lethality in mice and delays the formation of small subunit ribosomal RNA in human cells in vitro. Hum Mol Genet. 2011;20:422–435. doi: 10.1093/hmg/ddq478. [DOI] [PubMed] [Google Scholar]
- Gariano RF. Cellular mechanisms in retinal vascular development. Prog Retin Eye Res. 2003;22:295–306. doi: 10.1016/s1350-9462(02)00062-9. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Geeraets R. An electron microscopic study of the closure of the optic fissure in the golden hamster. Am J Anat. 1976;145:411–431. doi: 10.1002/aja.1001450402. [DOI] [PubMed] [Google Scholar]
- Geng X, Oliver G. Pathogenesis of holoprosencephaly. J Clin Invest. 2009;119:1403–1413. doi: 10.1172/JCI38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Speirs C, Lagutin O, Inbal A, Liu W, Solnica-Krezel L, Jeong Y, Epstein DJ, Oliver G. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell. 2008;15:236–247. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesemann M, Lesslauer A, Maurer CM, Schonthaler HB, Neuhauss SC. Phylogenetic analysis of the vertebrate excitatory/neutral amino acid transporter (SLC1/EAAT) family reveals lineage specific subfamilies. BMC Evol Biol. 2010;10:117. doi: 10.1186/1471-2148-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestri G, Carl M, Appolloni I, Wilson SW, Barsacchi G, Andreazzoli M. Six3 functions in anterior neural plate specification by promoting cell proliferation and inhibiting Bmp4 expression. Development. 2005;132:2401–2413. doi: 10.1242/dev.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestri G, Osborne RJ, Wyatt AW, Gerrelli D, Gribble S, Stewart H, Fryer A, Bunyan DJ, Prescott K, Collin JR, Fitzgerald T, Robinson D, Carter NP, Wilson SW, Ragge NK. Reduced TFAP2A function causes variable optic fissure closure and retinal defects and sensitizes eye development to mutations in other morphogenetic regulators. Hum Genet. 2009 doi: 10.1007/s00439-009-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, John SW. Anterior segment dysgenesis and the developmental glaucomas are complex traits. Hum Mol Genet. 2002;11:1185–1193. doi: 10.1093/hmg/11.10.1185. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Gray MP, Smith RS, Soules KA, John SW, Link B. The aqueous humor outflow pathway of zebrafish. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiling TM, Clark JI. The transparent lens and cornea in the mouse and zebra fish eye. Semin Cell Dev Biol. 2008;19:94–99. doi: 10.1016/j.semcdb.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiling TM, Aose M, Clark JI. Cell fate and differentiation of the developing ocular lens. Invest Ophthalmol Vis Sci. 2010;51:1540–1546. doi: 10.1167/iovs.09-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Gross JM, Perkins BD. Zebrafish mutants as models for congenital ocular disorders in humans. Mol Reprod Dev. 2008;75:547–555. doi: 10.1002/mrd.20831. [DOI] [PubMed] [Google Scholar]
- Gross JM, Perkins BD, Amsterdam A, Egana A, Darland T, Matsui JI, Sciascia S, Hopkins N, Dowling JE. Identification of zebrafish insertional mutants with defects in visual system development and function. Genetics. 2005;170:245–261. doi: 10.1534/genetics.104.039727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, Kramer C, Nowak M, Herzog W, Wittbrodt J. Loss of maternal Smad5 in zebrafish embryos affects patterning and morphogenesis of optic primordia. Dev Dyn. 2003;227:128–133. doi: 10.1002/dvdy.10281. [DOI] [PubMed] [Google Scholar]
- Harding RL, Howley S, Baker LJ, Murphy TR, Archer WE, Wistow G, Hyde DR, Vihtelic TS. Lengsin expression and function during zebrafish lens formation. Exp Eye Res. 2008;86:807–818. doi: 10.1016/j.exer.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, Dale TC, Wilson SW, Stemple DL. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393. [DOI] [PubMed] [Google Scholar]
- Hero I. The optic fissure in the normal and microphthalmic mouse. Exp Eye Res. 1989;49:229–239. doi: 10.1016/0014-4835(89)90093-6. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Raymond PA. The teleost retina as a model for developmental and regeneration biology. Zebrafish. 2004;1:257–271. doi: 10.1089/zeb.2004.1.257. [DOI] [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–265. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol. 1999;207:309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Huh S, Hatini V, Marcus RC, Li SC, Lai E. Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression. Dev Biol. 1999;211:53–63. doi: 10.1006/dbio.1999.9303. [DOI] [PubMed] [Google Scholar]
- Imai F, Yoshizawa A, Fujimori-Tonou N, Kawakami K, Masai I. The ubiquitin proteasome system is required for cell proliferation of the lens epithelium and for differentiation of lens fiber cells in zebrafish. Development. 2010;137:3257–3268. doi: 10.1242/dev.053124. [DOI] [PubMed] [Google Scholar]
- Inbal A, Kim SH, Shin J, Solnica-Krezel L. Six3 represses nodal activity to establish early brain asymmetry in zebrafish. Neuron. 2007;55:407–415. doi: 10.1016/j.neuron.2007.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Luby-Phelps K, Link BA, Besharse JC. Analysis of IFT kinesins in developing zebrafish cone photoreceptor sensory cilia. Methods Cell Biol. 2009;93:219–234. doi: 10.1016/S0091-679X(08)93012-0. [DOI] [PubMed] [Google Scholar]
- Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–170. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Baye LM, Amsterdam A, Besharse JC, Link BA. Analysis of a zebrafish dync1h1 mutant reveals multiple functions for cytoplasmic dynein 1 during retinal photoreceptor development. Neural Dev. 2010;5:12. doi: 10.1186/1749-8104-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings AR, Kirilak Y, Carroll WM. In situ characterisation of oligodendrocyte progenitor cells in adult mammalian optic nerve. J Neurocytol. 2002;31:27–39. doi: 10.1023/a:1022567414294. [DOI] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010;81:349–358. doi: 10.1016/j.brainresbull.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Harris WA. Ptf1a is expressed transiently in all types of amacrine cells in the embryonic zebrafish retina. Neural Dev. 2009;4:34. doi: 10.1186/1749-8104-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PJ, Schmidt AD, Santella A, Khairy K, Bao Z, Wittbrodt J, Stelzer EH. Fast, high-contrast imaging of animal development with scanned light sheet-based structured-illumination microscopy. Nat Methods. 2010;7:637–642. doi: 10.1038/nmeth.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BN, Alvarez Y, Brockerhoff SE, Stearns GW, Sapetto-Rebow B, Taylor MR, Hurley JB. Identification of a zebrafish cone photoreceptor-specific promoter and genetic rescue of achromatopsia in the nof mutant. Invest Ophthalmol Vis Sci. 2007;48:522–529. doi: 10.1167/iovs.06-0975. [DOI] [PubMed] [Google Scholar]
- Kennedy BN, Stearns GW, Smyth VA, Ramamurthy V, van Eeden F, Ankoudinova I, Raible D, Hurley JB, Brockerhoff SE. Zebrafish rx3 and mab21l2 are required during eye morphogenesis. Dev Biol. 2004;270:336–349. doi: 10.1016/j.ydbio.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitambi SS, McCulloch KJ, Peterson RT, Malicki JJ. Small molecule screen for compounds that affect vascular development in the zebrafish retina. Mech Dev. 2009;126:464–477. doi: 10.1016/j.mod.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev Biol. 2001;232:315–326. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- Koke JR, Mosier AL, Garcia DM. Intermediate filaments of zebrafish retinal and optic nerve astrocytes and Muller glia: differential distribution of cytokeratin and GFAP. BMC Res Notes. 2010;3:50. doi: 10.1186/1756-0500-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachmer JH, Palay DA. Corneal disease. N Engl J Med. 1991;325:1804–1806. doi: 10.1056/NEJM199112193252510. [DOI] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock BL, Bilotta J, Perkins BD. Noncell-autonomous photoreceptor degeneration in a zebrafish model of choroideremia. Proc Natl Acad Sci U S A. 2007;104:4600–4605. doi: 10.1073/pnas.0605818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger RH, Wagner HJ. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol A. 1996;179:837–842. doi: 10.1007/BF00207362. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–3592. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med. 2003;5:1–39. doi: 10.1017/S1462399403006628. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Langenberg T, Kahana A, Wszalek JA, Halloran MC. The eye organizes neural crest cell migration. Dev Dyn. 2008;237:1645–1652. doi: 10.1002/dvdy.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Oliver G. Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development. 2008;135:441–450. doi: 10.1242/dev.010082. [DOI] [PubMed] [Google Scholar]
- Lee J, Gross JM. Laminin beta1 and gamma1 containing laminins are essential for basement membrane integrity in the zebrafish eye. Invest Ophthalmol VisSci. 2007;48:2483–2490. doi: 10.1167/iovs.06-1211. [DOI] [PubMed] [Google Scholar]
- Lee J, Willer JR, Willer GB, Smith K, Gregg RG, Gross JM. Zebrafish blowout provides genetic evidence for Patched1-mediated negative regulation of Hedgehog signaling within the proximal optic vesicle of the vertebrate eye. Dev Biol. 2008;319:10–22. doi: 10.1016/j.ydbio.2008.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dowling JE. Zebrafish visual sensitivity is regulated by a circadian clock. Vis Neurosci. 1998;15:851–857. doi: 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE. Effects of dopamine depletion on visual sensitivity of zebrafish. J Neurosci. 2000a;20:1893–1903. doi: 10.1523/JNEUROSCI.20-05-01893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dowling JE. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. J Neurosci. 2000b;20:1883–1892. doi: 10.1523/JNEUROSCI.20-05-01883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YN, Matsui JI, Dowling JE. Specificity of the horizontal cell-photoreceptor connections in the zebrafish (Danio rerio) retina. J Comp Neurol. 2009;516:442–453. doi: 10.1002/cne.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Joseph NM, Easter SS., Jr The morphogenesis of the zebrafish eye, including a fate map of the optic vesicle. Dev Dyn. 2000;218:175–188. doi: 10.1002/(SICI)1097-0177(200005)218:1<175::AID-DVDY15>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. [DOI] [PubMed] [Google Scholar]
- Link BA, Gray MP, Smith RS, John SW. Intraocular pressure in zebrafish: comparison of inbred strains and identification of a reduced melanin mutant with raised IOP. Invest Ophthalmol Vis Sci. 2004;45:4415–4422. doi: 10.1167/iovs.04-0557. [DOI] [PubMed] [Google Scholar]
- Loosli F, Staub W, Finger-Baier KC, Ober EA, Verkade H, Wittbrodt J, Baier H. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003;4:894–899. doi: 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F, Winkler S, Burgtorf C, Wurmbach E, Ansorge W, Henrich T, Grabher C, Arendt D, Carl M, Krone A, Grzebisz E, Wittbrodt J. Medaka eyeless is the key factor linking retinal determination and eye growth. Development. 2001;128:4035–4044. doi: 10.1242/dev.128.20.4035. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios J, Tessmar K, Loosli F, Wittbrodt J, Bovolenta P. Six3 and Six6 activity is modulated by members of the groucho family. Development. 2003;130:185–195. doi: 10.1242/dev.00185. [DOI] [PubMed] [Google Scholar]
- Luo W, Williams J, Smallwood PM, Touchman JW, Roman LM, Nathans J. Proximal and distal sequences control UV cone pigment gene expression in transgenic zebrafish. J Biol Chem. 2004;279:19286–19293. doi: 10.1074/jbc.M400161200. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Scholes J, Strahle U, Brennan C, Holder N, Brand M, Wilson SW. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development. 1997;124:2397–2408. doi: 10.1242/dev.124.12.2397. [DOI] [PubMed] [Google Scholar]
- Maggs A, Scholes J. Reticular astrocytes in the fish optic nerve: macroglia with epithelial characteristics form an axially repeated lacework pattern, to which nodes of Ranvier are apposed. J Neurosci. 1990;10:1600–1614. doi: 10.1523/JNEUROSCI.10-05-01600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangrum WI, Dowling JE, Cohen ED. A morphological classification of ganglion cells in the zebrafish retina. Vis Neurosci. 2002;19:767–779. doi: 10.1017/s0952523802196076. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Wittbrodt J. Shaping the vertebrate eye. Curr Opin Genet Dev. 2009;19:511–517. doi: 10.1016/j.gde.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Masai I, Stemple DL, Okamoto H, Wilson SW. Midline signals regulate retinal neurogenesis in zebrafish. Neuron. 2000;27:251–263. doi: 10.1016/s0896-6273(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Masse K, Bhamra S, Eason R, Dale N, Jones EA. Purine-mediated signalling triggers eye development. Nature. 2007;449:1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Pellerin I, Dupe V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:140–148. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Maurer CM, Schonthaler HB, Mueller KP, Neuhauss SC. Distinct retinal deficits in a zebrafish pyruvate dehydrogenase-deficient mutant. J Neurosci. 2010;30:11962–11972. doi: 10.1523/JNEUROSCI.2848-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86:E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- McMahon C, Gestri G, Wilson SW, Link BA. Lmx1b is essential for survival of periocular mesenchymal cells and influences Fgf-mediated retinal patterning in zebrafish. Dev Biol. 2009;332:287–298. doi: 10.1016/j.ydbio.2009.05.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunsky JM, Maher TA, Zhao G, Roberts AE, Stalker HJ, Zori RT, Burch MN, Clemens M, Mulliken JB, Smith R, Lin AE. TFAP2A mutations result in branchio-oculo-facial syndrome. Am J Hum Genet. 2008;82:1171–1177. doi: 10.1016/j.ajhg.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens CB, Donn TM, Wolf-Saxon ER, Ma TP. Reverse genetics in zebrafish by TILLING. Brief Funct Genomic Proteomic. 2008;7:454–459. doi: 10.1093/bfgp/eln046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosajee M, Gregory-Evans K, Ellis CD, Seabra MC, Gregory-Evans CY. Translational bypass of nonsense mutations in zebrafish rep1, pax2.1 and lamb1 highlights a viable therapeutic option for untreatable genetic eye disease. Hum Mol Genet. 2008;17:3987–4000. doi: 10.1093/hmg/ddn302. [DOI] [PubMed] [Google Scholar]
- Morris AC, Schroeter EH, Bilotta J, Wong RO, Fadool JM. Cone survival despite rod degeneration in XOPS-mCFP transgenic zebrafish. Invest Ophthalmol Vis Sci. 2005;46:4762–4771. doi: 10.1167/iovs.05-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Pollack IP. Glaucoma: science and practice. New York, NY: Thieme; 2003. [Google Scholar]
- Morriss-Kay G, Tuckett F. The role of microfilaments in cranial neurulation in rat embryos: effects of short-term exposure to cytochalasin D. J Embryol Exp Morphol. 1985;88:333–348. [PubMed] [Google Scholar]
- Mueller KP, Neuhauss SC. Quantitative measurements of the optokinetic response in adult fish. J Neurosci Methods. 2010;186:29–34. doi: 10.1016/j.jneumeth.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Nadauld LD, Chidester S, Shelton DN, Rai K, Broadbent T, Sandoval IT, Peterson PW, Manos EJ, Ireland CM, Yost HJ, Jones DA. Dual roles for adenomatous polyposis coli in regulating retinoic acid biosynthesis and Wnt during ocular development. Proc Natl Acad Sci U S A. 2006;103:13409–13414. doi: 10.1073/pnas.0601634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss SC, Biehlmaier O, Seeliger MW, Das T, Kohler K, Harris WA, Baier H. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J Neurosci. 1999;19:8603–8615. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss SCF. Structure and Function of the Zebrafish Visual System. In: Perry F, Ekker M, editors. Zebrafish. Elsevier; 2010. pp. 81–122. [Google Scholar]
- Ott M, Walz BC, Paulsen UJ, Mack AF, Wagner HJ. Retinotectal ganglion cells in the zebrafish, Danio rerio. J Comp Neurol. 2007;501:647–658. doi: 10.1002/cne.21269. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Shirai S, Nozaki M, Ikeda K, Ogura Y. Maldevelopment of neural crest cells in patients with typical uveal coloboma. J Pediatr Ophthalmol Strabismus. 1999;36:337–341. doi: 10.3928/0191-3913-19991101-09. [DOI] [PubMed] [Google Scholar]
- Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the “refracton” hypothesis. Cornea. 2001;20:853–858. doi: 10.1097/00003226-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Picker A, Cavodeassi F, Machate A, Bernauer S, Hans S, Abe G, Kawakami K, Wilson SW, Brand M. Dynamic coupling of pattern formation and morphogenesis in the developing vertebrate retina. PLoS Biol. 2009;7:e1000214. doi: 10.1371/journal.pbio.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober BR, Longoni M, Noonan KM. A review of Donnai-Barrow and facio-oculo-acoustico-renal (DB/FOAR) syndrome: clinical features and differential diagnosis. Birth Defects Res A Clin Mol Teratol. 2009;85:76–81. doi: 10.1002/bdra.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Hitchcock PF, Palopoli MF. Neuronal cell proliferation and ocular enlargement in Black Moor goldfish. J Comp Neurol. 1988;276:231–238. doi: 10.1002/cne.902760207. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold M, Loosli F, Adams RJ, Wittbrodt J. Individual cell migration serves as the driving force for optic vesicle evagination. Science. 2006;313:1130–1134. doi: 10.1126/science.1127144. [DOI] [PubMed] [Google Scholar]
- Reynaud EG, Krzic U, Greger K, Stelzer EH. Light sheet-based fluorescence microscopy: more dimensions, more photons, and less photodamage. Hfsp J. 2008;2:266–275. doi: 10.2976/1.2974980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Parpaillon L, Heligon C, Chesnel F, Boujard D, Philpott A. The IGF pathway regulates head formation by inhibiting Wnt signaling in Xenopus. Dev Biol. 2002;244:407–417. doi: 10.1006/dbio.2002.0605. [DOI] [PubMed] [Google Scholar]
- Ritch R, Shields MB, Krupin T. The Glaucomas, Basic Science. 2. St. Louis, MO: Mosby, Inc; 1996. [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Muenke M. The molecular genetics of holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:52–61. doi: 10.1002/ajmg.c.30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Munoz A, Dahm R, Nusslein-Volhard C. chokh/rx3 specifies the retinal pigment epithelium fate independently of eye morphogenesis. Dev Biol. 2005;288:348–362. doi: 10.1016/j.ydbio.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Samardzija M, Neuhauss SCF, Joly S, Kurz-Levin M, Grimm C. In: Animal Models for Retinal Degeneration In: Animal Models of Retinal Diseases. Pang IH, Clark AF, Pang I-H, Clark AF, editors. Humana Press; New York, USA: New York, USA: Humana Press; 2010. pp. 51–80.pp. 51–80. [Google Scholar]
- Sapieha P, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early eye morphogenesis in the zebrafish, Brachydanio rerio. J Comp Neurol. 1994;344:532–542. doi: 10.1002/cne.903440404. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999;404:515–536. [PubMed] [Google Scholar]
- Schonthaler HB, Lampert JM, von Lintig J, Schwarz H, Geisler R, Neuhauss SC. A mutation in the silver gene leads to defects in melanosome biogenesis and alterations in the visual system in the zebrafish mutant fading vision. Dev Biol. 2005;284:421–436. doi: 10.1016/j.ydbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Schonthaler HB, Franz-Odendaal TA, Hodel C, Gehring I, Geisler R, Schwarz H, Neuhauss SC, Dahm R. The zebrafish mutant bumper shows a hyperproliferation of lens epithelial cells and fibre cell degeneration leading to functional blindness. Mech Dev. 2010;127:203–219. doi: 10.1016/j.mod.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Schonthaler HB, Fleisch VC, Biehlmaier O, Makhankov Y, Rinner O, Bahadori R, Geisler R, Schwarz H, Neuhauss SC, Dahm R. The zebrafish mutant lbk/vam6 resembles human multisystemic disorders caused by aberrant trafficking of endosomal vesicles. Development. 2008;135:387–399. doi: 10.1242/dev.006098. [DOI] [PubMed] [Google Scholar]
- See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009;325:94–105. doi: 10.1016/j.ydbio.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- Semina EV, Bosenko DV, Zinkevich NC, Soules KA, Hyde DR, Vihtelic TS, Willer GB, Gregg RG, Link BA. Mutations in laminin alpha 1 result in complex, lens-independent ocular phenotypes in zebrafish. Dev Biol. 2006;299:63–77. doi: 10.1016/j.ydbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–4837. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- Shen W, Vijayan M, Sivak JG. Inducing form-deprivation myopia in fish. Invest Ophthalmol Vis Sci. 2005;46:1797–1803. doi: 10.1167/iovs.04-1318. [DOI] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. JNeurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa T, Fimbel SM, Mallory DE, Maaswinkel H, Spritzer SD, Sand JA, Li L, Hyde DR, Stenkamp DL. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Bosenko DV, Zinkevich NS, Foley S, Hyde DR, Semina EV, Vihtelic TS. Zebrafish pitx3 is necessary for normal lens and retinal development. Mech Dev. 2005;122:513–527. doi: 10.1016/j.mod.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Shi X, Luo Y, Howley S, Dzialo A, Foley S, Hyde DR, Vihtelic TS. Zebrafish foxe3: roles in ocular lens morphogenesis through interaction with pitx3. Mech Dev. 2006;123:761–782. doi: 10.1016/j.mod.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sivak JG. The role of the lens in refractive development of the eye: animal models of ametropia. Exp Eye Res. 2008;87:3–8. doi: 10.1016/j.exer.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Skarie JM, Link BA. The primary open-angle glaucoma gene WDR36 functions in ribosomal RNA processing and interacts with the p53 stress-response pathway. Hum Mol Genet. 2008;17:2474–2485. doi: 10.1093/hmg/ddn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarie JM, Link BA. FoxC1 is essential for vascular basement membrane integrity and hyaloid vessel morphogenesis. Invest Ophthalmol Vis Sci. 2009;50:5026–5034. doi: 10.1167/iovs.09-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Song PI, Matsui JI, Dowling JE. Morphological types and connectivity of horizontal cells found in the adult zebrafish (Danio rerio) retina. J Comp Neurol. 2008;506:328–338. doi: 10.1002/cne.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules KA, Link BA. Morphogenesis of the anterior segment in the zebrafish eye. BMC Dev Biol. 2005;5:12. doi: 10.1186/1471-213X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Satterfield R, Muhunthan K, Sherpa T, Vihtelic TS, Cameron DA. Age-related cone abnormalities in zebrafish with genetic lesions in sonic hedgehog. Invest Ophthalmol Vis Sci. 2008;49:4631–4640. doi: 10.1167/iovs.07-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigloher C, Ninkovic J, Laplante M, Geling A, Tannhauser B, Topp S, Kikuta H, Becker TS, Houart C, Bally-Cuif L. Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Development. 2006;133:2925–2935. doi: 10.1242/dev.02450. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Crawford MA, Robison WG, Jr, Kanungo J, Piatigorsky J. Adaptive differences in the structure and macromolecular compositions of the air and water corneas of the “four-eyed” fish (Anableps anableps) Faseb J. 2003;17:1996–2005. doi: 10.1096/fj.03-0122com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada N, Kucenas S, Appel B. Sox10 is necessary for oligodendrocyte survival following axon wrapping. Glia. 2010;58:996–1006. doi: 10.1002/glia.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Takechi M, Seno S, Kawamura S. Identification of cis-acting elements repressing blue opsin expression in zebrafish UV cones and pineal cells. J Biol Chem. 2008;283:31625–31632. doi: 10.1074/jbc.M806226200. [DOI] [PubMed] [Google Scholar]
- Tamimi Y, Skarie JM, Footz T, Berry FB, Link BA, Walter MA. FGF19 is a target for FOXC1 regulation in ciliary body-derived cells. Hum Mol Genet. 2006;15:3229–3240. doi: 10.1093/hmg/ddl400. [DOI] [PubMed] [Google Scholar]
- Tang J, Gokhale PA, Brooks SE, Blain D, Brooks BP. Increased corneal thickness in patients with ocular coloboma. J Aapos. 2006;10:175–177. doi: 10.1016/j.jaapos.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Tittle RK, Sze R, Ng A, Nuckels RJ, Swartz ME, Anderson RM, Bosch J, Stainier DY, Eberhart JK, Gross JM. Uhrf1 and Dnmt1 are required for development and maintenance of the zebrafish lens. Dev Biol. 2010;350:50–63. doi: 10.1016/j.ydbio.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Tschopp M, Takamiya M, Cerveny KL, Gestri G, Biehlmaier O, Wilson SW, Strahle U, Neuhauss SC. Funduscopy in adult zebrafish and its application to isolate mutant strains with ocular defects. PLoS One. 2010;5:e15427. doi: 10.1371/journal.pone.0015427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004a;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Genetics of photoreceptor development and function in zebrafish. Int J Dev Biol. 2004b;48:925–934. doi: 10.1387/ijdb.041890mt. [DOI] [PubMed] [Google Scholar]
- van Rooijen E, Voest EE, Logister I, Bussmann J, Korving J, van Eeden FJ, Giles RH, Schulte-Merker S. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis Model Mech. 2010;3:343–353. doi: 10.1242/dmm.004036. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–3509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Vavvas D, Azar NF, Azar DT. Mechanisms of disease: cataracts. Ophthalmol Clin North Am. 2002;15:49–60. doi: 10.1016/s0896-1549(01)00015-3. [DOI] [PubMed] [Google Scholar]
- Veldman MB, Bemben MA, Goldman D. Tuba1a gene expression is regulated by KLF6/7 and is necessary for CNS development and regeneration in zebrafish. Mol Cell Neurosci. 2010;43:370–383. doi: 10.1016/j.mcn.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312:596–612. doi: 10.1016/j.ydbio.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Verbruggen V, Ek O, Georlette D, Delporte F, Von Berg V, Detry N, Biemar F, Coutinho P, Martial JA, Voz ML, Manfroid I, Peers B. The Pax6b homeodomain is dispensable for pancreatic endocrine cell differentiation in zebrafish. J Biol Chem. 2010;285:13863–13873. doi: 10.1074/jbc.M110.108019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veth KN, Willer JR, Collery RF, Gray MP, Willer GB, Wagner DS, Mullins MC, Udvadia AJ, Smith RS, John SW, Gregg RG, Link BA. Mutations in zebrafish lrp2 result in adult onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7:e1001310. doi: 10.1371/journal.pgen.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic TS. Teleost lens development and degeneration. Int Rev Cell Mol Biol. 2008;269:341–373. doi: 10.1016/S1937-6448(08)01006-X. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Wagner E. Amacrine cells in the retina of a teleost fish, the roach (Rutilus rutilus): a Golgi study on differentiation and layering. Philos Trans R Soc Lond B Biol Sci. 1988;321:263–324. doi: 10.1098/rstb.1988.0094. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Walls GL. The vertebrate eye and its adaptive radiation. New York, New York: Hafner Publishing Company; 1942. [Google Scholar]
- Wang JS, Kefalov VJ. The Cone-specific Visual Cycle. Progress in Retinal and Eye Research. 2011 doi: 10.1016/j.preteyeres.2010.11.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PG, Young RD. Scleral structure, organisation and disease. A review. Exp Eye Res. 2004;78:609–623. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of theforebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Wittenberg JB, Wittenberg BA. The choroid rete mirabile of the fish eye. I. Oxygen secretion and structure: comparison with the swimbladder rete mirabile. Biol Bull. 1974;146:116–136. doi: 10.2307/1540402. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dowling JE. Retinal bipolar cell input mechanisms in giant danio. III. ON-OFF bipolar cells and their color-opponent mechanisms. J Neurophysiol. 2005;94:265–272. doi: 10.1152/jn.00271.2004. [DOI] [PubMed] [Google Scholar]
- Wong KY, Cohen ED, Dowling JE. Retinal bipolar cell input mechanisms in giant danio. II. Patch-clamp analysis of on bipolar cells. J Neurophysiol. 2005a;93:94–107. doi: 10.1152/jn.00270.2004. [DOI] [PubMed] [Google Scholar]
- Wong KY, Adolph AR, Dowling JE. Retinal bipolar cell input mechanisms in giant danio. I. Electroretinographic analysis. J Neurophysiol. 2005b;93:84–93. doi: 10.1152/jn.00259.2004. [DOI] [PubMed] [Google Scholar]
- Xiao T, Baier H. Lamina-specific axonal projections in the zebrafish tectum require the type IV collagen Dragnet. Nat Neurosci. 2007;10:1529–1537. doi: 10.1038/nn2002. [DOI] [PubMed] [Google Scholar]
- Xie J, Farage E, Sugimoto M, Anand-Apte B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev Biol. 2010;10:76. doi: 10.1186/1471-213X-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM. Neurochemical anatomy of the zebrafish retina as determined by immunocytochemistry. J Neurocytol. 2001;30:551–592. doi: 10.1023/a:1016512617484. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, Norcom E, Nakamura H, Yee RW, Zhao XC. Transgenic analysis of the anterior eye-specific enhancers of the zebrafish gelsolin-like 1 (gsnl1) gene. Dev Dyn. 2007;236:1929–1938. doi: 10.1002/dvdy.21197. [DOI] [PubMed] [Google Scholar]
- Young TL. Molecular genetics of human myopia: an update. Optom Vis Sci. 2009;86:E8–E22. doi: 10.1097/OPX.0b013e3181940655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XC, Yee RW, Norcom E, Burgess H, Avanesov AS, Barrish JP, Malicki J. The zebrafish cornea: structure and development. Invest Ophthalmol Vis Sci. 2006;47:4341–4348. doi: 10.1167/iovs.05-1611. [DOI] [PubMed] [Google Scholar]
- Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002;129:2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- Zucker CL, Dowling JE. Centrifugal fibres synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987;330:166–168. doi: 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]