Abstract
The outcome of neonatal herpes simplex (HSV) infection, even after therapy with high dose acyclovir (ACV), is not optimum. We therefore evaluated N-methanocarbathymidine ((N)-MCT) using the guinea pig model of neonatal herpes. Treatment with ACV (60 mg/kg/day) was compared to doses of 1, 5, and 25 mg/kg/day of (N)-MCT initiated 1, 2 or 3 days post inoculation (dpi). Both ACV and (N)-MCT significantly improved survival, but only (N)-MCT significantly reduced the number of animals with symptoms when begun at 1 dpi. When therapy was begun at 2 dpi, only (N)-MCT (1, 5, or 25 mg/kg/day) significantly increased survival. In fact, (N)-MCT improved survival up to 3 dpi, the last time point evaluated. (N)-MCT was highly effective and superior to high dose ACV therapy for the treatment of neonatal herpes in the guinea pig model.
Keywords: Herpes simplex virus; neonatal herpes; (N)-MCT, N-methanocarbathymidine
Neonatal herpes simplex virus (HSV) infections are rare, about 1,500 cases/year, but have devastating consequences for the newborn (Corey and Wald, 2009; Kimberlin, 2007). Following infection, the disease may be limited to the skin, eye, and/or mouth (SEM disease). However, both treated and especially untreated infections may progress to involve multiple organs including the liver, lung, adrenal glands (disseminated disease) and the brain (CNS disease) (Whitley et al., 1988). Current therapy with high dose (60 mg/kg/day) acyclovir (ACV) is more effective than vidarabine (Whitley et al., 1980) and lower dose (30 mg/kg/day) ACV (Skoldenberg et al., 1984; Whitley et al., 1991a). However, the reduction in morbidity and mortality for CNS and disseminated disease is still not optimal (Kimberlin et al., 2001a). High dose ACV is also commonly associated with neutropenia that can require treatment to be halted (Kimberlin et al., 2001a). Therefore,it is clear that there is a need for improved therapy.
N-methanocarbathymidine ((N)-MCT) is a thymidine analog that incorporates a pseudosugar with a fixed Northern conformation. It has previously been shown to have potent antiviral activity against HSV-1 and HSV-2 (Marquez et al., 2006; Prichard et al., 2006; Quenelle et al., 2010; Zalah et al., 2002). The compound is selectively phosphorylated by the HSV thymidine kinase (TK) (Schelling et al., 2004) but phosphorylation is different from ACV. The HSV-1 TK catalyzes the conversion of (N)-MCT monophosphate to (N)-MCT diphosphate, whereas for ACV it creates the monophosphate (Zalah et al., 2002). (N)-MCT has a thousand fold-greater affinity for the HSV TK than the human homolog and thus is highly selective for virus-infected cells (Prichard et al., 2006; Prota et al., 2000). We chose to evaluate (N)-MCT in the guinea pig model of neonatal herpes because it closely mimics human disease (Bravo et al., 1996; Bravo et al., 1994). After infection, animals develop SEM disease with or without CNS and systemic involvement with a high but not universal mortality.
For these experiments, (N)-MCT was kindly provided by N & N Scientific Co. (Rockville, MD) and dissolved in saline(Prichard et al., 2006). ACV was obtained from Sigma Co. (St. Louis, MO). Female Hartley guinea pigs (250–350 g) were obtained from Charles River Breeding Laboratories (Wilmington, MA) and housed under AAALAC approved conditions. We used HSV-2 strain MS (ATCC-VR540) as previously described (Bourne et al., 2000). Newborn guinea pigs were inoculated intranasally within 48 hours after birth with 8.7 × 105 pfu of HSV-2 (Bravo et al., 1996; Bravo et al., 1994). Animals were randomly assigned within litters to receive doses of 1, 5, or 25 mg/kg/day of (N)-MCT, placebo (saline) or the recommended dose of ACV for neonates, 60 mg/kg/day (Bravo et al., 1996; Bravo et al., 1994). Treatments were administered intraperitoneally (IP), twice daily for 10 days beginning 1–3 days post inoculation (dpi).
Animals were followed daily for clinical evidence of herpetic disease which consist of vesicular lesions on the nose, mouth or eyelids (skin, eye and mouth, or SEM disease). Animals were also evaluated for the presence of respiratory symptoms (nasal flaring, subcostal or intercostal retractions, decreased respiratory rate) and general signs of disease (weight loss, ruffled fur, lethargy and dehydration (Bravo et al., 1996; Bravo et al., 1994).
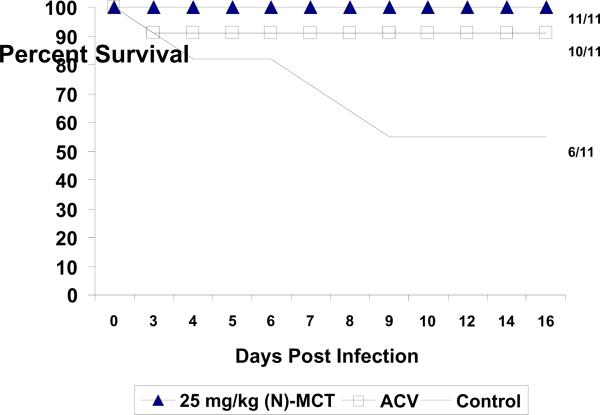
Based on the results obtained in previous mouse evaluations (data not shown), we selected a dose of 25 mg/kg/day of (N)-MCT begun at 1 dpi for the initial evaluation. As seen in Figure 1, (N)-MCT treatment significantly increased survival of newborn guinea pigs compared to the control group (11/11 vs. 6/11, p=0.035) and was equivalent to ACV (10/11,). However, only 2/11 newborns treated with (N)-MCT exhibited any sign of infection compared to 9/11 newborns with symptoms in the ACV group (p=0.009) (Table 1). We next evaluated a delay in the onset of drug therapy to 2 dpi. As seen in Table 1 (experiment 2), 25 mg/kg/day (N)-MCT remained protective, while ACV was not protective, similar to previous results in this model, (Bravo et al., 1996; Bravo et al., 1994). This experiment was followed by evaluations of lower doses of (N)-MCT and extended delays in initiation of therapy. For simplicity and because the mortality in the placebo group was consistent, we combined the results for the last two experiments which evaluated doses of 25 mg/kg of (N)-MCT initiated at 3 dpi, 5 mg/kg/day of (N)-MCT initiated at 2 and 3 dpi, and 1 mg/kg/day of (N)-MCT initiated at 2 dpi. A control group with ACV was not included since ACV therapy begun at 2 dpi was not protective in this model (above and Bravo et al., 1996; Bravo et al., 1994). As seen in Table 2, the 25 mg/kg/day of (N)-MCT dose remained effective when therapy was begun at 3 dpi, with 100% survival (6/6, p=0.009 vs. control). Similarly, the 5 mg/kg/day dose of (N)-MCT initiated at 2 dpi was also 100% protective (11/11 survival, p=0.001 vs. control), and also protected most newborns from death (8/10, p<0.05 vs. control) when treatment was begun at 3 dpi. Lastly, even a dose of 1 mg/kg/day of (N)-MCT provided significant protection (8/9 survival, p=0.009 vs. control) when begun at 2 dpi. Significant decreases in the number of newborns exhibiting symptoms was, however, only seen in the group receiving 5 mg/kg/day of (N)-MCT when treatment was begun at 2 dpi (Table 2). For example, SEM disease and respiratory symptoms were seen in all placebo recipients but in only 4/11 (36.4%, p=0.004 vs. placebo) and 2/11 (18.2%, p=0.0002 vs. placebo) (respectively for SEM disease and respiratory symptoms) of the animals treated with 5 mg/kg/day of (N)-MCT beginning at 2 dpi.
Figure 1.
Effect of N-methanocarbathymidine ((N)-MCT) treatment on neonatal HSV-2 infection in the guinea pig. Animals were treated with 25 mg/kg/day of (N)-MCT or 60 mg/kg/day of ACV beginning one day after virus inoculation and followed for 14 days. The numbers on the right show the number of surviving animals for each group.
Table 1.
Effect of 25 mg/kg/day of (N)-MCT when administered beginning 1–2 days post HSV-2 inoculation of newborn guinea pigs
| Experiment One | |||
|---|---|---|---|
| Group | N | Survival (% Survival) | # Without Symptomsa (% Without Symptoms) |
| Control | 11 | 6/11 (55%) | 1/11 (9.1%) |
| ACV 60 mg/kg/day @ 1 dpi × 10 Days | 11 | 10/11 (91%) | 2/11 (18.2%) |
| (N)-MCT 25 mg/kg @1 dpi × 10 days | 11 | 11/11 (100%) b | 9/11 (81.8%)c |
| Experiment Two | |||
|---|---|---|---|
| Group | N | Survival (% Survival) | # Without Symptoms a (% Without Symptoms) |
| Control | 11 | 3/11 (27.3%) | 0/11 (0%) |
| ACV 60 mg/kg @ 2 dpi × 10 Days | 10 | 3/10 (30%) | 0/10 (0%) |
| (N)-MCT 25 mg/kg @ 2 dpi × 10 Days | 11 | 10/11 (90.9%) c | 4/11 (36.4%) |
dpi: Day post inoculation
As described in methods this includes: Vesicular Lesions, respiratory, and other general symptoms
p<0.05 vs. Control
p<0.01 vs. Control and ACV
Table 2.
Effect of 1–25 mg/kg/day of N-MCT when administered beginning 2–3 days post HSV-2 inoculation of newborn guinea pigs
| # With Symptomsa (% With symptoms) |
||||||
|---|---|---|---|---|---|---|
| Group | N | Survival (% survival) | # Without Symptoms d (% Without symptoms) | SEM | Respiratory | General Symptoms |
| Control | 11 | 3/11 (27.3%) | 0/11 (0%) | 11/11 (100%) | 11/11 (100%) | 6/11 (54.5%) |
| N-MCT 1 mg/kg @ 2 dpi × 10 days | 9 | 8/9 (88.9%)b | 1/9 (11.1%) | 7/9 (77.8%) | 5/9 (55.6%) | 1/9 (11.1%) |
| N-MCT 5 mg/kg @ 2 dpi × 10 days | 11 | 11/11 (80.0% c | 5/11 (45.5%)d | 4/11 (36.4%) b | 2/11 (18.2%)c | 0/11 (0%)b |
| N-MCT 5 mg/kg @ 3 dpi × 10 days | 10 | 8/10 (80.0%)d | 0/10 (0%) | 8/10 (80%) | 9/10 (90%) | 3/10 (30%) |
| N-MCT 25 mg/kg @ 3 dpi × 10 days | 6 | 6/6 (100%)b | 2/6 (33.3%) | 4/6 (66.7%) | 3/6 (50%) | 0/6 (0%) |
dpi: Day post inoculation
As described in methods this includes: Vesicular Lesions, respiratory, eye disease and general symptoms
p≤0.01 vs. Control
p<0.001 vs. Control
p<0.05 vs. Control
Although high dose ACV therapy significantly improves the outcome of neonatal HSV infections, the morbidity and mortality remain unacceptably high (Corey and Wald, 2009; Kimberlin, 2007; Kimberlin et al., 2001a; Kimberlin et al., 2001b). In the the investigations presented here, we investigated a new drug with potent anti-HSV activity, (N)-MCT using our guniea pig model of neonatal herpes. (Bravo et al., 1996; Bravo et al., 1994). The guinea pig model of neonatal HSV mimics human disease with the development of local vesicular disease (SEM disease) which progresses to systemic and CNS infection with high but not universal mortality if untreated (Bravo et al., 1994). Because the outcome of neonatal HSV infection has been correlated to the time of inititiation of therapy in relation to symptoms onset (Whitley et al., 1991b), we compared (N)-MCT to ACV at various times post HSV infection.
In these experiments, we found that (N)-MCT at doses of 5 or 25 mg/kg/day significantly decreased mortality when begun as late as 3 dpi while ACV at 60 mg/kg/day was only effective when initiated within 1 day of virus inoculation. Further, the lowest dose that we evaluated, 1 mg/kg/day of (N)-MCT, provided significant protection when begun at 2 days post inoculation. When (N)-MCT was evaluated in a lethal HSV-2 mouse model, doses as low as 0.01 mg/kg/day of (N)-MCT provided protection when initiated within 1 day of intranasal inoculation) while a dose of 25 mg/kg/day significantly reduced virus replication in the brain (Quenelle et al., 2010). Similarly, (N)-MCT has been shown to reduce both brain and lung virus titers in mice infected with vaccinia virus, albeit at higher doses, 100–500 mg/kg, than evaluated for HSV (Smee et al., 2007). Safety studies of (N)-MCT conducted in mice have revealed no toxicity at doses up to 1,000 mg/kg/day (Smee et al., 2007).
The increased efficacy of (N)-MCT as compared to ACV may be due differences in potency, bioavailability, and/or the distribution of these two compounds especially CNS penetration. When evaluated for in vitro activity, the EC50 of (N)-MCT against HSV was 0.07 μg/ml compared to 0.3 μg/ml for ACV (Prichard et al., 2006). The pharmacokinetic properties of ACV in rodent models are well characterized (Biron et al., 1982; Sutton and Boyd, 1993) however, only one study has been conducted with (N)-MCT (Noy et al., 2002). Both ACV and (N)-MCT appear to have good selective indices (SI) and little cytotoxicity at the doses we evaluated. Thus, the SI for (N)-MCT is >182 and the cytotoxicity >100 μg/ml (Pritchard et al 2006). ACV penetration into the brain is impaired in guinea pigs (Myerson and Hsiung, 1983) and humans (package insert; Lycke et al., 2003), perhaps limiting the efficacy of treatment for CNS diseases such as neonatal HSV. The data on (N)-MCT concentrations in the brain is limited. In one report (N)-MCT levels in the brain were low compared to serum or other organs (Noy et al., 2002). Further pharmacokinetic studies of (N)-MCT are warranted for the development of (N)-MCT as a potential therapy for HSV infections as well as other herpesviruses such as Epstein-Barr virus (EBV) and Kaposi sarcoma-associated herpesvirus (KSHV) (Prichard et al., 2006; Zhu et al., 2005).
In summary, treatment of newborn guinea pigs with (N)-MCT was highly efficacious in protecting the guinea pigs from disease and symptoms of neonatal herpes infection, even when the onset of treatment is delayed. In this regards, (N)-MCT was more effective than ACV. Thus, (N)-MCT is a promising new drug for neonatal HSV infections in the newborn infant.
Highlights
-
>
We compared (N)-MCT to ACV for the treatment of guinea pig neonatal HSV infections.
-
>
(N)-MCT was effective at lower doses than ACV.
-
>
Both ACV and (N)-MCT improved survival when begun on day one after infection
-
>
Only (N)-MCT reduced the number of animals with symptoms when begun on day one.
-
>
Only (N)-MCT was effective when begun on day 2 or 3 after infection
Acknowledgments
Funding This work was supported by the NIH/NIAID antiviral testing program (National Institute of Health Contract No.: AI 15438) to Cincinnati Children's Hospital Medical
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Robert Glazer and Aquilur Rahman are employed by N & N Scientific Inc. All others have no conflict.
References
- Biron KK, Noblin JE, de Miranda P, Elion GB. Uptake, distribution, and anabolism of acyclovir in herpes simplex virus-infected mice. Antimicrob Agents Chemother. 1982;21:44–50. doi: 10.1128/aac.21.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo FJ, Bourne N, Harrison CJ, Mani C, Stanberry LR, Myers MG, Bernstein DI. Effect of antibody alone and combined with acyclovir on neonatal herpes simplex virus infection in guinea pigs. J Infect Dis. 1996;173:1–6. doi: 10.1093/infdis/173.1.1. [DOI] [PubMed] [Google Scholar]
- Bravo FJ, Myers MG, Stanberry LR. Neonatal herpes simplex virus infection: pathogenesis and treatment in the guinea pig. J Infect Dis. 1994;169:947–955. doi: 10.1093/infdis/169.5.947. [DOI] [PubMed] [Google Scholar]
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376–1385. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin DW. Herpes simplex virus infections of the newborn. Semin Perinatol. 2007;31:19–25. doi: 10.1053/j.semperi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar Arvin, A.M., Gutierrez K, Shelton M, Weiner LB, Sleasman JW, de Sierra TM, Weller S, Soong SJ, Kiell J, Lakeman FD, Whitley RJ. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001a;108:230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Frenkel LM, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar M, Arvin AM, Gutierrez K, Shelton M, Weiner LB, Sleasman JW, de Sierra TM, Soong SJ, Kiell J, Lakeman FD, Whitley RJ. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001b;108:223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- Lycke J, Malmestrom C, Stahle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother. 2003;47:2438–2441. doi: 10.1128/AAC.47.8.2438-2441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez VE, Hughes SH, Sei S, Agbaria R. The history of N-methanocarbathymidine: the investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent. Antiviral Res. 2006;71:268–275. doi: 10.1016/j.antiviral.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Myerson D, Hsiung GD. Prophylactic and therapeutic treatment with acyclovir of genital herpes in the guinea pig. Proc Soc Exp Biol Med. 1983;174:147–152. doi: 10.3181/00379727-174-41717. [DOI] [PubMed] [Google Scholar]
- Noy R, Ben-Zvi Z, Elezra M, Candotti F, Ford H, Jr, Morris JC, Marquez VE, Johns DG, Agbaria R. Pharmacokinetics and organ distribution of N-methanocarbathymidine, a novel thymidine analog, in mice bearing tumors transduced with the herpes simplex thymidine kinase gene. Cancer Chemother Pharmacol. 2002;50:360–366. doi: 10.1007/s00280-002-0505-8. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Keith KA, Quenelle DC, Kern ER. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob Agents Chemother. 2006;50:1336–1341. doi: 10.1128/AAC.50.4.1336-1341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prota A, Vogt J, Pilger B, Perozzo R, Wurth C, Marquez VE, Russ P, Schulz GE, Folkers G, Scapozza L. Kinetics and crystal structure of the wild-type and the engineered Y101F mutant of Herpes simplex virus type 1 thymidine kinase interacting with (North)-methanocarba-thymidine. Biochemistry. 2000;39:9597–9603. doi: 10.1021/bi000668q. [DOI] [PubMed] [Google Scholar]
- Quenelle D, Glazer R, Rahman A, Collins D, Rice T. In vivo efficacy of twice daily oral treatment with N-MCT against Herpes Simplex virus type 2 in Balb/c mice, 23rd International Conference on Antiviral Research (ICAR); San Francisco, CA. 2010. Antiviral Research 2010. [Google Scholar]
- Schelling P, Claus MT, Johner R, Marquez VE, Schulz GE, Scapozza L. Biochemical and structural characterization of (South)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells. J Biol Chem. 2004;279:32832–32838. doi: 10.1074/jbc.M313343200. [DOI] [PubMed] [Google Scholar]
- Skoldenberg B, Forsgren M, Alestig K, Bergstrom T, Burman L, Dahlqvist E, Forkman A, Fryden A, Lovgren K, Norlin K, et al. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet. 1984;2:707–711. doi: 10.1016/s0140-6736(84)92623-0. [DOI] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Glazer RI, Rahman A, Sidwell RW. Efficacy of N-methanocarbathymidine in treating mice infected intranasally with the IHD and WR strains of vaccinia virus. Antiviral Res. 2007;76:124–129. doi: 10.1016/j.antiviral.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D, Boyd MR. Comparative activity of penciclovir and acyclovir in mice infected intraperitoneally with herpes simplex virus type 1 SC16. Antimicrob Agents Chemother. 1993;37:642–645. doi: 10.1128/aac.37.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R, Arvin A, Prober C, Burchett S, Corey L, Powell Plotkin, S., Starr S, Alford C, Connor J, et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991a;324:444–449. doi: 10.1056/NEJM199102143240703. [DOI] [PubMed] [Google Scholar]
- Whitley R, Arvin A, Prober C, Corey L, Burchett S, Plotkin S, Starr S, Jacobs R, Powell D, Nahmias A, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991b;324:450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Corey L, Arvin A, Lakeman FD, Sumaya CV, Wright PF, Dunkle LM, Steele RW, Soong SJ, Nahmias AJ, et al. Changing presentation of herpes simplex virus infection in neonates. J Infect Dis. 1988;158:109–116. doi: 10.1093/infdis/158.1.109. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics. 1980;66:495–501. [PubMed] [Google Scholar]
- Zalah L, Huleihel M, Manor E, Konson A, Ford H, Jr., Marquez VE, Johns DG, Agbaria R. Metabolic pathways of N-methanocarbathymidine, a novel antiviral agent, in native and herpes simplex virus type 1 infected Vero cells. Antiviral Res. 2002;55:63–75. doi: 10.1016/s0166-3542(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Zhu W, Burnette A, Dorjsuren D, Roberts PE, Huleihel M, Shoemaker RH, Marquez VE, Agbaria R, Sei S. Potent antiviral activity of north-methanocarbathymidine against Kaposi's sarcoma-associated herpesvirus. Antimicrob Agents Chemother. 2005;49:4965–4973. doi: 10.1128/AAC.49.12.4965-4973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]