Abstract
Among people exposed to major psychological stressors in early life, there are elevated rates of morbidity and mortality from chronic diseases of aging. The most compelling data come from studies of children raised in poverty or maltreated by their parents, who show heightened vulnerability to vascular disease, autoimmune disorders, and premature mortality. These findings raise challenging theoretical questions. How does childhood stress get under the skin, at the molecular level, to affect risk for later diseases? And how does it incubate there, giving rise to diseases several decades later? Here we present a Biological Embedding Model, which attempts to address these questions by synthesizing knowledge across several behavioral and biomedical literatures. This model maintains that childhood stress gets “programmed” into macrophages through epigenetic markings, post-translational modifications, and tissue remodeling. As a consequence these cells are endowed with pro-inflammatory tendencies, manifest in exaggerated cytokine responses to challenge and decreased sensitivity to inhibitory hormonal signals. The model goes on to propose that over the lifecourse, these pro-inflammatory tendencies are exacerbated by behavioral proclivities and hormonal dysregulation, themselves the products of exposure to early stress. Behaviorally, the model posits that childhood stress gives rise to excessive threat vigilance, mistrust of others, poor social relationships, impaired self-regulation, and unhealthy lifestyle choices. Hormonally, early stress confers altered patterns of endocrine and autonomic discharge. This milieu amplifies the pro-inflammatory environment already instantiated by macrophages. Acting in concert with other exposures and genetic liabilities, the resulting inflammation drives forward pathogenic mechanisms that ultimately foster chronic disease.
Keywords: Stress, inflammation, cortisol, HPA axis, childhood, heart disease, epigenetics
Across the behavioral and biomedical sciences, there is mounting interest in the hypothesis that psychosocial stress in the early years of life has a lingering influence on physical health (Gluckman and Hanson, 2006; Matthews and Gallo, 2011; Matthews, 2005; Repetti et al., 2002; Shonkoff et al., 2009). This interest has been fueled by the results of several recent studies showing that, among people exposed to major psychological stressors in childhood, there are elevated rates of morbidity and mortality from chronic diseases of aging. For instance, one study compared the medical outcomes of 17,000+ adults who did versus did not experience stressors like familial violence, abuse, and neglect as children. It found a 1.5–2.0 fold greater incidence of cardiovascular disease, autoimmune disorders, and premature mortality among those exposed to early adversity (Anda et al., 2009; Dong et al., 2004; Dube et al., 2009). Another study tracked the onset of coronary heart disease (CHD) over 40 years in medical students enrolled at Johns Hopkins University (Kittleson et al., 2006). Even among these educated, affluent physicians, childhood adversity was associated with worse health in adulthood. The rates of CHD by age 50 were 2.4 times higher in physicians who had been raised in households that were low versus high in socioeconomic status (SES). And recently, a study of cancer in Israelis who had emigrated from Europe appeared (Keinan-Boker et al., 2009). It found that cancer rates were elevated in immigrants who arrived after World War 2, many of whom were Jews persecuted during the Holocaust. The largest effects were seen in people born 1940–1945, who would have been exposed to horrific conditions before age 5. Their cancer risk was elevated 3.5-fold, relative to same-aged immigrants who arrived pre-war.
Findings like these raise challenging theoretical questions. How does psychosocial stress in childhood get under the skin, at the molecular level, to affect risk for later diseases? And how is it able to do so in a fashion that persists across multiple decades? Researchers working at the interface of the behavioral and biomedical sciences have developed models for explaining the mechanistic basis of mind-body effects (Glaser and Kiecolt-Glaser, 2005; Kop and Cohen, 2001; Miller et al., 2009b; Pressman and Cohen, 2005). However, the focus of these models is generally on the immediate biological sequelae of stress, and it is thus unclear how they would explain pathology that develops over such a lengthy incubation period (Baum et al., 1993; Miller et al., 2009b; Mohr and Pelletier, 2006). By contrast, researchers working in developmental psychobiology have made significant progress in delineating how early events shape life-long response tendencies of the hypothalamic-pituitary-adrenocortical (HPA) axis (Gunnar and Quevedo, 2007; Heim et al., 2008; Levine, 2005; Zhang et al., 2006). However, the focus of this work has been on how disrupted HPA axis functioning could give rise to psychopathology. There has been an implicit assumption that the HPA axis could also be a gateway to medical problems (Cameron et al., 2005), but little effort has been made to detail the pathophysiologic mechanisms by which such effects might occur.
The current article has two primary objectives. The first is to provide an overview of the literature on psychosocial stress in childhood and vulnerability to chronic disease in adulthood. Drawing on studies of humans exposed to socioeconomic disadvantage and/or parental maltreatment, and relevant animal models of early stress, we ask whether there is evidence for a causal influence of childhood adversity on later disease. The paper’s second objective is to present a model that attempts to explain how such effects could occur mechanistically. To do that, we introduce a Biological Embedding of Childhood Adversity Model, and go on to review evidence for its major propositions. The model represents a synthesis of thinking from various theoretical perspectives, including the fetal-origins literature (Barker, 1992), lifecourse epidemiology (Lynch and Smith, 2005), socioemotional development (Pollak, 2008; Repetti et al., 2002), stress physiology (McEwen, 1998), and behavioral immunology (Coe and Lubach, 2007; Raison and Miller, 2003). Briefly, it posits that childhood stress establishes a pro-inflammatory phenotype in cells of the immune system called monocytes/macrophages. 1 As a result, these cells are permanently endowed with pro-inflammatory tendencies, as manifest in exaggerated responses to challenge and decreased sensitivity to inhibitory signals. Over the lifecourse, these pro-inflammatory tendencies are exacerbated by behavioral proclivities and hormonal dysregulation, themselves the products of exposure to childhood stress. Behaviorally, the model posits that early stress fosters vigilance for threat and mistrust of others, traits which make it difficult to form deep social ties. Early stress also impairs self-regulation, creating a proclivity for unhealthy behaviors, and alters patterns of endocrine and autonomic discharge. As a result of the latter, release patterns of various hormones, transmitters, and peptides is dysregulated, consigning monocytes/macrophages to operate in a milieu that accentuates their pro-inflammatory tendencies. The ensuing chronic inflammation drives forward pathogenic mechanisms that ultimately contribute to chronic disease.
Background: Childhood Stress and Later Disease
The history of science is filled with debates about the best way to define stress. Here we adopt an integrative definition advanced by Cohen, Kessler, and Underwood (1995), which treats stress as a process that entails a stimulus, appraisals of it, and a response. This view draws on a classic model holding that when stimuli, commonly referred to as stressors, are appraised as threatening and unmanageable, they elicit a psychological state that is experienced as stress, as well as a cascade of behavioral and biological adjustments, commonly referred to as responses (Lazarus and Folkman, 1984). Thus, in the rest of the paper we use “stress” as an umbrella term, meant to capture times when a person has been exposed to a stimulus and judged it to be a threat s/he cannot manage. Given the paper’s focus on early-life contributions to later health, we deal with stress that is experienced during childhood. We focus on stress that is both severe and chronic in nature. By severe, we mean difficulties that fall outside the range of what children normatively experience in developed countries (e.g., maltreatment). We define “chronic” stress as an experience where the stimulus remains present in a child’s life over a lengthy period of time (e.g., recurring conflict between parents, or a lack of material resources due to poverty). Stress can also be chronic when the threat posed by a stimulus looms for an extended period of time, even if the stimulus itself does not (e.g., the sense of danger that follows being physically abused; see Baum et al., 1993). We do not discuss stressors of acute duration, as they generally do not make lasting imprints on physiology (Dickerson and Kemeny, 2004; Segerstrom and Miller, 2004). The exception is when acute stress continues to be viewed as a threatening (Baum et al., 1993), in which case it falls under our definition of chronic. Finally, our focus is on stress that is mainly psychological in nature. In adopting this focus we omit a host of classically physical stressors, like inadequate nutrition and infectious disease, which also play a role in shaping disease risks. These exposures are important for health, both in their own right, and as potential mediators/moderators of psychological stress. However, they are beyond the scope of this paper.
There are many severe chronic stressors to which children could be exposed. However, nearly all of the extant research linking early stress to adult health has focused on 1 of 2 experiences: parental maltreatment and socioeconomic disadvantage. While both of these experiences are chronic psychological stressors under the definition proposed above, they obviously differ in some critical respects – the kind of threats they pose, the duration, frequency, severity of those threats, and the opportunities for coping. On the other hand, it is clear that disadvantage and maltreatment co-occur more often than expected by chance alone (Crouch et al., 2000; Leventhal and Brooks-Gunn, 2000; Taylor et al., 2000). And they can share a number of overlapping features, which may include cold, unresponsive parents who use harsh discipline, routine exposure to conflict and violence in the home and/or the neighborhood, and limited access to basic material resources (Repetti et al., 2002). As we argue later, these features could set into motion biobehavioral cascades with long-term health implications. Consistent with this idea are the results of a recent prospective study, focusing on whether various forms of childhood stress presage the clustering of risk factors for CHD in adulthood (Danese et al., 2009). It found that both maltreatment and disadvantage in early life were associated with risk factor clustering at age 32. Covariance analyses showed the effects of these experiences to be partially overlapping – suggesting that they operated to some degree through common mechanisms, as we suggest they should. But there was also evidence that each linked to CHD risk through unique pathways, suggesting an element of equifinality, or a similar outcome achieved via different mechanisms (Cicchetti and Blender, 2006). To us, these patterns suggest that it is reasonable to proceed on the assumption that maltreatment and disadvantage share enough common features that they can be aggregated under the rubric of chronic stress, at least for the purposes of generating a broad mechanistic model to guide research in this nascent area. Of course, as the field develops it will be important to reevaluate this assumption, and refine the model accordingly should the data suggest it is necessary.
Because of the financial and logistical difficulties associated with following participants over multiple decades, most studies have used retrospective assessments of childhood stress. The interpretational difficulties associated with this approach are well known and should be kept in mind as the evidence is presented. That said, we agree with the conclusions of two reviews of retrospective assessments of early stress – that concerns about their reliability and validity are often overstated, particularly when people are being asked to report on salient things (e.g., whether or not they were physically abused; what their father’s job was when they were adolescents), and not the details or timing of a specific event (Brewin et al., 1993; Hardt and Rutter, 2004).
On the health side of the equation, studies have assessed everything from the frequency of minor physical symptoms like headaches and constipation, to rates of and death from various conditions. As much as possible we focus on morbidity (the diagnosis of a disease, or a clinical manifestation of it) and mortality (death, either as a general outcome, or traced to a specific cause). The advantages of these endpoints are that they can be ascertained objectively, and viewed as reflecting differences in an underlying disease process. This makes them much simpler to interpret than outcomes reported by patient themselves, which tend to be heavily shaped by individual differences in symptom perception, labeling, and reporting (Feldman et al., 1999; Pennebaker, 1983). Thus, by focusing on morbidity and mortality, we can reduce the possibility that early stress is linked to adult health because it alters symptom perception and reporting, rather than influencing the underlying pathogenic mechanisms. Because the paper’s emphasis is on conditions that arise in adulthood, most of the evidence reviewed deals with what are known as chronic diseases of aging, namely CHD, stroke, diabetes, cancers, and autoimmune disorders. Focusing on these core diseases is important because they are a pressing public health concern in developing countries today. They also have a reasonably well understood pathogenesis, which can be leveraged here to construct a biologically plausible model of mechanisms through which early stress contributes to later disease.
Long-Term Health Effects of Maltreatment
Evidence
A recent meta-analysis examined the long-term health consequences of childhood maltreatment (Wegman and Stetler, 2009). Drawing on a group of studies that collectively included 48,000 individuals, it considered exposure to childhood neglect, as well as physical, sexual, and emotional abuse. The disease endpoints included cardiovascular, respiratory, metabolic, musculoskeletal, and autoimmune conditions. The aggregate effect size across conditions was d=.42, meaning those maltreated as children had outcomes that were almost ½ SD more severe or more common than those not exposed. However, in some studies the outcome was self-reported diagnosis or symptom severity, raising questions about how much maltreatment contributed to the disease process itself versus subjective perceptions of it. To address this question, the authors stratified the studies according to how health status was ascertained. Results indicated that the magnitude of effect sizes was identical in studies that used objective versus subjective methodology. Moreover, maltreatment had a significant relationship with some fairly salient outcomes, like history of stroke and myocardial infarction (d=.66), which people could be expected to recall in a reliable fashion. These findings help assuage concerns that the observed associations are simply a reflection of maltreatment shaping the way that people experience or communicate their symptoms.
The most rigorous work in this area has come from the Adverse Childhood Experiences (ACE) Study, a large-scale project that assessed maltreatment retrospectively in 17,337 adults. Participants were queried about whether they were exposed to various kinds of abuse, neglect, and household dysfunction before the age of 18. They also were asked a series of questions about their history of CHD. There was evidence of a dose-response association, with a 20% increase in CHD incidence for each additional kind of early adversity experienced. Moreover, a 2–3 fold increase in CHD was found among those who reported more than 4 types of early life adversity (Dong et al., 2004). The individual forms of abuse and neglect (sexual, physical, emotional) were associated with 1.3–1.7 fold increases in CHD risk. These associations were independent of a variety of traditional CHD risk factors, including demographics, smoking, exercise, adiposity, diabetes, and hypertension.
The investigators of this project recently published further analyses which provide a stronger basis for causal inference (Anda et al., 2009). In this latest paper the outcome variable was the extent of premature mortality (before age 65, excluding suicides) among family members of the respondent. The assumption here was that the respondent’s siblings or parents would have been exposed to a similar household environment, so any long-term adverse health effects should be apparent in them as well. The analyses revealed an OR of 1.13, indicating that for every additional type of adversity, the likelihood of premature death of a family member rose by 13%. For those who had experienced 4 or more types of childhood adversity, the odds of a premature death ranged from 1.5–2.9 depending on age. These effects were independent of age, sex, race, and SES. Although these data are still based on the self-report of a single respondent, they are more compelling because the outcome of interest was experienced by a third-party. This design feature makes it improbable that early stresss imply biased the way respondents attend to, interpret, or report their symptoms.
Another recent analysis from this dataset used hospital discharge records to explore the prevalence of autoimmune diseases in a subset of the original cohort (Dube et al., 2009). It found that the odds of a first hospitalization for any autoimmune disease was higher among adults with 2 or 3 kinds of childhood adversity compared with those with none; however, the relationship was statistically significant only for women. In linear trend analysis there was a dose-response effect for both genders; for every additional kind of childhood adversity, the odds of an adult hospitalization rose by 20% for women and 10% for men. Because the outcome here was based upon hospital records, it is again improbable that biases introduced by self-report of health underlie the findings.
More recently, analyses from the Nurses Health Study II have begun to appear. This large-scale study has followed 91,000+ females over 20+ years. Recently a subset of 61,000+ participants reported on maltreatment experiences in childhood. Analyses suggest that sexual and physical abuse during childhood increased later risks for diabetes and hypertension (Rich-Edwards et al., 2010; Riley et al., 2010). Although the endpoints used in these analyses were ascertained by self-report, the authors presented validation studies testifying to the veridicality of nurses’ accounts.
Interpretation
Collectively, this research is suggestive of the conclusion that childhood maltreatment has long-term effects on health. That said, the literature has some important weaknesses that preclude a definitive interpretation. First, many of the studies rely upon one-time assessments, during which early stress and later health are measured via retrospective self-report. For many readers these methods will raise concerns about the reliability of reporting. This certainly has the potential to be a problem, especially if the reporting of both predictors and outcomes is systematically biased. However, the most likely scenario is that retrospective self-report assessments lead to under-reporting of maltreatment (Hardt and Rutter, 2004). Assuming this is true, the net effects would be to add noise to the data and artificially constrain variance on the maltreatment variable. In both cases this would reduce statistical power, making the study less likely to detect associations. Another concern is that the associations observed reflect maltreatment-related differences in the perception or reporting of symptoms, rather than effects on disease per se. However, the ACE study data on autoimmune disorders and premature mortality assuage these concerns. And as we will see below, the studies of low SES go a long ways towards doing so. More problematic from our perspective is the possibility of associations being inflated by confounds. The ACE studies measured and controlled for many of the more plausible confounds, like age, race, SES, medical history, but there remains the possibility that other factors are playing a role, like a familial genetic liability that contributes to abusive parenting and disease vulnerability. Unless the locus of such an effect was known, this explanation would be impossible to rule out in human studies. But as we shall see below, in animal models, early stress can be manipulated experimentally, and concerns like this can be eliminated. Thus, we conclude that the evidence here is suggestive of a causal influence of childhood maltreatment on later health problems, but more work needs to be done to definitively rule out alternative explanations and clarify what the associations reflect in terms of underlying pathophysiology. More attention also needs to be paid to what transpires between exposure to maltreatment and later manifestations of disease; few studies have considered the role of behavioral and biological processes that unfold during these intervening years.
Long-Term Health Effects of Low SES
Evidence
There is a more extensive literature on childhood SES and its implications for adult health. Galobardes and colleagues reviewed ~40 studies of childhood SES and mortality in adulthood (Galobardes et al., 2004; Galobardes et al., 2008). All were prospective in the sense that health outcomes were tracked over time, but in some instances early-life SES was assessed retrospectively when respondents were adults. Most studies used paternal occupation to index childhood SES, and focused on individuals who were born in the early to middle of the 20th century. The samples were drawn from Europe, Asia, and North America, and collectively included millions of respondents. In most instances the studies made a point of statistically controlling for SES in adulthood. When studying the long-term health effects of childhood SES, this is an important explanation to consider. There are two reasons for this. First, SES tends to be fairly stable over the lifecourse, with poor children becoming poor adults far more often than would be expected by chance (Hertzman, 1999). Second, SES in adulthood is strongly related to morbidity and mortality from the chronic diseases we consider here (Adler and Rehkopf, 2008; Lynch and Smith, 2005). Thus, to convincingly show that early-life SES is shaping risks for disease later in life, studies need to establish that childhood SES is not simply rendering people more or less advantaged in adulthood.
Galobardes and colleagues (2004; 2008) reported that 28/33 studies found an increased risk of all-cause mortality, typically of 20–40%, among individuals reared in low versus high-SES households. Adjustments for adult SES generally attenuated these associations, but did not eliminate them. In terms of disease-specific outcomes, low childhood SES emerged as a risk factor for CHD mortality in 7/10 studies and stroke mortality in 4/6 large studies. Again, these effects were attenuated but not eliminated by adjustment for adult SES. Deaths from violence, accidents, and drugs/alcohol were also more common among those from low SES backgrounds, particularly if they were male offspring. There was some evidence of childhood SES making independent contributions to cancer mortality, particularly in stomach and smoking-related neoplasms.
The same group also reviewed studies of childhood SES and cardiovascular disease (CVD; Galobardes et al., 2006). The studies it drew upon were done in the United Kingdom, Europe, and the United States, and mainly used father’s occupation to index childhood SES. 19/24 prospective studies found significant inverse associations, with the excess risk attributable to low-SES in the 30–60% range. When SES in adulthood was controlled these associations were attenuated, but the excess CVD risk persisted in the 20–40% range. Mirroring the mortality data, the effects were stronger for stroke than for CHD, though the latter were reliable.
Interpretation
The studies of SES have notable strengths compared to the maltreatment literature. They make use of large and representative samples from multiple continents, use prospective designs, have objective SES indicators, and ascertain outcomes through medical records or healthcare databases, eliminating the concern that reporting styles are driving the observed associations. They also generally control for the many plausible alternative explanations for disease outcomes, like socially patterned differences in lifestyle factors such as smoking, adiposity, etc. And finally, the fact that findings emerge across so many different countries suggests some universalism in the phenomenon, and makes it unlikely that culture-specific factors (like class-related differences in access to health care in the U.S.) are driving the findings. These study strengths bolster confidence that the observed associations reflect a causal process, though they do not prove it definitively.
Indeed, there are several weaknesses in this literature that complicate its interpretation. The most important is the observational design of the studies. The possibility remains that the observed associations are due to unmeasured confounds, such as a common genetic liability that predisposes offspring to low SES and poor health. The only way to definitively evaluate this alternative explanation is through an experimental manipulation of childhood SES, which is neither ethical nor feasible in humans. That said, as we shall see below, animal studies can be used to address these concerns. Second, most of the studies are not fully prospective in the sense that they do not assess childhood health at the point of exposure to SES (Cohen et al., 2010). This would be difficult to do in many cases, however, because the diseases of interest have either not begun yet, or are in preclinical stages where prognosis remains unclear. Nonetheless, it would bolster confidence if these studies collected data on health at the time SES was assessed, to address the possibility that poor children start out life sicker, which affects both their parents’ earnings and their own long-term health.
A final limitation of the literature here is that it does not specifically implicate childhood exposure to stress as critical for later disease. Although studies link childhood SES with later medical outcomes, it remains unclear whether these effects are specifically attributable to early-life exposure in the way our interpretation presupposes. In other words the studies do not answer questions like: Is childhood a sensitive period for exposure? Could low SES at other junctures in the lifecourse be equally detrimental? And perhaps even more critically: Might childhood SES predict outcomes because it is acting as a proxy for cumulative disadvantage, which many argue is likely to be the most influential sociodemographic determinant of disease and disability (Cohen et al., 2010)?
Studies that have sought to address this question have generally found support for the hypothesis that childhood is a sensitive period, during which time low SES has potent and lasting effects on health. This conclusion derives from analyses suggesting that the excess disease risk associated with low childhood SES persists even when people experience upward social mobility, and is not generally accounted for by their cumulative exposure to disadvantage (Hart et al., 2000; Kuh et al., 2002; Ljung and Hallqvist, 2006; Pensola and Martikainen, 2003; Power et al., 2005b; Smith et al., 1998). Such findings are seen as proof that “it matters how and when” people are exposed to low SES (p. 1082; Ljung & Hallqvist 2006). But in reality these effects are exceedingly difficult to disentangle (Hallqvist et al., 2004). That is because in most populations, it is impossible to form mutually exclusive categories of individuals who had the relevant exposures necessary for comparisons to be made, i.e., those who were low SES in childhood vs. had upward mobility in adulthood vs. those who were persistently low SES across the lifespan. That said, work in this area would be more informative if it mapped SES trajectories across the lifecourse. Many existing studies ignore the lengthy period between exposure to low childhood SES and later disease outcomes.
All of these problems can be circumvented with experimental studies of animals, which manipulate the timing of exposure to stress to determine whether sensitive periods exist. Such work suggests the existence of sensitive periods for a number of processes and outcomes relevant to the current argument. For example, in rodents the first 8 days of life represent a specific window during which variations in the quality of maternal care shape subsequent responsivity of the HPA axis to stress (Meaney, 2001). Lower maternal care during this juncture leads animals to have larger HPA responses to stress, which persist over the lifecourse. Mechanistically, this response tendency persists because lower maternal care downregulates glucocorticoid receptor (GR) expression in the hippocampus (Liu et al., 1997). Interestingly, the influence of maternal care is especially potent during the first week of life. When care quality is altered during week 2 of life, the effects on GR expression are significantly less pronounced. Moreover, care manipulations in week 3 of life have no influence whatsoever on long-term GR expression (Meaney and Aitken, 1985) Even more relevant to arguments about the potential influence of cumulative exposure, these studies revealed that care manipulations had identical effects on adult GR expression regardless of whether they were restricted to week 1 of life vs. persisted for weeks 1–3. In both conditions the effects on GR were larger than those produced by care manipulations during postnatal week 2 or week 3 (Meaney and Aitken, 1985). Similar patterns were found in studies where corticosteroid responses to stress in adulthood were measured (Hess et al., 1969). Besides this work on HPA activity, studies of disease have pointed to specific developmental windows during which maternal separation enhances later vulnerability to peptic ulcers and implanted tumors (Ackerman et al., 1975; Ader et al., 1960). Collectively, this work suggests that early stress has direct and lasting influences on some disease-relevant biological processes, which are not simply a function of boosting total lifetime exposure to stress. When coupled with human studies, these results are suggestive of childhood being a sensitive period for effects of stress to become “embedded” in some physiological systems for the long term.
Early Stress and Adult Health in Animals
As noted above, it is difficult, and perhaps impossible, to evaluate whether early stress causally influences adult disease in humans, because it would be unethical and unfeasible to manipulate the relevant human experiences. However, studies of this nature can be done in animal models, and it is instructive to consider what they have revealed, as this evidence can serve as “proof of principle” that early stress can (or can not) causally influence later disease risk. So in this section we provide an overview of the available research in this area, focusing on studies that ask whether animals exposed to early-life stress have differential susceptibility to medical illness later in life.
Most work in this area has modeled early stress through paradigms that wean rodents from their mothers prematurely. Early weaning is achieved by permanent removal of offspring from their mothers. This manipulation deprives the offspring of maternal care from an early stage in its life.. But as Hofer has observed, when an animal is weaned from its mother prematurely, it loses more than just emotional nurturance. During the early post-natal period caregivers function as external regulators, maintaining a homeostatic equilibrium the offspring’s physiology is too immature to support itself. By providing body warmth, nutrients, and tactile stimulation, mothers regulate their newborn offspring’s heart rate, oxygen consumption, HPA axis activity, growth hormone secretion, and other processes (Hofer, 1984; Hofer, 1987). In this regard, the manipulation has some parallels to disadvantage and maltreatment. On the whole, children exposed to these stressors receive less sensory, cognitive, and emotional stimulation, and are more likely to be deprived of basic necessities like food and heat, than more privileged youth (Evans, 2004; Maulik and Darmstadt, 2009; McLoyd, 1998). That said, it is always difficult to ascertain how closely deprivation in rodent models resembles the human experience. Permanent maternal separation is a relatively uncommon in humans, even in families where maltreatment and disadvantage are present. So at best, the animal studies model what is fairly extreme human stress. Species differences in development are another major challenge. At the time of birth, rodents are much less mature than humans. As a result, their physiology may need external regulation by caregivers in a manner that is not paralleled in full-term human newborns.
Those caveats aside, a number of studies indicate that premature weaning can have long-term effects on animals’ vulnerability to disease. In a typical study rats were permanently separated from their mothers at either 15 or 21days of life, corresponding to early versus normal weaning (Ader and Friedman, 1965). A few weeks later, all offspring were implanted with a tumor (Walker 256 carcinosarcoma), and followed to assess mortality. Analyses revealed shorter times to death among prematurely separated animals. The early-weaned rats died a median of 21 days after tumor receipt; the parallel figure was 25 days in rats separated from their mothers at normal weaning age. In another study rats were placed in a stressful situation at 100 days of life (corresponding to early adulthood in humans). The situation required the rats to incur electric shocks to obtain food and drink for 4 days (Ader et al., 1960). Nearly all of the rats developed gastric ulcers following this experience. However, the density of these ulcers was five-fold greater in rats who had been prematurely separated from their mothers at 15 days of life. In a follow-up study, a third group was added to evaluate whether nutritional disparities were responsible for the health effects of the separation manipulation. To test this hypothesis, an additional group of rodents was tested: the mother was removed from her offspring at 15 days, had her nipples cauterized to prevent nursing, and was then returned until 21 days. The median number of ulcers in this group was similar to the control rats. Because both groups in which the mother remained present had significantly fewer ulcers than the prematurely separated and weaned rats, these data suggest the effects were due to the absence of maternal nurturance rather than nutritional deficiencies per se (Ader, 1962).
This pattern was later replicated and extended by Weiner and colleagues (Ackerman et al., 1975; Ackerman et al., 1978; Skolnick et al., 1980). They focused on gastric ulcers, which we now know to be caused by infection with the bacterium helicobacter pylori and the inflammatory responses that it provokes (Portal-Celhay and Perez-Perez, 2006). This work showed that early life stress accelerates the emergence of gastric ulcers in rodents. Among those prematurely separated and weaned from their mothers, ulcer vulnerability peaked near 30 days of life (childhood in humans). Among those who were separated and weaned normally, ulcers tended not to appear until early adulthood, at roughly 100 days (Ackerman et al., 1975; Ackerman et al., 1978).
More recent studies have focused on common infectious diseases. In one study mice pups were separated from their dams for 6 hours daily over the first two weeks of life (Avitsur et al., 2006). As adults, the mice were challenged intranasally with an influenza virus. Compared to controls which remained with their mothers until weaning, the separated mice had greater viral replication and worse symptoms of infection. This was a result of an overly aggressive inflammatory response to the virus. Separated mice had a relative increase in pro-inflammatory mediator expression in the lungs five days post-infection. Interestingly, some of the inflammatory mediators continued to be elevated nine days post-infection, a time when viral particles had declined to the point of being almost undetectable. These findings suggest that early stress calibrated the immune system to mount overly aggressive and extended inflammatory responses to the influenza virus.
There also has been mounting interest in early life influences on asthma in animal models. One study randomized mice to 1 of 3 conditions: one in which they received regular footshocks for 1 hour on 3 days during the fourth week of life; another in which they watched and heard other mice undergo this experience, but were not shocked themselves; and a control arm in which mice remained undisturbed in their home cages (Chida et al., 2007). When the mice reached young adulthood (i.e., at 8 and 10 weeks of life) they were sensitized to ovalbumin, a protein in eggs that causes allergic reactions. At 11 weeks all mice were given airway challenges with ovalbumin. Relative to controls, those that received or observed footshocks showed greater airway inflammation and more bronchial reactivity to the challenge. Similar patterns were observed in another study of rats which, over the first month of life, were either separated from their mothers daily for 2 hours, and then reunited, or remained undisturbed in their home cages (Kruschinski et al., 2008). When the rats were 5-month old adults, asthma was induced by sensitizing subjects to ovalbumin, and airway tissue was collected. Analyses revealed striking differences. Adult rats that had been repeatedly separated from their mothers early in life showed more severe airway pathology than adult controls, with increased numbers of eosinophils, T-cells, and other pro-inflammatory mediators found in their lungs upon dissection.
Conclusions
Although the animal literature on this topic is not extensive, the available studies provide consistent evidence linking early stress and later health, and do so across a broad array of diseases. Because these studies used experimental manipulations of stress, they provide leverage for making definitive causal inferences in humans. And while the findings from animal models do not prove that the human studies are capturing a causal process, they do provide quite strong proof of the principle that such effects can occur. When considered alongside the more rigorous studies of maltreatment and disadvantage, which rule out the influence of a number of plausible confounders, the animal studies lead us towards a cautious causal interpretation of the human findings.
The Biological Embedding of Childhood Adversity Model
The overview suggests that childhood stress has consequences for vulnerability to chronic disease in adulthood. What is needed at this point is a model to explain how and why this happens. To be successful, such a model will have to address two questions raised by the studies we have just reviewed. First, how does childhood stress get under the skin, at the level of tissues and organs, to affect risk for later diseases? To address this question, a model needs to connect processes occurring at multiple levels of analysis. It will have to consider the social and economic contexts in which children develop, and explain how and why they give rise to particular behavioral and biological tendencies. The model will also have to specify in a biologically plausible manner how these tendencies give rise to pathogenic processes that result in excess morbidity and mortality.
Second, the model will need to explain the lengthy incubation period between exposure to childhood stress and the clinical manifestation of disease. In some studies this interval lasted 4–5 decades. Existing models offer insights into the mechanistic basis of mind-body interactions (Glaser and Kiecolt-Glaser, 2005; Kop and Cohen, 2001; Miller et al., 2009b; Pressman and Cohen, 2005), but focus on the immediate biological sequalae of stress. To explain how early stress operates, a model must specify how it “incubates” in the body, manifesting in disease several decades later.
Overview of Model
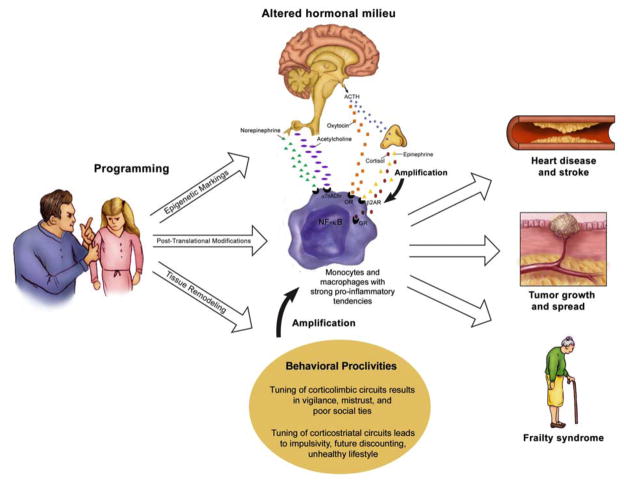
To address these issues we propose a Biological Embedding of Childhood Adversity Model. As noted it represents a synthesis of theoretical perspectives from the fetal-origins literature (Barker, 1992), lifecourse epidemiology (Lynch and Smith, 2005), socioemotional development (Pollak, 2008; Repetti et al., 2002), stress physiology (McEwen, 1998), and behavioral immunology (Coe and Lubach, 2007; Raison and Miller, 2003). The model’s basic premise is that when stress occurs during sensitive periods of development, it calibrates how certain bodily systems operate going forward. This is the notion of biological programming, which grew out of research on nutritional deprivation (Barker, 1992), but now is applied to a variety of perinatal experiences, including stress in the psychosocial domain (Cameron et al., 2005; Cottrell and Seckl, 2009; Drake et al., 2007; Hertzman, 1999; Wright, 2010). The focus in our model is on how stress programs the response tendencies of cells of the monocyte/macrophage lineage, which play a key role in initiating and maintaining inflammation, a process that is central to a number of chronic diseases of aging. The model specifies three mechanisms – epigenetic markings, post-translational modifications, and tissue remodeling – responsible for this programming. As a result, stress gets “embedded” in these immune cells, causing them to mount excessive inflammatory responses to microbial challenges, and be insensitive to inhibitory hormonal signals. This fosters a chronic inflammatory state in the body.
The model goes on to propose that over the lifecourse, these pro-inflammatory tendencies are exacerbated through behavioral proclivities and hormonal dysregulation, themselves brought about through exposure to childhood adversity. Behaviorally, early stress leads people to become vigilant for threat and mistrusting of others. These traits shape the manner in which people engage their social worlds, making them more likely to elicit conflict and rejection, and less likely to garner warmth and support. They have persistent difficulties forming and keeping relationships. Early stress also leads people to develop poor self-regulation skills, wherein the future is highly discounted in favor of immediate gratification, and there is a resulting propensity to engage in unhealthy behaviors. Together, these social difficulties and unhealthy lifestyle serve to amplify the chronic inflammatory state. Also contributing to this process are dysregulated patterns of endocrine and autonomic discharge, which consign monocytes/macrophages to operate in a milieu that accentuates their pro-inflammatory tendencies. Depending on the individual’s genetic liabilities and other relevant exposures (e.g., to pollutants, toxicants, carcinogens, etc.), the ensuing chronic inflammation drives forwards various mechanisms of pathogenesis. Those can include high blood pressure, insulin resistance, plaque growth, tissue destruction, and tumor progression. These processes eventuate in chronic diseases like diabetes, CHD, autoimmunity, and cancers.
Functional Significance
What would be the functional significance of programming a child’s physiology in this manner upon exposure to chronic stress early in life? To address this question we draw on the concept of a predictive adaptive response (PAR) from behavioral ecology (Gluckman and Hanson, 2006). PAR’s are biological adaptations made in response to anticipated environmental circumstances. They occur during sensitive periods of development, when bodily tissues have maximal plasticity to environmental inputs. Typically PAR’s become embedded in physiology on a permanent basis. PAR’s function to calibrate physiology in a manner that best matches the demands of the ecology in which the organism will ultimately reside. Functionally, PARs enable the organism to better defend itself, make optimal use of resources, and reproduce more successfully. In so doing, they confer enhanced fitness and, at least through reproductive age, survival benefits (Gluckman and Hanson, 2006).
We view the phenotype depicted in the Embedding model as a vestigial PAR, which evolved to meet the demands of perilous ancestral ecologies (Zhang et al., 2006). Inherent in these ecologies were a host of recurring difficulties that posed threats to survival and reproductive fitness. These difficulties are likely to have included predation, conflict with other humans and the ensuing injuries, limited access to nutrients and resources, and accidents and infections. To the extent that our ancestors’ bodies could have anticipated these difficulties, and made the phenotypic adjustments specified in the model, they may have been better equipped to survive and reproduce in a perilous environment. For example, in a setting where predators abound, high vigilance for threat might confer a survival advantage by helping the organism to more rapidly perceive and respond to impending danger. Biologically, when fighting and fleeing were necessary, robust endocrine activity would also prove valuable, providing metabolic support for these behaviors. Finally, when fighting occurred, organisms which possessed the model’s pro-inflammatory tendencies might enjoy a survival advantage, as these traits would accelerate the healing of wounds and the clearing of secondary infections. From a metabolic perspective, endowing monocytes/macrophages with these aggressive tendencies would be relatively efficient, at least by comparison to the energetically costly lymphocytes that comprise the adaptive immune system (Segerstrom, 2010). Also, having stress get embedded in innate immune cells such as monocytoes/macrophages would limit the scope of collateral damage the organism might incur; at least in the short-term, over-activated adaptive immune cells can do far more damage to bodily tissue than macrophages. Finally, in contexts where nutrients and resources were scarce, the impulsive and appetitive tendencies the model specifies would have adaptive value, motivating approach behaviors when resources are found and helping to sustain the organism through periods of famine (by gorging when resources were available).
Today, few Western children reside in settings that resemble the ancestral ecology. However, we suspect that when such children are reared in sufficiently perilous ecologies, a vestigial PAR is cued that gives rise to the behavioral and biological phenotype depicted in the model. These traits served to promote fitness in ancestral ecologies, and may continue to do so today. But these traits also set into motion an ongoing inflammatory state that, over the increased duration of a modern human lifespan, fosters pathogenic mechanisms that eventuate in chronic diseases of aging. In ancestral times this apparent downside would have been inconsequential from the perspective of natural selection, since these humans both differentially reproduced and died of injuries, starvation, and infection decades before chronic diseases would typically manifest. In this regard the collection of tendencies our model envisions can be understood as a classic life-history tradeoff, wherein the organism allocates resources towards an especially defensive phenotype, in the service of managing threats that would compromise reproductive success (McDade, 2005). However, in doing so it leverages these potential benefits against a greater long-term liability for chronic diseases of aging.
Insights from Other Models
Like most other models in psychology, ours is primarily a synthesis of knowledge from other spheres, rather than a fully novel set of propositions. We draw heavily on concepts from other theories, and assemble them into an elaborated, integrated framework. Thus, before reviewing evidence for the model, we outline a handful of other relevant theories, specifying where they have provided insights critical to our thinking, and where our model builds upon their contributions.
The “fetal-origins” hypothesis grew out of research by David Barker and his colleagues (Barker, 1992). This work showed that children of low birth weight are at risk for obesity, metabolic syndrome, and coronary disease in adulthood (Godfrey, 2006). Barker and others have argued that low birth weight reflects nutritional deprivation in utero, which arises because of poor maternal diet and/or insufficient nutrient transfer across the placenta (Barker, 1992; Gluckman et al., 2008). The fetus responds to these deficiencies with metabolic adjustments (e.g., changes in secretion of, sensitivity to, and breakdown of hormones that regulate growth) thought to optimize development in a nutritionally compromised environment. These adjustments spare critical organs like the heart, brain, and pancreas and favor the emergence of offspring with small body size, low skeletal muscle, and high visceral fat (Gluckman and Hanson, 2006). Because the metabolic adjustments are programmed into physiology during sensitive periods of development, they are thought to persist over the lifespan in a fashion that is permanent and immutable. In the original formulations of the model, physiological adjustments were thought to cause wear and tear on organs over time, which acting with genetic liabilities, gave rise to metabolic and coronary diseases. In recent updates, there has been increasing emphasis on the interaction between the survival phenotype and its postnatal nutritional environment in shaping later disease outcomes (Gluckman et al., 2008)
The fetal-origins hypothesis provides a valuable framework for thinking about early-life experience. Through the concept of biological programming, it explains how and why nutritional imbalances in utero could give rise to later disease. Our model draws upon this concept to explain how stress could become “embedded” in the immune cells of a developing child in a lasting manner. (This parallels other models wherein stress is depicted as a programming agent; e.g., Cameron et al., 2005; Cottrell and Seckl, 2009; Drake et al., 2007; Hertzman, 1999; Wright, 2010). Where our framework builds on these ideas is in its depiction of people as active agents that continue to shape their environments throughout life. That is, it suggests that the experiences people have in early life shapes the kinds of environments they seek out (and create) for habitation later in life (in adulthood), as well as the ways in which they respond to challenges in those environments. From their early social context people develop likes and dislikes, patterns of interacting with others, and strategies for regulating their desires and emotions (Bowlby, 1969; Cassidy and Shaver, 2008; Luecken and Lemery, 2004). Across the lifespan these tendencies, and the experiences that arise from them, modulate disease-relevant behavioral and biological processes in dynamic ways (Chen et al., 2002; Repetti et al., 2002; Shonkoff et al., 2009).
A second class of models that has been used to explain the health effects of childhood stress comes from lifecourse epidemiology (Lynch and Smith, 2005). These models emphasize the trajectories that childhood experience sets people upon (Pollitt et al., 2005), which are referred to as “chains of risk” (Kuh and Ben-Shlomo, 2004) and “accumulating chains of advantage or disadvantage” (Blane, 1999). The notion inherent in these models is that adversity begets adversity. A child raised in poverty is likely to attend a school with limited financial resources and receive a suboptimal education. This in turn makes it likely that s/he will be a low-income adult, have a job with routine exposure to pollutants and irritants, live in a neighborhood where fresh foods are hard to find, green spaces for exercise are not available, access to health care is limited, and so on. These exposures are presumed to accumulate over the lifecourse such that the more adversity a person experiences, the more likely he or she is to become ill (Hertzman, 1999; Pollitt et al., 2005).
The lifecourse models are appealing in their emphasis on trajectories. As such, our model draws on notions of “chaining” to explain how early stress sculpts psychological tendencies that influence the social environments people create for themselves. However, our model also extends lifecourse concepts by blending them with notions of biological programming, in order to explain why childhood is a sensitive period during which stress gets embedded in monocytes/macrophages (Cameron et al., 2005; Fenoglio et al., 2006a; Hertzman, 1999; Lyons et al., 2009).
Finally, the Risky Families Model offers a psychosocial account of how childhood stress impacts health (Repetti et al., 2002). It posits that some families are “risky” places for children to develop, because they tend to be unstable and conflictual, lacking in warmth and support, and make use of harsh discipline. These familial dynamics trigger a cascade of psychological vulnerabilities, including deficits in social competence and emotion regulation, and a propensity to compensate for them with health-compromising behaviors. They also lead to increased reactivity of the HPA axis and the sympathetic nervous system (SNS). Frequent activation of these circuits triggers the release of hormones like cortisol, epinephrine, and norepinephrine. With time the impact of these hormonal surges accumulates, leading to wear and tear on bodily systems known as allostatic load (McEwen, 1998), and subsequent health problems. In support of this view, adults who report having been reared in harsh family climates show relatively high levels of circulating inflammatory markers, more components of the metabolic syndrome, and increasing trajectories of blood pressure (Lehman et al., 2005; Lehman et al., 2009; Taylor et al., 2006b).
The Risky Families Model provides a valuable heuristic framework for considering the effects of early-life stress. Like the pathway models discussed earlier, it is instructive in highlighting the dependencies between experiences at different stages of the lifespan. It goes beyond the previously reviewed models by stitching together these experiences psychologically. For example, it pinpoints the features of early family climate that are likely to be “toxic” for health, specifies how they manifest developmentally in emotion-regulation and social-competence deficits, and suggests that their effects are sustained through dispositions like hostility over the lifespan. These concepts serve as a foundation for our model. We build on them here by proposing that childhood stress (a) gets programmed into the response tendencies of monocytes/macrophages through specific molecular pathways, and (b) favors the emergence of certain behavioral proclivities early in development, e.g., vigilance for threat, mistrust of others, impulsivity, poor self-regulation. These proclivities shape the kinds of environments people create for habitation later in life and the manner in which they respond to challenges in those environments. (In other words, the proclivities serve as antecedents to the kinds of emotion-regulation and social-competence difficulties featured in the Risky Families Model.) Finally, based on recent insights from neuroimaging, our model builds on Risky Families by highlighting some of the neural mechanisms likely to underlie these behavioral proclivities.
Like the Risky Families Model and a number of other accounts (Evans et al., 2007; Shonkoff et al., 2009; Worthman and Panter-Brick, 2008), our model draws on the concept of allostatic load physiologically. The core of this idea is that during stress, the body attempts to restore balance through change (allostasis; McEwen and Stellar, 1993). Allostatic maneuvers are viewed as being adaptive on an acute basis. But if they are triggered repeatedly, dysregulation across multiple systems ensues, causing declines in physical health. Indeed, there is mounting evidence that high levels of allostatic load presage declines in physical functioning and premature mortality (Karlamangla et al., 2002; Karlamangla et al., 2006; Seeman et al., 2001; Seeman et al., 2004).
Our model draws on the allostatic load concept, particularly for its explanation of how the biological consequences of stress accumulate. We suggest that childhood adversities give rise to lifelong behavioral proclivities, like vigilance for threat, poor social ties, ineffective self-regulation, and unhealthy lifestyle choices. These proclivities lead to frequent activation of the body’s stress-response systems, creating a hormonal milieu that requires adaptations by the immune system. Ultimately, these adaptations serve to exacerbate the pro-inflammatory tendencies already programmed into cells by childhood stress. Our model extends the concept of allostatic load, however, by positing a role for the biological embedding of early stress. Thus, the model supposes that lasting biological alterations can arise from relatively brief exposures if they are timed correctly, even in the absence of the kind of cumulative wear-and-tear that allostatic models depict as taxing the body. As reviewed previously, there is robust evidence of such effects in animal models (Hess et al., 1969; Meaney and Aitken, 1985). Our model also diverges from allostatic load in specifying inflammation as a single, common, and necessary pathway linking stress to disease.
Evaluating the Model
Having outlined the model’s structure, we now shift to assessing its validity. In the sections below we critically review evidence for each of its premises. Table 1 summarizes the key evidence.
Table 1.
Key Evidence for Propositions in The Biological Embedding of Childhood Adversity Model.
| Proposition & Evidence | Source of Evidence |
|---|---|
| 1. Disadvantage and maltreatment are chronic stressors for children. They have common features that can include unresponsive parents, harsh discipline, routine exposure to violence, and limited access to resources. | |
| -Low-SES families have limited material resources, poor living conditions, low job control, and limited coping options. |
Albelda (2001) Evans (2004) |
| -Due to these constraints, low-SES parents have less time to spend with their children and are less responsive & supportive |
Bradley et al. (2001) Dodge et al. (1994) |
| -Low-SES families tend to have more conflict, use harsher and more punitive discipline, and have inconsistent parenting. |
Leventhal & Brooks-Gunn (2000) McLoyd (1990) |
| -Children in low-SES settings are more likely to witness and experience violence, both inside and outside their homes. |
Crouch et al. (2000) Schubiner et al. (1993) |
| -Maltreatment is a “toxic” relational environment, in which children can incur physical or sexual abuse and neglect of their emotional or material needs. These different forms of maltreatment co-occur in a sizeable minority of cases. |
Arata et al. (2007) Cicchetti & Toth (2005) Dong et al. (2003) |
| 2. Chronic stress in childhood programs a pro-inflammatory phenotype in monocytes/macrophages. | |
| -Newborns exposed to maternal stress in utero have larger ex vivo inflammatory cytokine responses to microbial challenges. | Wright et al. (2010) |
| -Adults raised in low-SES families display larger ex vivo inflammatory cytokine responses to various microbial challenges. | Miller et al. (2009) |
| -Adults raised in low-SES families show reduced cortisol-mediated signaling in peripheral blood mononuclear cells, an indication of resistance to the anti-inflammatory properties of cortisol. | Miller et al. (2009) |
| -Over time, teenagers from harsh family climates display progressively larger ex vivo inflammatory cytokine responses to LPS, and greater resistance to cortisol’s anti-inflammatory effects. | Miller & Chen (2010) |
| -Adults raised in low-SES families show higher circulating levels of biomarkers like CRP and IL-6, which reflect the degree of chronic inflammation. | Danese et al. (2009), Lawlor et al. (2005), Loucks et al. (2010), Phillips et al. (2009), Pollitt et al. (2007), Schreier and Chen (2010), Tabassum et al. (2008) |
| -Adults who were maltreated in childhood show higher levels of CRP, IL-6, and other markers of chronic inflammation. | Danese et al. (2007), Kiecolt-Glaser et al. (2011), Slopen et al (2010) |
| -Adults raised in low-SES families show higher CRP, due in part to worse psychosocial functioning imparted by a harsh family of origin climate. | Taylor et al. (2006) |
| 3. Childhood stress gets embedded through epigenetic alterations to DNA, post-translational modification of proteins, and tissue remodeling. | |
| -Rats who receive low maternal care in early life show more methylation in a stretch of the glucocorticoid receptor gene promoter in the hippocampus. This change reduces expression of the gene, and by doing so increases cortisol reactivity to stress by interfering with negative feedback circuits. | Weaver et al. (2004) |
| -Cord blood cells of newborns exposed to maternal distress in utero show more methylation of a stretch of glucocorticoid receptor promoter. | Oberlander et al. (2008) |
| -Post-mortem, hippocampal slices of persons abused in youth utero show more methylation of a stretch of glucocorticoid receptor promoter. | McGowan et al. (2009) |
| -In rat pups, less nurturant parenting days 2–8 of life triggers post-translational modifications to a kinase that regulates the expression of CRH, a key trigger of HPA axis activity. | Fenoglio et al. (2006) |
| -In rat pups, less nurturant parenting days 2–8 of life results in more excitatory glutamatergic innervation of hypothalamic area centrally involved in regulating HPA axis activity. | Korosi et al. (2010) |
| -In rat pups, less nurturant parenting days 2–14 of life is associated with more extensive dendritic branching in locus coeruleus, which plays key role in regulating SNS responses to stress. | Swinny et al. (2009) |
| 4. Chronic stress in childhood gives rise to behavioral proclivities that accentuate inflammation. One route for this includes tuning of the corticolimbic circuitry, which leads to vigilance, mistrust, and subsequent difficulties with social relationships. | |
| -Children from low-SES families read more threat into situations that are ambiguous. This trait persists in adulthood. | Chen et al. (2003a, 2003b, 2004b, 2006); Miller (unpublished data) |
| -Maltreated children have selective attention for anger, perceive it more in ambiguous situations, are slower to disengage from it, and orient towards others’ conflict. | Pollak & Kistler (2002), Pollak & Sinha (2002), Pollak et al. (2005) |
| -Adults from low-SES backgrounds are more likely to endorse hostile and mistrusting beliefs about others. | Barefoot et al. (1991), Lynch et al. (1997), Scherwitz et al. (1991) |
| -Adults reared in low-SES families or maltreated as kids have smaller social networks, more conflict, and less social support. |
Graves et al. (1998) Repetti et al. (2002) |
| -Adults raised in low-SES families have larger amygdala responses when matching angry faces to neutral targets. | Gianaros et al. (2008) |
| -During emotional labeling tasks in scanner, adults from harsh families show ineffective prefrontal regulation of amygdala. | Taylor et al. (2006) |
| -Mistrust is associated with greater ex vivo pro-inflammatory cytokine production following LPS exposure, as well higher levels of the inflammatory markers CRP and IL-6. | Graham et al. (2006), Marsland et al. (2008), Suarez et al. (2002, 2004) |
| -Abrasive social encounters and poor social ties are associated with higher levels of CRP and IL-6 in circulation, activation of pro-inflammatory transcription control pathways, and reduced cortisol-mediated signaling. | Cole (2007), Fuligni et al. (2009), Loucks (2006a, 2006b), Kiecolt-Glaser et al. (2005) |
| 5. Chronic stress in childhood brings about behavioral proclivities that accentuate inflammation. Another route for this includes tuning of the corticostriatal circuitry. The resulting phenotype is impulsive, discounts the future, and has a proclivity for unhealthy behaviors. | |
| -Adults raised in low-SES families are more impulsive and tend to discount the future. | Griskevicius (2011), McLoyd (1994), Sweitzer et al. (2008) |
| -Adults from low-SES families are more likely to smoke, drink to excess, have poor diets and inactive lifestyles, and be obese. |
Lynch et al. (1997) Power et al. (2005) |
| -Impulsivity and discounting mediate a portion of the association between low SES and unhealthy lifestyles. |
Adams & White (2009) Wardle & Steptoe (2003) |
| -Adults who were maltreated in childhood more likely to smoke, be alcohol dependent, use illicit drugs, and be obese. | Felitti et al. (1998) |
| -During a monetary gain task, adults from low-SES families show reduced activity in lateral and dorsomedial prefrontal cortex, and reduced connectivity of this area with left ventral striatum. | Gianaros et al. (2010) |
| -An unhealthy lifestyle, in the form of smoking, heavy alcohol use, a poor diet, sedentary behavior, and obesity, is associated with more systemic inflammation as marked by CRP and IL-6. | Ghanim et al. (2004), Handschin (2008), Hotamisligil (2006), Kiecolt-Glaser (2010), O’Connor et al. (2009), Yanbaeva et al. (2007), Yudkin et al. (2001) |
| 6. Chronic stress in childhood alters autonomic and endocrine discharge in a durable manner, creating a hormonal milieu that accentuates inflammation. | |
| -Adults raised in low-SES families show greater diurnal output of salivary cortisol as they go about day-to-day life. | Gustafsson et al. (2009), Li et al. (2007), Miller et al. (2009) |
| -Over time, low-SES children show progressively greater output of cortisol. |
Chen, Cohen, & Miller (2010) Evans et al. (2007) |
| -Adults who were abused or neglected in childhood have altered cortisol output in daily life. In most studies cortisol levels are reduced relative to non-exposed controls, though in some cases increased output has been described. | Heim et al. (2000), Luecken et al. (2004), Lupien et al. (2009), Meewisse et al. (2007), Miller et al. (2007), Troxel & Matthews (2004), van der Vegt et al. (2009) |
| -Prolonged exposure to altered cortisol output, with low or high, can facilitate inflammatory responding of macrophages. | Raison & Miller (2003), Sapolsky et al. (2000), Sternberg (2006) |
| -Children from low-SES families have greater urinary levels of the SNS hormonal end-product, epinephrine. | Evans & English (2002) |
| -Adults raised in low-SES families show increased expression of genes switched on by SNS endproducts, epinephrine and/or norepinephrine. | Miller et al. (2009) |
| - SNS endproducts accelerate departure of pro-inflammatory myeloid progenitors into circulation. | Avitsur et al. (2005), Engler et al. (2005), Katayama et al. (2006) |
| - Norepinephrine upregulates pro-inflammatory gene expression in monocytes. Catecholamines have mixed effects on cells engaged with microbial stimuli, in some cases enhancing production of inflammatory cytokines, but in others doing the opposite. | Cole et al. (2010), Grisanti et al., (2010), Gruber-Olipitz et al. (2004), Mohamed-Ali et al. (2001), Rontgen et al., 2004, von Patay et al. (1998) |
| -Maltreatment associated with lower heart-rate variability, an index of PNS regulation of cardiac rhythms. Some evidence these links persist into adulthood. | Dale et al. (2009), Miskovic et al., (2009), Oosterman et al. (2010), Shenk et al., (2010) |
| -Cholinergic signaling inhibits macrophage production of inflammatory cytokines in mice. In humans, markers of PNS activity associates inversely with CRP and IL6 production. | Haensel et al. (2008), Marsland et al. (2007), Pavlov & Tracey (2005) |
| -Oxytocin concentrations are lower in individuals who have experienced maltreatment relative to controls. | Fries et al. (2005), Heim et al. (2009) |
| -Oxytocin attenuates magnitude of in vivo inflammatory response to LPS in humans. | Clodi et al. (2008) |
| -Oxytocin reduces inflammation and atherosclerosis in mice at risk for CHD. | Nation et al. (2010), Szeto (2008) |
| 7. Inflammation accelerates pathogenesis of chronic diseases of aging. | |
| -Inflammation contributes to development of components of the metabolic syndrome. | Hotamisligil et al. (2006) |
| -Inflammation plays a role in each stage of atherosclerosis, the condition underlying myocardial infarctions and some strokes. | Libby & Theroux (2005) |
| -Inflammation contributes to growth and spread of some tumors. | Mantovani et al. (2008) |
| -Inflammation promotes a “frailty syndrome” marked by softening of bone, loss of muscle mass, strength and function, and a decline in cognitive functions. | Chung et al. (2009) |
Note. ACTH = adrenocorticotropic hormone; CRH = corticotropin releasing hormone; CRP = C-reactive protein; HPA = hypothalamic-pituitary-adrenocortical axis; IL-6 = interleukin-6; LPS = lipopolysaccharide; PNS = parasympathetic nervous system; SES = socioeconomic status; SNS = sympathetic nervous system.
The Social Climate of Adversity
There are many forms of childhood stress. But as we argued earlier, they often have overlapping features, and this is particularly true of disadvantage and maltreatment, the main foci of research in this literature. These features can include cold, unresponsive parents who use harsh discipline, routine exposure to conflict and violence, and limited access to material resources (Conger and Donnellan, 2007; Leventhal and Brooks-Gunn, 2000; Repetti et al., 2002). Here we briefly review evidence that disadvantage and maltreatment are characterized by these challenges.
Parenting
Lower SES parents often face multiple demands that affect the quantity and quality of time they can spend with their children. These parents often work multiple jobs with undesirable hours (Presser and Cox, 1997) and have to commute long distances to work (Osterman, 1991). Low SES parents often have little control over their work schedules (Marmot et al., 1997), which may limit their ability to be at home with their children. In addition, they may have little leeway to take time off without being fired (Albelda, 2001). At home, low-SES parents deal with frequent stressors because they are more likely to live in dilapidated housing (Evans, 2004). They are also more likely to be single parents (Garfinkel and McLanahan, 1986). As a result of these demands, low SES parents spend less time each day with their kids (Bradley et al., 2001), and provide them with less support (Dodge et al., 1994) and emotion socialization (McLoyd, 1998). Low SES parents are also more likely to have mental health problems of their own (Conger and Donnellan, 2007), reducing their ability to attend to their children’s needs. These types of family environments have been characterized as ‘cold’ and ‘neglectful’ (Repetti et al., 2002), although it is important to note that while the descriptive labels may be accurate, the behaviors they refer to often stem from the life circumstances that low SES families face, rather than representing intentional parenting styles.
Maltreatment is by definition a toxic relational environment (Cicchetti and Toth, 2005), though it can take a number of different forms. These manifestations include physical abuse, in which a child is injured by non-accidental means; sexual abuse, which involves sexual contact between a child and adult for the latter’s gratification; neglect, in which a child does not receive adequate care from his/her parental figures. A child can also be emotionally maltreated, when his/her basic affective needs go unmet by caregivers (Cicchetti and Toth, 2005). A sizeable minority of maltreated children are subjected to multiple forms of abuse and neglect (Arata et al., 2007; Dong et al., 2003).
Harshness, conflict, and violence
Lower SES families have more frequent conflict and poorer quality interactions compared to higher SES families (Conger and Elder, 1994). Low SES parents also discipline their children differently. They are more likely to use harsh strategies, like corporal punishment (Bradley et al., 2001; Dodge et al., 1994; McLoyd et al., 1994), and to impose demands on their children, without explaining why. Low-SES parents also tend to be inconsistent in their parenting, punishing sometimes but not others for the same offense (Conger and Donnellan, 2007; McLoyd, 1990). Finally, in low SES families conflict is more likely to escalate, resulting in marital violence or abusive behavior towards children (Crouch et al., 2000; Leventhal and Brooks-Gunn, 2000; Taylor et al., 2000). These patterns likely stem from the social constraints under which parents live (McLoyd, 1990). The multiple demands that low-SES parents face, and the reduced time and energy they have for their children, make it difficult for them to explain the reasoning behind every punishment, and to apply rules consistently. In addition, parenting approaches may also stem from different philosophies about what is best for children. Whereas high-SES parents often encourage independent thinking and questioning, lower SES parents often see obedience as critical (Adams, 1998; Kohn, 1977; McLoyd, 1990), reasoning that it helps insure that children avoid danger, do not stray into bad behaviors, or fall under the influence of delinquent peers.
Low-SES children are also exposed to more outside-the-home conflict and violence than their high-SES counterparts. Low-SES children are more likely to witness violence and personally experience it. By the time children reach their teenage years, 50% of low-SES children have witnessed at least one violent attack on another person, and 24% have themselves been the victim of a physical assault (Crouch et al., 2000). Rates of experiencing violence are about half that level among higher SES children (Crouch et al., 2000). Low-SES children also subjectively rate their neighborhoods as more stressful (Overstreet and Braun, 2000; Wright, 2006). For example, 50% of low SES children report concerns about being attacked in their neighborhood (Schubiner et al., 1993). Furthermore, low SES parents are more likely to keep their children indoors because of concerns about violence (Wright et al., 2004b), resulting in greater exposure to family conflict and other adverse features of home environment described above (Overstreet and Braun, 2000).
Maltreatment often entails harsh parenting, conflict and violence. In sexual and physical abuse, violence is by definition involved, or at least the threat of it. Children who are neglected are not necessarily treated violently by caregivers. However, neglect often co-occurs with other conditions, like alcohol and substance disorders, marital difficulties, and intimate partner violence, where the child witnesses conflict and aggression between others (Leonard and Eiden, 2007).
Resources
According to most theories, low SES is defined by limited access to material resources (Lynch and Kaplan, 2000). As Evans (2004) describes, this is particularly true for poor children. In comparison to their more advantaged peers, children from low-SES families are more likely to live in run-down housing, with poor quality water, heating, and sewage. Their homes tend to be noisy, crowded, and unsafe, as do the schools they attend and the neighborhoods in which they reside. Low-SES children have limited access to toys, books, computers, learning materials, and recreational activities in the community. Healthy food is also scarcer in low-SES households, due to its high costs and its absence in local markets. In sum, although most low-SES families residing in Western society today have their most basic needs for food and shelter met, they still face a good deal of material deprivation relative to the expected standards of modern industrialized nations.
Maltreatment and disadvantage co-occur far more often than expected by chance alone, meaning that many children who are abused or neglected will also lack material resources (Crouch et al., 2000; Leventhal and Brooks-Gunn, 2000; Taylor et al., 2000). And a central feature of neglect is the caregiver’s failure to meet basic needs like food, shelter, and emotional warmth for the child. All that said, not all maltreated children will experience deprivation. Particularly in cases of abuse, the child may have ready access to good housing, nutrition, resources, and activities.
Programming of Pro-Inflammatory Tendencies
The model’s central premise is that childhood stress gets embedded in the operating tendencies of the cells that regulate inflammation. It suggests that to the extent that children spend their early years in settings where parenting is harsh, conflict and violence are common, and material resources are scarce, their monocytes/macrophages will develop response tendencies that give rise to a chronic pro-inflammatory state. Because the stress occurs during a sensitive period when immune function is highly plastic, we further maintain that such tendencies become embedded in a durable manner. In the section that follows we review evidence for this proposition, focusing on human studies linking childhood stress and later inflammatory responding. Before turning to the evidence, however, we provide a brief primer on inflammation.
When bodily tissue has been injured by trauma or infected with a pathogen, the immune system launches an inflammatory response, which causes white blood cells to accumulate at the afflicted site. The major players in this response are cells of the innate immune system, principally neutrophils, dendritic cells, and macrophages. (Some of the macrophages will have been present in tissue already, whereas others will have been recruited from circulation, where they were residing in an immature form as monocytes. Upon migrating into tissue spaces monocytes differentiate into macrophages.) Together the innate cells work to eliminate the pathogen, rid the body of infected tissue, repair any damage the pathogen has caused, and begin the process of healing. These activities are coordinated by molecules called pro-inflammatory cytokines, which are released by damaged tissues and infiltrating immune cells. The most critical cytokines are interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). They have wide-ranging functions that include facilitating the migration of cells to sites of infection/injuries, signaling them to proliferate and differentiate, activating effector functions like killing and repair, and initiating systemic adaptations such as fever and changes in fluid balance.
The acute inflammatory response is essential for survival - without it, minor injuries or infections would become lethal. However, its duration and magnitude must be carefully regulated, so that it ends once the infection is cleared or the wound is healed. If this regulation does not take place or the stimulus itself persists, the inflammation can become chronic, maintained by macrophages caught in cytokine-mediated positive-feedback loops. (T-cells also frequently become involved in chronic inflammation.) To slow down inflammation, bodily tissues release a number of inhibitory molecules, many of which emanate from immune cells. However, an especially powerful strategy the body uses to regulate inflammation is to systemically release cortisol. (This happens when cytokines like IL-1β trigger the HPA axis to release cortisol.) In a classic negative feedback circuit, cortisol binds to glucocorticoid receptors (GR’s) located within macrophages and other immune cells, and slows down the inflammatory processes. Mechanistically, this happens because the ligated GR moves into a cell’s nucleus, and ties up a protein called nuclear factor-kappa B (NF-κB), a key part of the molecular machinery that switches on pro-inflammatory genes.
In recent decades, it has become evident that early life represents a period of marked plasticity for the immune system. Starting in utero and lasting through the early years of childhood, long-term patterns of immune responding are established by exposures to pathogens, allergens, and irritants (Finch and Crimmins, 2004; Prescott, 2006). These findings have fueled interest in whether the psychosocial environment might also have a role in calibrating the immune system’s response tendencies (Bilbo and Schwarz, 2009; Coe and Lubach, 2005; Coe and Lubach, 2007; Finch and Crimmins, 2004; McDade, 2005; Prescott, 2006; Wright, 2007). Our model builds on this work by positing that early stress acts as a programming agent that shapes the operating tendencies of monocytes/macrophages. This programming manifests in two ways: the cells show more pronounced inflammatory responses when exposed to challenge, and they are less able to terminate these responses because they have reduced sensitivity to inhibitory hormonal signals. As a consequence, persons exposed to early stress show chronic inflammation throughout life.
Increased microbial reactivity
The model’s assertions about childhood influences on long-term monocyte/macrophage responding are supported by recent studies. One study enrolled healthy adults aged 25–40 who were either low or high in childhood SES, as defined by the prestige of their parents’ occupations (Miller et al., 2009a). To prevent early and later SES from becoming confounded, the groups were balanced on the prestige of participants’ current occupations. Participants’ white blood cells were cultured in vitro with a series of microbial products, and the magnitude of the cell responses to these stimuli was indexed by subsequent production of IL-6, which is one of the primary cytokines that orchestrates inflammation. Those participants who had been reared in low-SES families showed more pronounced IL-6 production in response to flagellin, a bacterial product, and to poly I:C, a viral analogue, compared with participants from high-SES families. These associations persisted after adjustment for various demographics and biomedical confounds. And as noted above, the study’s design eliminated the possibility that participants’ current SES was responsible for these associations. These findings suggest that low childhood SES may program monocytes to have more pronounced inflammatory responses to certain microbial stimuli. (Monocytes are probably responsible for the observed disparities because they are present in human peripheral blood, whereas macrophages are only found in tissue. Moreover, monocytes are known to generate much of the IL-6 in cultures stimulated with flagellin and poly I:C.)
Other research has begun to trace the developmental trajectories of inflammatory responding as a function of variations in early stress. One project collected cord blood cells from newborns, cultured them with microbial products, and measured subsequent production of pro-inflammatory cytokines (Wright et al., 2010). During pregnancy the newborns’ mothers had been assessed for chronic stress, and these data were used to form a cumulative indicator reflecting persistent interpersonal, housing, and neighborhood problems. To the extent that their mothers were high in chronic stress prenatally, the newborns displayed greater production of TNF-α (following stimulation with a viral RNA analogue) and IL-8 (following stimulation with bacterial DNA). Another project from the same team measured stimulated cytokine production in 2 year-old children at risk for asthma (Wright et al., 2004a), and whose parents had completed bimonthly reports of perceived stress from the time of their birth. Children from families who reported higher cumulative stress produced more TNF-α following stimulation of their cells with allergic triggers. Since neither project followed participants in adulthood, it remains unclear whether the observed phenotype persists in the manner our model envisions. Nevertheless, the findings are provocative in suggesting that even very early stress may establish a trajectory of pro-inflammatory responding.
Effects of early stress were also observed in a recent project on trajectories of inflammatory responding in adolescents (Miller and Chen, 2010). 135 teenage females reported on the degree of parental harshness in their early years of life. Every six months for the next 1.5 years, blood was collected to assess monocyte IL-6 production following in vitro challenge with lipopolysaccharide (LPS), a component of gram-negative bacteria. To the extent that participants were reared in harsh families, they showed increasingly pronounced IL-6 responses over time. (That is, their cells seemed to become progressively more responsive to LPS as the study went on.) These associations persisted after adjustment for SES, demographic and biomedical characteristics, and depressed mood. Because these findings derive from prospective longitudinal analyses, they provide fairly strong evidence that family climate plays a role in establishing the monocyte response tendencies the model envisions. That said, because the follow-up period ended when participants were still in adolescence, the durability of the phenotype over the lifecourse remains unclear.
Resistance to inhibitory signals
The Embedding Model also specifies that childhood stress renders macrophages relatively insensitive to inhibitory hormonal signals. Two of the projects described above directly evaluated this hypothesis by examining the anti-inflammatory effects of cortisol. In the study of healthy adults who were low vs. high in childhood SES, Miller et al. (2009) also conducted genome-wide transcriptional profiling of peripheral blood mononuclear cells. This technology enabled them to quantify how “loudly” cortisol signals were being registered by the genomes of these cells. Levels of RNA for more than 18,000 transcripts were quantified. Bioinformatic techniques were then used to quantify the prevalence of transcription factor binding motifs in the promoters of genes that were differentially expressed according to childhood SES. These analyses revealed that among participants from low-SES families, there was significant upregulation of genes with response elements for the transcription factor NF-κB. As mentioned previously NF-κB is a key part of the molecular machinery that switches on pro-inflammatory genes. Participants from low-SES backgrounds also showed a significant downregulation of genes with response elements for GR. As noted above, one of GR’s key roles in the immune system is to transduce cortisol’s anti-inflammatory signals for cells; it does so by inhibiting the production and activation of NF-κB and molecules like it. These findings led the authors to conclude that early-life SES calibrates the sensitivity of GR in monocytes, such that children reared in disadvantaged environments become resistant to anti-inflammatory signals transmitted via this pathway. The consequence of a phenotypic adjustment like this would be to amplify and extend the inflammatory response through reciprocal, permissive activation of NF-κB.
In the study of early family climate in teenagers, Miller and Chen (2010) more directly assessed monocyte sensitivity to cortisol inhibition. As noted above, blood was drawn from the 135 participants four times over a 1.5-year period. On each occasion whole blood was cultured with the bacterial product LPS in the context of varying dosages of cortisol. Subsequent production of IL-6 was measured as a reflection of cellular sensitivity to cortisol inhibition. Analyses suggested an influence of family climate. To the extent that participants reported being raised in a harsh early family climate, they showed progressive desensitization to cortisol’s anti-inflammatory properties over the follow-up period. In other words the inflammatory response of their monocytes became increasingly resistant to inhibition by cortisol. These associations persisted following adjustment for SES, demographic and biomedical characteristics, and depressed mood. Again, because these findings derive from prospective longitudinal analyses, they provide fairly strong evidence that early stress can establish the kind of inflammatory phenotype the model proposes.
Consequences for chronic inflammation
Next, the model suggests that by programming these response tendencies into macrophages, childhood stress fosters a chronic inflammatory state in the body. There is mounting evidence to support this hypothesis, from studies that measure cytokines like IL-1β, IL-6, and TNF-α in circulation. However, because these proteins have short half-lives, research usually focuses on C-reactive protein (CRP) as a proxy. CRP is an acute phase protein released by the liver in response to IL-6. Though its precise biological activities are not well understood (Gabay and Kushner, 1999), CRP is widely used as a marker of inflammation (Ridker, 2003). It has a long half-life, can be detected at low levels, and values across the observed range are useful prognostically. For example, even in apparently healthy adults with normal CRP values, there is a dose-response relationship with CHD morbidity and mortality (Ridker and Cook, 2004).
Consistent with the model’s assumptions, there is a good deal of evidence showing that markers of inflammation are relatively elevated in persons exposed to childhood stress, even when they reach older ages. For example, in a study of 12,000+ US adults from diverse backgrounds, early-life SES was inversely associated with CRP independent of current SES (Pollitt et al., 2007). Similar patterning of inflammatory biomarkers by childhood SES has been observed in numerous other studies (Danese et al., 2009; Lawlor et al., 2005; Loucks et al., 2010; Phillips et al., 2009; Schreier and Chen, 2010; Tabassum et al., 2008), though not all (Gimeno et al., 2008; Miller et al., 2009a).
Research on family climate has also yielded evidence of a linkage between childhood adversity and subsequent inflammation. One project followed 972 members of a birth-cohort from Dunedin, NZ into adulthood (Danese et al., 2007; Danese et al., 2009), periodically gathering reports of maltreatment from subjects and their parents, and via behavioral observations. Analyses revealed that log-CRP values at age 32 were 3-fold higher in those who had vs. had not been maltreated in the first decade of life. Maltreatment was broadly defined in this project to include maternal rejection, harsh discipline, and caregiver changes, as well as abuse. Those who came from low-SES backgrounds, and were socially isolated as children, were also more likely to show high CRP, and these effects were independent of each other. The same group recently provided evidence that maltreatment’s link with inflammation emerges quite early in life. In that study, CRP was measured in 12-year olds whose family climate had been assessed repeatedly since birth. CRP values were nearly twice as high in maltreated vs. nonexposed children, but only if those in the former group also endorsed depressive symptoms (Danese et al., 2010).
Several recent studies have assessed inflammation in samples of adults who were asked to report on experiences of childhood adversity retrospectively. One study compared older adults who did vs. did not endorse being abused as children. The former group showed higher circulating IL-6 and TNF-α levels than the latter (Kiecolt-Glaser et al., 2011). Remarkably, these patterns were detectable even though some participants were dealing with a severe chronic stressor – caring for a family member with dementia – at the time their blood was collected for the assessment of inflammation. In another study, the authors formed a composite indicator of early-life adversity, based on respondents’ recollections of the quality of their relationships with parents, other stressors the family may have faced, and more traditional items that assess maltreatment (Slopen et al., 2010). Higher scores on the adversity composite were positively associated with four markers of inflammation: IL-6, fibrinogen, endothelial leukocyte adhesion molecule-1, and soluble intercellular adhesion molecule-1 (but not CRP). Interestingly, these associations were evident among African-American participants, but not those of European descent, a pattern the authors attributed to accelerated physiological aging brought on by institutionalized discrimination. Finally, another study used structural equation modeling to explicate the lifecourse pathways linking early adversity with later inflammation (Taylor et al., 2006b). Focusing on 3671 middle-aged adults in the CARDIA study, it reported that low SES in childhood was related to higher CRP in adulthood. Structural modeling was consistent with the hypothesis that low SES fostered a harsh family-of- origin environment, which in turn led to diminished psychosocial functioning in adulthood, and then to chronic low-grade inflammation as indexed by CRP. These findings are helpful in pointing to the kinds of lifecourse psychosocial trajectories that children reared in stress get set upon.
Conclusions
The model posits that childhood stress programs monocytes/macrophages to have pro-inflammatory tendencies. The evidence from studies of disadvantage and maltreatment is consistent with this notion. To the extent they have been exposed to childhood stress, adults display heightened cytokine responses to certain microbial stimuli, and are less sensitive to cortisol-mediated signals that shut down this process. Presumably as a consequence, these adults also display evidence of mild, chronic inflammation, as marked by CRP and IL-6 levels in circulation.
Adaptive immunity
Though our model focuses on how childhood stress affects the function of monocytes/macrophages, it is important to keep in mind that these cells do not operate in isolation. How they respond to stimuli has important consequences for the behavior of other leukocytes, particularly the T-and B-cells that orchestrate adaptive immune responses. These responses come into play when cells of the innate immune system cannot eradicate a pathogen from the body. They provide a more targeted and powerful response to the pathogen. Moreover, a subgroup of these cells is maintained in circulation as an immunologic memory of previously encountered pathogens, so that the next time the intruder is encountered a swifter and stronger response can be mounted.
There is much crosstalk between innate and adaptive immune cells. The details of these exchanges are beyond the scope of this review. However, it does bear noting that the chronic inflammatory state our model posits would likely have consequences for the way adaptive immune cells behave. In particular, there is mounting evidence that with persistent exposure to inflammatory cytokines, some routine functions that T-cells perform are partially suppressed (Baniyash, 2006). This has fostered speculation that by suppressing adaptive immune functions, chronic inflammation renders the host vulnerable to infection and tumor growth (Baniyash, 2006).
Consistent with this reasoning, there is abundant evidence from animal models that early-life stress exposure can disrupt adaptive immune functions (Coe and Lubach, 2005; Coe and Lubach, 2007). However, nearly all this work has assessed immune outcomes in childhood, so whether stress has the kinds of durable effects our model supposes remains unclear. Two relevant studies of humans have touched on this issue, though indirectly. For instance, in one study adults were exposed to rhinoviruses that cause the common cold, and quarantined for 5 days while illness symptoms were monitored (Cohen et al., 2004). To the extent they were reared in low-SES households, participants were more likely to become infected with the rhinovirus, and to develop symptoms that physicians judged as clinically significant. The study did not measure the functions of T-or B-cells directly. However, it is highly likely that SES exerted some of its influence by affecting the behavior of these cells, as they are integral to the immune system’s capacity to resist infection with rhinoviruses. Other indirect evidence for early-life influences on adaptive immunity comes from a recent study of maltreatment and herpes virus activity (Shirtcliff et al., 2009). This study assessed three groups of adolescents: those who had been reared in foreign orphanages, but later adopted into American families (earlier adversity), those who had experienced sustained maltreatment, but remained living with their families (ongoing adversity), and healthy control participants who were similar demographically. Saliva was collected repeatedly to assess levels of immunoglobulin A (sIgA) to the herpes-simplex 1 (HSV-1) virus. Levels of these antibodies rise when HSV-1, which is usually maintained in a latent state, begins to replicate within the oral cavity. This reactivation is often caused by impaired T-cell control of the virus and, if unchecked, can result in the formation of oral lesions known as cold sores (Glaser and Gottlieb-Stematsky, 1982). The results suggested that adversity has lingering influences on HSV-1 activity, as participants who had been exposed to either earlier or ongoing adversity had elevated virus-specific sIgA levels relative to healthy controls, even after the influence of various potential confounds was considered.
Both of these findings suggest that childhood stress may have lasting effects on adaptive immune functions. However, as we noted above this evidence is indirect, and must be corroborated with specific measures of T- and B-cell function before any definitive conclusions are made. That said, if durable influences of childhood stress are detected, it will be important to incorporate adaptive immunity into future versions of the model. In the meantime, these findings are important to keep in mind for another reason: They suggest that early stress enables viruses to replicate more extensively. If these infections become chronic, even at a low-level, they could function as a further stimulus for inflammation (Leinonen and Saikku, 2002; Shirtcliff et al., 2009).
Mechanisms of Embedding
Having shown childhood stress endows monocytes/macrophages with pro-inflammatory tendencies, we now turn to the question of how this programming occurs mechanistically. Three potential mechanisms are highlighted: epigenetic alterations to DNA, post-translational modification of proteins, and remodeling of tissue. Through these pathways early stress gets embedded in the cells, setting the bounds of how they operate going forwards. Note that the model does not suggest that monocytes/macrophages get locked into a functional setpoint from which they cannot deviate. Rather, it assigns early stress a role in setting the upper and lower bounds of how these cells will deal with challenge going forwards. Within these programmed boundaries, later experiences are assumed to fine-tune these operating tendencies to meet more immediate demands.
Epigenetic pathways
Epigenetics describes stable changes in the activity of a gene that arise without changes to its DNA sequence (Jirtle and Skinner, 2007; for a non-technical background on the how genes work, see Clark & Russell, 2005). (The absence of changes to the DNA sequence is what distinguishes epigenetics from polymorphisms and other forms of allelic variation that influence the activity of genes.) A primary function of epigenetic alterations is to allow cells to develop and maintain specialized functions. This is necessary because all the cells in a person’s genome have an identical DNA sequence, which is established at conception and fixed for life, save for acquired mutations. The DNA sequence serves as a blueprint for transcription, the process whereby cells synthesize RNA molecules. This process is also called “gene expression.” RNA molecules are later translated into proteins, which cells use for structural and functional purposes. Epigenetic alterations modify a cell’s ability to transcribe a particular gene into RNA. Because RNA serves as a template and catalyst for translation, these alterations have downstream influences on how much of the gene’s protein is ultimately synthesized. When this process takes place across many different genes, it can give rise to significant phenotypic diversity among cells of the body.
Epigenetic alterations occur in two main ways: methylation of the DNA itself or remodeling of the chromatin structure (Whitelaw and Garrick, 2006). In methylation, enzymes cause methyl groups to bind to cytosine residues, often in a stretch of the gene’s DNA called the promoter, which controls whether the gene is switched on. The methyl groups can prevent regulatory molecules from binding to the promoter, the effect of which is to either suppress or enhance the rate of transcription. Chromatin remodeling involves various chemicals attaching to (or detaching from) the histone proteins around which DNA is wrapped in the cell’s nucleus. These chemicals cause the DNA near the gene to become more or less tightly wrapped around the histones. This affects how easily regulatory molecules can access the promoter to initiate transcription. As is evident, both types of epigenetic alterations operate by changing the rate at which a particular gene is transcribed, and as a result, the amount of its mRNA and protein that will be available to the cell.
Over the course of development, the DNA of mammalian species undergoes several phases of epigenetic remodeling (Reik, 2007). This process enables the organism to modulate the activation of specific gene expression routines at developmentally appropriate junctures, i.e. to keep a gene silent until the time comes to build some tissue, and then switch it on briefly so that the task can be completed. It also enables cells that are initially pluripotent to mature into specialized phenotypes with divergent functions. Some of the epigenetic alterations that occur during the later stages of embryonic development appear to be mitotically and meiotically heritable (Richards, 2006). This means that they can be passed across generations of both somatic and germline cells, and potentially have long-term effects on the function of the cells in which they are embedded. The highly stable nature of these changes makes epigenetics a conceptually attractive mediating process for researchers focused on early-life influences on later health. They offer a solution to the problem of “incubation” we outlined at the outset of the article, i.e. how stress in early life can “get inside the body” in a manner that is sufficiently persistent to bring about disease many decades later.
Epigenetic alterations have become even more attractive to researchers in recent years, as evidence has mounted that they can be induced by some early-life physical and social exposures. For example, studies in animals have shown that perinatal exposures to cigarette smoke, vitamin B12, and folic acid can induce epigenetic alterations in metabolic processes that persist through the lifecourse (Jirtle and Skinner, 2007). In some cases these alterations contribute to phenotypic differences and vulnerability to disease (Dolinoy et al., 2007). A recent study found that human monozygotic twin pairs were epigenetically similar during early life, but showed diverging patterns of DNA methylation and histone acetylation as they aged (Fraga et al., 2005).
Most germane herein is a research program by Meaney and Szyf (Szyf et al., 2008). This work built upon a long line of studies showing that variation in maternal care produces permanent alterations in the offspring phenotype. Of particular interest to this audience, neonatal rodents who receive low maternal care in the first week of life have exaggerated cortisol responses to stress when they reach adulthood (see review by Levine, 2005). In studies aimed at elucidating the mechanisms underlying this pattern, Meaney’s group found that low maternal care attenuates expression of GR in the hippocampus (Liu et al., 1997). As noted earlier GR in this structure plays a key role in regulating cortisol output during stress. Using techniques from molecular biology, they later showed that DNA methylation and histone deacetylation in the GR gene were among the mechanisms responsible for this phenomenon (Weaver et al., 2004). These epigenetic modifications occurred along a stretch of DNA in the GR promoter where nerve growth factor inducible protein A (NGFIA) usually binds to initiate transcription. To verify the causal structure of these findings, the authors later performed cross-fostering studies, in which the offspring of low- or high-care dams were reared by “adoptive” mothers, who themselves had been identified as low-or high in maternal care. These studies yielded a similar pattern of findings, providing the basis for a strong causal inference that via epigenetic mechanisms, low maternal care in early-life can have long-lasting influences on hippocampal gene expression, which manifests in greater HPA axis reactivity to stress.
Two recent studies have extended these findings to humans. In one, hippocampal tissue from suicide victims was assessed post-mortem (McGowan et al., 2009). Greater methylation of GR’s exon 1F was detected in sections of those who had vs. had not been abused as children. (Exon 1F contains a promoter thought to be active in the hippocampus, and is the human homologue of the locus Meaney’s group studied in rodents.) These methylation differences appeared to be functionally significant, as abuse victims had less GR mRNA and less efficient NGFIA binding to the GR promoter. However, the methylation findings are difficult to reconcile with other studies of post-mortem hippocampal tissue, which have reported that exon 1F of the GR is completely unmethylated (Alt et al., 2010; Herbeck et al., 2010; Moser et al., 2007). Because the latter reports were post-mortem studies of other psychiatric conditions, it is possible that 1F methylation is a consequence unique to child abuse. This does not seem especially likely, however, as control brains in the abuse study had some methylation of IF. Until these discrepancies are resolved, it is difficult to know how to interpret these epigenetic findings on childhood abuse.
The other study of humans assessed maternal mood during pregnancy and its association with offspring GR methylation (Oberlander et al., 2008). To the extent they were exposed to depressed/anxious mood states in the 3rd trimester of pregnancy, newborn children tended to show greater methylation of GR exon IF at a predicted binding site for NGFIA, the same locus identified in the rodent studies by Meaney and colleagues. Methylation at this locus was in turn related to greater cortisol reactivity to stress at 3 months of age, suggesting that it may have functional implications for HPA axis regulation. These findings are provocative in suggesting that in utero exposure to dysphoria can potentially influence the epigenetic landscape of the offspring’s GR promoter, and do so in a manner that is similar to the effects seen in tightly controlled studies of animals. That being said, these results are somewhat difficult to interpret because DNA methylation was assessed in cord blood, and it is unknown how well readouts in these cells would map onto those taken in hippocampal neurons. Also, the fact that methylation analyses were done in bulk pools of cord blood cells introduces some potential alternative explanations. Specifically, it is known that (a) there are individual differences in the balance of cell types that comprise the cord blood population, (b) low mood and life stress are known to shift that balance, at least in the peripheral blood cell pool, and (c) the degree of methylation at a site often varies from one cell type to another (Herbert and Cohen, 1993; Ohgane et al., 2008; Segerstrom and Miller, 2004). Thus, the observed associations could be driven by mood-related variations in the balance of cell types analyzed, rather than methylation per se. Follow-up studies that assess these relations in isolated cell populations, or control statistically for variations in cell type balance, are needed to sort out this issue.
Together, these studies are provocative in suggesting that epigenetic markings can operate as a mechanism through which early stress gets programmed into the genome of certain tissues in a lasting way. From the perspective of our model it is particularly noteworthy that epigenetic modifications to GR have been found, because this protein conveys the anti-inflammatory signals of cortisol to monocytes and macrophages. That being said, the issues raised above complicate interpretation of the human findings. Moreover, these data come from neural tissue and cord blood. Given that epigenetic markings are often tissue-specific (Ohgane et al., 2008), similar patterns in monocyte/macrophages cannot be assumed. Nonetheless, studies have found that epigenetic processes are active in cells of the immune system, and have a role in regulating expression of genes that orchestrate inflammation, including GR and NF-κB (Vanden Berghe et al., 2006). Research is now needed to determine whether exposure to childhood stress influences these processes.
Post-translation modifications
After protein molecules have been synthesized, they often undergo chemical alterations, known as post-translation modifications (PTMs), that expand their functional repertoire. PTMs occur when a molecule attaches to one or more of the amino acids that comprise the protein. Dozens of different molecules can attach to a target, the most common of which are salts like phosphate and proteins like ubiquitin. These additions can modify a protein’s binding capacity, change its ability to pass through membranes, accelerate its degradation, or recruit new molecules towards it. PTM’s can have a marked impact on cellular behavior by altering the dynamics of higher-level processes like signal transduction, transcriptional efficiency, DNA replication, and cell cycle progression (Hochstrasser, 2009). (The chromatin remodeling process outlined earlier, whereby chemicals attach to the histones that package DNA, and in doing so affect how tightly it is coiled, is an example of a PTM catalyzed by acetyl or methyl groups).
Research indicates that a number of the molecules relevant to the arguments made here are subject to PTM’s, including GR, NF-κB, and receptors for other hormones released during stress (Galliher-Beckley and Cidlowski, 2009; Tobin et al., 2008; Vanden Berghe et al., 2006). For instance, the attachment of phosphate groups to specific amino acid residues on the GR has a marked influence on the efficiency of cortisol-mediated signaling (Barnes, 2004; Pace et al., 2007). Recall that after cortisol is released into circulation, it must bind to GRs in the cytosol of target tissues. The ligand-receptor complex then undergoes a complex series of changes in structure, after which it can migrate into the cell’s nucleus to regulate gene expression (Sternberg, 2006). Studies in human cell lines have shown that phosphorylation of one GR residue, Serine 203, can block the later stages of this process by inhibiting nuclear migration (Galliher-Beckley and Cidlowski, 2009). This means that even when cortisol levels rise in a tissue, this signal would not necessarily be propagated into the genomes of its cells. In other words, the cells would become unresponsive to signals they receive from cortisol. As noted, a pattern like this is seen in persons exposed to childhood stress, who fail to show appropriate cortisol-mediated suppression of inflammatory cytokine production (Miller and Chen, 2010; Miller et al., 2009a). PTMs like Serine 203 phosphorylation could mediate such effects.
There is indirect evidence from animal models suggestive of a role for PTMs. In one study, rat pups were removed from their cages and handled for 15 minutes daily over the first week of life, (Fenoglio et al., 2006b). This procedure elicits high levels of care from the pups’ dams (in the form of licking, grooming, and arched-backed nursing) and as a consequence dampens their cortisol reactivity to future stressors in adulthood (Levine, 2005). This particular study aimed to elucidate the behavioral and molecular mediators of this effect. It found that relative to a non-disturbed control condition, handling reduced mRNA for corticotropin releasing hormone (CRH) in the paraventricular nucleus (PVN). CRH released from this region instigates HPA axis activity. The study also found that handling reduced the degree of phosphorylation of extracellular-signal regulated kinase, a transcription factor that plays a key role in initiating expression of the CRH gene. These findings are provocative in suggesting a role for PTM’s in programming the effects of early handling into the molecular machinery that drives the HPA axis. Whether similar dynamics occur in monocytes/macrophages remains unclear, but research of this nature is needed.
A question that arises about the role of PTMs is how they can be maintained over time. Most cells in the body, with exception of those in the CNS, have fast rates of turnover. And even in stable tissues like the CNS, proteins usually degrade within weeks-months. So how might a PTM induced by early stress get embedded in cells in a lasting manner? The most plausible answer to this question is that PTMs initiate positive-feedback loops in cells that become self-sustaining. For example, cytokine exposure can induce phosphorylation of some GR residues in white blood cells (Pace et al., 2007). As noted above PTMs of this nature have the capacity to render cells functionally unresponsive to cortisol-mediated signaling. Because cortisol is one of the body’s primary agents for regulating inflammation, the downstream consequence of this PTM would likely be to facilitate cytokine release. This process would induce more GR phosphorylation, and presumably the cycle would begin again (Miller et al., 2009b). Moreover, because cytokines can have paracrine influences – i.e., effects on cells that are nearby – this cycle of inflammation and phosphorylation could spread.
Tissue remodeling
The other plausible mechanism through which early stress could become embedded in monocytes/macrophages is tissue remodeling. Mounting evidence suggests that in some bodily tissues, exposures during sensitive periods of development can trigger a local restructuring process, and do so in a manner with lasting functional implications. Effects of this nature have been repeatedly documented in studies of airway disease (Prescott, 2006). This work suggests that exposure to noxious stimuli early in life, such as cigarette smoke, viral infections, and air pollution, can damage the epithelial lining of the airways. These injuries trigger a local immune response aimed at repairing the damage. However, in some cases the immune response overshoots, which causes scarring beneath the epithelium, thickening of the walls of the airway, and the growth of new blood vessels. These changes not only reduce the person’s resting lung function, but also render his/her airway more reactive to future stimuli (see review by Prescott, 2006).
There have been a handful of studies with animals showing that early stress can trigger remodeling of neural tissues. In one study rats were briefly separated from their mothers daily for the first two weeks of life, using the handling procedure known to increase maternal licking and grooming and which subsequently dampens stress responding in adult offspring (Swinny et al., 2009). When offspring reached early adolescence, brain sections were collected from the CNS region that houses the locus coeruleus (LC), a structure that plays a key role in regulating autonomic responses to stress. Compared to undisturbed controls, the rats handled in early life had less extensive dendritic branching, and shorter dendrites in general. And when those rats’ LC slices were treated with CRH (a hormone that initiates HPA and autonomic responses to stress) their firing rate did not increase significantly. Another study used this paradigm to explore the morphology of CRH-releasing neurons (Korosi et al., 2010). Results indicated that handled rats had lower PVN CRH during adolescence than controls. This was in part due to a handling-induced reduction in excitatory glutamatergic innervation of the PVN. Together, these findings are provocative in suggesting that early social context can, to some degree, shape the architecture of the developing hypothalamus, with implications for how neurons in this structure propagate stress-related signals.
Besides shaping the architecture of circuits within the brain itself, childhood stress could also influence functional connections between the nervous system and the lymphoid organs where immune cells mature and/or respond to microbes. Through the fibers of the autonomic nervous system (ANS), the brain has connections with almost every organ in the periphery. And recent studies indicate that stress itself can modify the density of these connections. One study assigned rhesus macaques to 39 weeks of social interactions with a peer group that was either constant or changed daily (Sloan et al., 2007). At the end of the manipulation, macaques in unstable conditions had significantly greater SNS innervation of the parenchyma of axillary lymphoid nodes, a change that allowed more stress-induced norepinephrine signal to reach these structures. To evaluate the functional significance of this change, the authors infected animals with simian immunodeficiency virus (SIV), a monkey analogue of the pathogen that causes AIDS in humans. They found that SIV replicated more rapidly at lymph node sites adjacent to the new SNS fibers. These findings suggest that stress can “rewire” functional connections between the nervous and immune systems (Cole, 2009), and in doing so modify the host’s capacity to resist the progression of a serious infection.
One kind of remodeling that could be especially relevant here involves sympathetic innervation of bone marrow. The bone marrow is where myeloid progenitor cells are born, and mature into monocytes prior to being released into circulation. Recent studies show that signals emanating from SNS neurons, in the form of norepinephrine, can accelerate the departure of myeloid progenitors from bone marrow (Katayama et al., 2006). In mice, stress has been shown to mimic these effects (Engler et al., 2004). Notably, the monocytes that egress from bone marrow under stress tend to have exaggerated pro-inflammatory cytokine responses upon exposure to LPS, and be relatively insensitive to inhibition by glucocorticoids (Avitsur et al., 2005; Engler et al., 2005). Together, these findings suggest a plausible remodeling scenario, wherein childhood stress boosts the density of SNS fibers entering the bone marrow, allowing more norepinephrine signal to reach myeloid progenitors there. These signals favor the migration of specific progenitor subtypes into circulation, which are disposed towards aggressive, prolonged inflammatory responding.
Childhood Adversity Engenders Behavioral Tendencies that Accentuate Inflammation
The second major premise of our model is that childhood stress engenders behavioral proclivities that persist across the lifecourse, and accentuate the pro-inflammatory tendencies that have been programmed into monocytes/macrophages. This process serves to perpetuate and exacerbate chronic inflammation. Specifically, we argue that early stress fosters vigilance for threat, mistrust of others, and poor self-regulation of appetitive behaviors. Mechanistically, this occurs because stress molds the corticolimbic circuits that process threat and the corticostriatal pathways that support self-regulation. Below we review evidence for these propositions.
Corticolimbic circuitry, vigilance, and mistrust
The model suggests that by molding the response tendencies of the brain’s corticolimbic circuitry, childhood stress will engender vigilance for threat and mistrust of others. These tendencies will render people more likely to read threat into ambiguous situations, be more sensitive to recognizing and responding to anger, and be cynical about others’ intentions and behaviors. Over the long term, these traits will give rise to abrasive social exchanges and make it difficult to garner support from others. As noted above, the Risky Families Model envisions a similar cascade, wherein harsh family climates undermine children’s social competence and emotion-regulation skills, and in doing so predispose them to interpersonal difficulties (Repetti et al., 2002). Here we identify antecedents to the constructs featured in the Risky Families model by proposing that deficits in emotion regulation and social competence stem from heightened vigilance for, and appraisal of, threat. We also build on recent discoveries from functional neuroimaging to suggest that the neural basis of these effects resides in disrupted communication between the amygdala and the prefrontal cortex (PFC).
Though multiple brain systems are involved in processing threat, research on its neural underpinnings has emphasized the role of corticolimbic circuits. Of special relevance within these circuits is the amygdala. This cell complex plays a role in evaluating the biological significance and emotional salience of stimuli, shaping the way ambiguous information is processed, and organizing biobehavioral responses to situations perceived as threatening (Davis and Whalen, 2001; Phelps, 2006; Whalen et al., 2001). Also of importance here are regions like the ventromedial (vmPFC) and ventrolateral (vlPFC) prefrontal cortices, which exert top-down influences on the amygdala and the messages it exchanges with output centers elsewhere in the brain (Price and Drevets, 2010).
The amygdala is comprised of multiple reciprocally connected nuclei (Davis and Whalen, 2001; Ernst and Fudge, 2009). The lateral, basal, and accessory nuclei receive most of the amygdala’s input. Visual, auditory, and olfactory stimuli registered in the sensory cortices are transferred to these nuclei via excitatory glutamatergic projections. Input about the biological relevance and emotional salience of stimuli arrives from regions of the PFC and limbic structures like the hippocampus. These signals are aggregated and integrated in cell groups acting under the influence of GABA, norepinephrine, dopamine, serotonin, acetylcholine, and glucocorticoids (Rodrigues et al., 2009). These molecules “gate” the delivery of signals to the amygdala’s major output regions, the medial and central nuclei. Ultimately, these cell groups project to brainstem circuits involved in movement, to striatal circuits that process reward, and to PFC regions that mediate appraisal, self-regulatory efforts, decision-making, and other processes. Most critically for the discussion here, cell groups in the amygdala project to hypothalamic centers that regulate outflow from the ANS, the HPA axis, and other neuroendocrine circuitry. By altering patterns of outflow from these systems, the amygdala shapes the hormonal milieu in which monocytes/macrophages operate in the periphery. As we discuss later, this has implications for inflammation.
Research suggests that corticolimbic circuits undergo significant maturation in the early postnatal years and are subject to both experience-expectant (species typical) and experience-dependent (species atypical) modulation (Leppanen and Nelson, 2009). There is structural variation in the timing and duration of this process, with nuclei in the amygdala maturing in childhood and prefrontal regions continuing through adolescence and early stages of adulthood (Ernst and Fudge, 2009). Given this developmental plasticity, researchers have speculated that the long-term structure and function of these circuits could be shaped by stress in the early years of life, via the kinds of epigenetic and remodeling processes discussed earlier (Cicchetti and Blender, 2006; Pollak, 2005).
Consistent with this view, recent studies have linked childhood stress to patterns of corticolimbic activity in adulthood. For example, one study had college students perform an emotion-matching task in the scanner (Gianaros et al., 2008). Participants had to match a target face, which was either angry, surprised, or neutral, with a face at the bottom of the monitor. Early adversity was indexed by subjective reports of childhood SES, using a standard measure that depicts parental social status on a 10-rung ladder (Goodman et al., 2001). Analyses revealed an inverse linear gradient such that, to the extent that participants were raised in a low-SES household, they had greater amygdala activity when matching the angry faces in particular, presumably reflecting heightened sensitivity to threatening emotional information. This association persisted after adjustment for participants’ current SES, neuroticism, and distress, consistent with the hypothesis that the response tendencies of amygdalar circuitry is shaped, to some degree, by childhood SES.
Another study examined whether being reared in a harsh family context was associated with neural responses to a similar task (Taylor et al., 2006a). In a within-subjects design, adults viewed faces bearing negative emotions, and depending on the trial were asked to assign emotion labels, assign gender labels, or merely observe. During the observe condition those from harsh families had lower amygdala reactivity, which the authors hypothesized was a result of habituation to, or detachment from, negative emotional stimuli. Early family climate was unrelated to amygdala reactivity during the emotion- labeling condition. It was also unrelated to activity in the right vlPFC, a structure typically engaged by this task, and which can exert top-down effects on the amygdala. However, follow-up analyses of connectivity between these structures revealed disparities related to family climate. Among participants reared in warmer families, there was a negative correlation between amygdala and vlPFC activity. This relationship was strongly positive among persons from harsher families, a pattern the authors suggest is indicative of a failure to effectively recruit the relevant areas for top-down regulation of the amygdala.
Vigilance for threat
Behaviorally, the model proposes that in molding corticolimbic circuitry, childhood adversity engenders heightened threat vigilance. Consistent with this view, research shows that both disadvantage and maltreatment are associated with greater vigilance. Pollak (2008) argues that physically abused children develop increased perceptual sensitivity for anger, which leads them to identify this emotion more readily and rapidly in others’ faces, even when limited contextual information is available to support these judgments (Pollak and Sinha, 2002). This account have been consistently supported in studies using a variety of paradigms to capture different features of emotional processing. Specifically, physically abused youngsters have selective attention for angry facial displays (Pollak, 2008), and are slower to disengage from them than unexposed controls (Pollak and Tolley-Schell, 2003). They also more readily identify anger in faces with ambiguous expressions (Pollak and Kistler, 2002) and in situations where there is minimal supporting perceptual information (Pollak and Sinha, 2002). Physically abused children also orient their attention towards others’ conflictual interactions more sothan controls (Pollak et al., 2005).
In a similar vein, Chen has argued that because children from low SES backgrounds are frequently exposed to life events that are both uncontrollable and at times unpredictable, they come to view the world as a threat-laden place that requires high levels of vigilance (Chen et al., 2004). This belief makes them more likely to appraise situations as threatening, particularly when the outcomes are ambiguous (i.e., when it is unclear whether another person is intentionally acting negatively). This hypothesis has been evaluated by having adolescents watch a series of videos with outcomes that are ambiguous (e.g., a sales clerk paying close attention to a shopper). During subsequent interviews about the storyline, consistent SES differences in interpretation emerge (Chen and Matthews, 2003; Chen et al., 2003; Chen et al., 2004; Chen et al., 2006). Participants from low-SES families read more threat into the situation (e.g., “she thinks I’m about to steal something”) than do those from high-SES families (e.g., “she is trying to be helpful and make a sale”). Chen argues that this appraisal style reflects an underlying vigilance, wherein low-SES children closely monitor their world for danger, and maintain a low threshold for judging situations as threatening.
Because all the findings from Chen’s work come from studies of adolescents, it remains unclear whether the appraisal tendencies persist through the lifecourse in the manner proposed by our model. To resolve this issue, we had 246 adults (mean age = 45.3, SD = 6.6) watch the videos and report on their parent’s educational attainment. A robust SES gradient was apparent. To the extent that they were raised in less educated households, middle-aged adults made more threatening appraisals of the ambiguous situations [for omnibus effect, F(4,241) = 4.63, p < .001; for linear trend, contrast = .69, SE = .21, p = .001]. Because social class tends to be fairly stable from childhood to adulthood, one alternative explanation is that any differences in appraisal tendencies are rooted in participants’ current SES, and not their level of early disadvantage. However, a re-analysis controlling for current familial SES (indexed by the highest degree of educational attainment) suggests this is not the case. Even with the covariate included in the model, the disparities related to early SES persisted, F(4,240) = 2.93, p = .02. These findings suggest that appraisal tendencies are shaped by people’s early SES and persist in a fairly stable fashion across the lifespan.
Mistrust of others
The model also proposes that children reared in stress will develop and maintain negative beliefs about others. There is much evidence to support this proposition. In large-scale studies across multiple countries, individuals who come from lower SES backgrounds are more likely to endorse hostile beliefs (e.g., believing that others will take advantage of them and mistreat them if they can; (Barefoot et al., 1991; Lynch et al., 1997; Scherwitz et al., 1991). This is not only true among adults. Even in children SES and hostility are inversely related, suggesting that these beliefs are established early in life (Gump et al., 1999). These patterns can even be seen at the neighborhood level: in communities that are more deprived of material resources, greater proportions of residents endorse the view that others cannot be trusted (one component of social capital) (Drukker et al., 2003; Kawachi et al., 1999). Taken together, these findings suggest that living in an environment with few resources promotes mistrust in others.
Influences on social interactions
The model suggests that vigilance and mistrust form the starting point of a self-promoting cycle that culminates in poor social relationships. The idea here is that these traits lead people to perceive and engage their social worlds in a manner that brings about conflict and rejection, and renders them less able to receive support and warmth from others. This pattern of exchanges serves to perpetuate the traits themselves, as people come to view their beliefs about others as justified by experience. Those beliefs provide the fuel for more abrasive encounters and, over the long-term, make it difficult for individuals to develop close social ties.
These notions come out of models of children’s social-information processing (Crick and Dodge, 1994). Such models posit a cyclical process, wherein appraisals shape behavior towards others, and in so doing elicit particular responses, which themselves shape future appraisals. For example, when children attribute hostile intent to others, they are more likely to respond aggressively, and by doing so cause others to escalate (Dodge & Pettit, 2003). The escalation is then taken as proof that the initial appraisal was accurate. The notion that individual styles and social interactions are inextricably and reciprocally linked is found in other theoretical perspectives as well. It is a central tenet of interpersonal approaches to personality (Kiesler, 1996) and the literature on continuities and consequences of interaction styles (Caspi et al., 1989).
Consequences for social relationships
We suggest that as a result of the way they engage the social world, persons exposed to childhood stress will have trouble developing and maintaining high-quality social bonds. As noted a similar assumption lies at the core of the Risky Families Model (Repetti et al., 2002). Consistent with both frameworks, research shows that children who are reared in harsher families have smaller social networks, more conflict with family and friends, and perceive less social support (Repetti et al., 2002). These effects persist across the lifecourse. One study followed youth prospectively from kindergarten through adolescence, and reported that over twelve years, children who had been maltreated in early life were twice as likely to develop major social problems as their peers (Lansford et al., 2002). Another study measured childhood SES in 539 medical students (Graves et al., 1998). More than 30years later the quality of their social networks was assessed. Individuals who came from lower SES households or lost a parent in childhood were less engaged with community organizations at midlife. Moreover, those who reported less closeness in their family of origin while in medical school reported at midlife that they had fewer friends and family whom they could “feel at ease with, can talk to about private matters, or can call for help (p. 1333).” Of course, these findings could reflect the underlying influence of neuroticism, or other individual differences that shape feelings about (or reporting on) social ties. But these findings are nonetheless consistent with the broader argument being made here, and in several other parallel literatures, that children’s early familial experiences have long-lasting effects on their capacity to form bonds with others (Bowlby, 1969; Cassidy and Shaver, 2008; Reis et al., 2000).
Consequences for inflammation
Finally, the model suggests that vigilance and mistrust, and the social problems they create, will accentuate the tendencies programmed into monocytes/macrophages, and thereby amplify chronic inflammation. There is a good deal of evidence to support the proposed linkages in this chain-of-events, though no studies have woven them all together in a single dataset. For example, many studies have focused on the construct of hostility, a core feature of which is cynical mistrust of other people. To the extent that healthy young adults endorse the trait of hostility, their monocytes produce larger volumes of pro-inflammatory cytokines following ex vivo LPS stimulation (Suarez et al., 2002; Suarez et al., 2004). Hostility is also positively related to CRP and IL-6 in circulation, suggesting that it is accompanied by mild, chronic inflammation (Graham et al., 2006; Marsland et al., 2008). Moving to social interactions, there is evidence that acute bouts of marital conflict increase levels of IL-6 and TNF-α in circulation, which are still evident 24 hours later (Kiecolt-Glaser et al., 2005). Research suggests that when abrasive social interactions occur regularly, they have longer-lasting effects on inflammation. For example, to the extent that adolescents experience difficult interactions with others, they show higher levels of CRP (Fuligni et al., 2009), and increased expression of NF-κB (Miller et al., 2009), the transcription factor that switches on genes that regulate inflammation. (Presumably, abrasive social interactions contribute to worsening inflammation through an allostatic process, wherein macrophages are attempting to restore balance after repeated exposure to conflict-related autonomic and endocrine discharge). Finally, if a pattern of abrasive interactions renders the individual socially isolated, this state might also amplify pro-inflammatory dynamics. Social network diversity is inversely associated with CRP and IL-6 in circulation, particularly in men (Loucks et al., 2006a; Loucks et al., 2006b). Subjective reports of social isolation, as reflected in loneliness, are also related to elevated CRP. Moreover, microarray profiling of leukocytes shows that loneliness is associated with same pattern of pro-inflammatory genomic activity as childhood stress. This profile is marked by upregulation of genes with response elements for NF-κB and downregulation of genes with response elements for GR (Cole et al., 2007).
Conclusions
The studies reviewed in this section are consistent with the predictions in the Embedding Model. Their findings suggest that children exposed to stress mature into adults with altered corticolimbic responsivity to emotional stimuli. These individuals also tend to be vigilant for threat and mistrusting of others. They engage others in a manner that leads to abrasive exchanges and makes it difficult to garner social support from others. They have persistent difficulties forming and keeping close social ties. These proclivities accentuate the tendencies already programmed into monocytes/macrophages, further contributing to the chronic inflammatory state in the body. (As noted earlier, this is possible because early stress is not assumed to “lock” cells at a permanent setpoint. Rather, early stress calibrates the parameters of how cells deal with challenge going forwards, with later experiences fine-tuning these operating tendencies.) Despite the plethora of support for the model’s predictions, it is important to remember that all of the relevant empirical evidence has been established in separate literatures. To determine whether the constructs are linked in the causal fashion our model suggests, future research that makes use of multi-method, longitudinal strategies will be necessary.
Corticostriatal circuitry and appetitive behaviors
The model postulates that childhood stress also shapes the functioning of the neural circuitry that underlies self-regulation of appetitive behaviors. This shaping process gives rise to a phenotype that highly discounts the future, behaves in an impulsive fashion, and readily seeks out appetitive stimuli. As a consequence, the individual has a propensity for engaging in health-compromising behaviors, like smoking cigarettes, eating high-fat foods, avoiding physical activity, and drinking excess alcohol.
There is mounting evidence to implicate the brain’s corticostriatal circuitry in the cognitive and behavioral elements of these appetitive tendencies (Kable and Glimcher, 2009; Somerville and Casey, 2010). Studies in animal models and patients with brain lesions, as well as functional neuroimaging paradigms, point to a key role for corticostriatal circuitries in mediating a variety of processes involved in the self-regulation of appetitive behavior. They include encoding motivationally relevant stimuli, judging their reward value, weighing alternative courses of action,, plotting a strategy for stimulus pursuit and, finally, directing motor regions to implement the plan (Haber and Knutson, 2010; Kable and Glimcher, 2009). The corticostriatal circuitry is comprised of anatomical and functional connections between regions of the prefrontal cortex (PFC) and the ventral striatum. Of particular importance is the nucleus accumbens (NAcc), a structure within the ventral striatum ascribed a key role in guiding behavior towards motivationally relevant stimuli. The NAcc receives excitatory glutamatergic inputs from multiple PFC regions and limbic structures such as the amygdala and hippocampus. Cell groups within the NAcc integrate these messages through dopamine signaling. In essence, dopamine functions to modulate the influence of other brain regions on the NAcc; it does so by differentially suppressing versus enhancing synaptic activity in cell groups activated by excitatory glutamatergic inputs. After integrating messages, cell groups in the NAcc can relay information back to the PFC via palidal-thalamic loops, and/or direct signals to the hindbrain structures that control motoric activity (Ernst and Fudge, 2009; Grace et al., 2007). In this way, the NAcc serves as an interface between cortical, limbic, and motoric regions, helping the organism select and realize behavioral priorities (Floresco, 2007).
Ontogenic studies have found that corticostriatal circuits undergo significant maturation during childhood (Ernst and Fudge, 2009; Somerville and Casey, 2010). There appears to be variation across structures in the onset and length of this period, with maturation of striatal and limbic regions occurring through late childhood and early adolescence. By contrast, maturation of the PFC is thought to continue well into the early stages of adulthood as mentioned previously.
Building on evidence of developmental plasticity in these circuits, a recent functional neuroimaging study examined whether early stress might have long-term effects on corticostriatal reward processing (Gianaros et al., in press). Seventy-six adults engaged in a monetary gain/loss paradigm in the scanner. To index childhood SES, the participants were queried about their parents’ educational attainment and occupations, and these data were used to stratify the sample into those reared in high- and low- SES families. Analyses revealed that childhood SES was associated with cortical activity during monetary reward processing. During reward processing, those from lower childhood SES backgrounds exhibited reduced activity in the lateral and dorsomedial PFC, as well as the perigenual anterior cingulate and inferior parietal cortex. There was also evidence of reduced functional connectivity between the dorsomedial PFC and the left ventral striatum among adults from low-SES backgrounds. These associations persisted following adjustment for participants’ own SES. Interestingly, childhood SES was not associated with neural responses to monetary loss, or directly related to activity in ventral striatal circuits during either gain or loss trials. In interpreting these results the authors suggest that early stress may bias maturational processes in these structures in a fashion that leads to “ineffective top-down regulation of limbic and forebrain circuits driving reward-processing in later life (p. 22).” These are provocative, albeit preliminary, findings. If replicated in future research, they could provide major insights about how and why early stress shapes behavioral proclivities around reward processing.
Manifestation in impulsivity
The model posits that by molding the corticostriatal circuitry, early stress brings about a tendency for impulsive behavior. There is mounting evidence to support this view. One study of 743 adults found inverse relationships between childhood SES and temporal discounting of monetary rewards (Sweitzer et al., 2008). To the extent that their parents had lower education, participants preferred smaller immediate over larger postponed rewards. These effects persisted following adjustment for the participants’ own educational attainment. Similar patterns emerged in three studies of college students, which linked temporal discounting with childhood disadvantage, particularly under conditions of mortality threat (Griskevicius et al., 2011). Other studies have also found evidence of temporal discounting in low-SES individuals, in parallel with greater cognitive impulsivity, marked by a failure to plan ahead, a present-minded orientation, and brief time horizons (de Wit et al., 2007; McLoyd, 1998; White et al., 1994). However, much of the latter research has focused on concurrent associations between these constructs, rather than testing whether early stress has lasting effects of impulsivity.
Implications for health practices
Consistent with the model’s chain-of-events concept, there is robust evidence that preferences for present over future rewards have implications for health behaviors. Children who are more future-oriented are less likely to use drugs and alcohol (Robbins and Bryan, 2004; Wills et al., 2001). Conversely, adolescents with higher impulsivity are more likely to smoke and drink alcohol (Robbins and Bryan, 2004). Across numerous samples of adolescents and young adults, present time perspective likewise predicts more frequent alcohol, drug, and tobacco use (Keough et al., 1999). Furthermore, this present focus partially explains disparate health behaviors across the SES gradient (Adams and White, 2009; Wardle and Steptoe, 2003).
In addition, there is evidence that childhood stress can have long-lasting effects on unhealthy lifestyle choices. Studies have found that across multiple countries, low childhood SES predicts a greater risk of current and persistent smoking, a lower probability of quitting smoking, and increased risk of obesity in adult women, even after controlling for current SES (Jefferis et al., 2004; Power et al., 2005a). Low childhood SES also is associated with more episodes of drunkenness, decreased physical activity, and poorer diets in adulthood (Lynch et al., 1997). In a large birth cohort study, Poulton and colleagues found that to the extent that individuals were raised in low-SES households, at the age of 26, they displayed higher body mass index and great waist: hip ratios, and were more likely to have a history of alcohol dependence (Poulton et al., 2002). These disparities were independent of the respondents’ achieved SES in adulthood. Similar patterns are evident for early life adversity defined by maltreatment. For example, the ACE study assessed maltreatment retrospectively in 9500+ adult members of an HMO. In nearly a dose-response fashion, childhood adversity was associated with cigarette smoking, severe obesity, infrequent exercise, alcohol dependence, and illicit drug use (Felitti et al., 1998).
Consequences for inflammation
The model proposes that unhealthy lifestyles amplify the chronic inflammation found in persons exposed to childhood stress. Consistent with this view, studies have found that smokers display low-grade inflammatory activity, which is still present 10–20 years after quitting (Yanbaeva et al., 2007). Diets that are high in fats and sugar also promote inflammation (Yudkin et al., 2001). There are also intriguing suggestions that diet and stress may act synergistically, together fostering more pronounced inflammation than either does alone (Kiecolt-Glaser, 2010). Excess weight is also associated with inflammation (Hotamisligil, 2006). NF-κB is upregulated in the monocytes of overweight individuals, suggesting these cells are primed for pro-inflammatory responding (Ghanim et al., 2004). Adipose tissue itself is one of the body’s major reservoir of inflammatory mediators, accounting for almost 1/3 of the IL-6 that is present in circulation (Mohamed-Ali et al., 1997). By contrast, regular exercise can reduce inflammation, and do so even when the individual’s weight remains stable (Handschin and Spiegelman, 2008).
Conclusions
The studies reviewed are consistent with the propositions in the model. There is initial evidence to suggest that adults who come from low-SES families show altered corticostriatal responses to appetitive stimuli. There also seems to be a lingering impulsivity in adults exposed to childhood adversity, marked by tendencies to discount the future and pursue immediate gratification. One of the implications of this style is that individuals develop unhealthy lifestyles, characterized by smoking, poor diet, physical inactivity, and obesity, which are likely to persist over the lifecourse and contribute to a pro-inflammatory environment. All of that said, most of these strands of evidence have emerged from separate literatures, so it remains to be seen whether they are connected in a manner that the model specifies.
Childhood Adversity Engenders a Pro-Inflammatory Hormonal Milieu
The model’s last major premise is that childhood stress has an enduring influence on everyday patterns of autonomic and endocrine discharge. As a consequence the tonic release patterns of various hormones, transmitters, and peptides is dysregulated, consigning monocytes/macrophages to operate in a milieu that accentuates their pro-inflammatory tendencies. Below we discuss five signaling molecules that could plausibly be involved in such dynamics – the adrenal steroid cortisol, the ANS neurotransmitters epinephrine, norepinephrine, and acetylcholine, and the hypothalamic peptide oxytocin. We focus on these molecules for two reasons, the first being that stress can modulate their activity. But more importantly, each has a cognate receptor in/on macrophages, which allows it to regulate the magnitude of these cells’ inflammatory responses to challenge. 2
Cortisol
The model posits that childhood stress has a durable influence on HPA activity, causing dysregulated patterns of cortisol output through the lifecourse. There is mounting evidence to support this assertion. For example, a handful of studies have monitored adults’ diurnal cortisol output for brief epochs of 1–3 days, and examined whether it associates with their childhood SES (Gustafsson et al., 2009; Li et al., 2007; Miller et al., 2009a). The results of these studies are consistent with the notion that being reared in a low-SES household leaves a long-lasting imprint on tonic HPA axis activity, which is manifest in greater diurnal cortisol output, though there is some inconsistency across studies about which time of day (s) this is most pronounced. More definitive evidence comes from a recent multiwave prospective study that followed teenagers over two years (Chen et al., 2010a). Every six months they collected salivary cortisol 4 times daily over two days. To the extent that their families were low in SES, participants displayed increasing daily cortisol output over the five waves of follow-up. These findings provide prospective evidence that low SES precedes, and possibly instigates, age-related increases in daily output of cortisol over two years.
Assuming low childhood SES does heighten cortisol output in a lasting manner, what might be the downstream consequences for inflammatory responding? At first blush one might expect the result to be salutary, because cortisol generally suppresses inflammation (Sternberg, 2006). However, as noted above low childhood SES seems to desensitize monocytes to cortisol’s anti-inflammatory properties (Miller and Chen, 2010). This causes the genomes of these cells to “hear” less cortisol signal than would be expected on the basis of hormonal availability (Miller et al., 2009a). As a result of this dampened cortisol-mediated signaling, pro-inflammatory transcription factors like NF-κB are reciprocally activated. This phenomenon illustrates how elevated cortisol output and achronic inflammatory state could occur simultaneously.
But how a child exposed to severe stress might arrive at this juncture is less clear. One plausible hypothesis is that stress initially activates the HPA axis. But with persistent exposure to high levels of cortisol over time, bodily tissues mount a counter-regulatory response of the kind envisioned by allostatic load models (McEwen, 1998). This would involve downregulation of GR, the receptor that binds cortisol under most conditions (Fries et al., 2005b; Miller et al., 2007). If this downregulation occurred in the hippocampal centers that regulate HPA outflow, it might enable cortisol to partially escape negative feedback inhibition, and lead to the relative increase in cortisol seen in persons from low-SES families. A similar dynamic in cells of the immune system would diminish GR’s capacity to inhibit NF-κB, as well as other pro-inflammatory transcriptional-control pathways. The result would be that an allostatic maneuver intended to restore balance (McEwen, 1998) accentuates the pro-inflammatory tendencies already present in monocytes/macrophages.
In addition to the work on SES, there is a large corpus of research that examines whether childhood maltreatment has lasting influences on patterns of cortisol release. However, much of this work is difficult to interpret for our purposes, because the influences of earlier maltreatment and current psychopathology cannot be separated (Heim et al., 2008). A handful of studies have sought to isolate the role of maltreatment by studying adults without current mental illness. In these samples 24-hour urinary cortisol is usually lower in persons with histories of childhood maltreatment versus non-exposed controls (see reviews by Heim et al., 2000; Meewisse et al., 2007; Miller et al., 2007). Lower cortisol output has also been found in studies of other potent childhood stressors, like parental conflict, loss, and divorce (Luecken and Lemery, 2004; Lupien et al., 2009; Troxel and Matthews, 2004). On the other hand, some work has linked maltreatment with greater cortisol output, as it is in the research on low SES. Probably the most ambitious research in this area collected salivary cortisol from 623 adults who had been adopted internationally as children (van der Vegt et al., 2009). To obtain accurate reports, participants’ adoptive parents were asked whether they believed their child had been subjected to maltreatment prior to adoption, based on his/her physical condition and any documentation parents had received from authorities. Those who allegedly had been abused or neglected prior to adoption had greater overall cortisol output, relative to those without a history of maltreatment. The largest disparities were among persons who had been both abused and neglected. Importantly, these findings persisted after adjustment for age, gender, and psychiatric difficulties, suggesting they stemmed from the effects of early stress per se.
The mixed nature of these findings makes it difficult to speculate about consequences for inflammation. If maltreatment does cause the HPA axis to become hypoactive (Heim et al., 2000), it might create a lymphoid milieu that is permissive of inflammation (Raison and Miller, 2003; Sapolsky et al., 2000). By contrast, a relative excess of cortisol in maltreated individuals might be expected to counter inflammatory tendencies. All of that said, even if a uniform and durable HPA response to maltreatment could be established, its consequences would hinge on how sensitive the GR’s of monocytes/macrophages were to cortisol, and how efficiently signals through this pathway were transmitted to the genome. In other words, the consequences for inflammation are likely to hinge more on how “loudly” cells register cortisol signals than the amount of hormone released per se. To clarify these issues, future studies could perform genome-wide transcriptional profiling in maltreated populations. As described above, this technique has proven quite useful in studies of SES, revealing much about how cells process cortisol signals and the implications for inflammation.
Catecholamines
The model also suggests that childhood stress has a durable influence on the SNS, resulting in greater tonic release of its catecholamine end-products, epinephrine and norepinephrine. Surprisingly, this hypothesis has received little direct attention over the years (Matthews and Gallo, 2011). Some of the best work available comes from a study of rural youth in New York, which collected overnight urine samples to measure cumulative output of epinephrine and norepinephrine. This study found higher levels of epinephrine among children whose family incomes were below the poverty line versus in the middle-class range (Evans and English, 2002). There were no disparities in norepinephrine. A portion of this sample was followed over the next 3–4 years. The primary question was whether cumulative adversity (a composite of poverty, family turmoil, crowding, poor housing, etc) was associated with accelerated progression of allostatic load over time (an index of dysregulation in multiple physiologic systems). Results suggested a prospective linear relationship between these processes. Children who had more cumulative risk at study entry showed larger increases in allostatic load scores over the follow-up, of which epinephrine and norepinephrine were constituents (Evans et al., 2007). There are a handful of studies reporting higher tonic epinephrine and norepinephrine in adults with a history of childhood maltreatment (Bunevicius et al., 2005; De Bellis et al., 1999; Girdler et al., 2003). However, in much of this work the participants have comorbid psychiatric conditions at the time of assessment, which make it difficult to ascertain whether the SNS dysregulation stems from earlier maltreatment or current symptomatology. (To be fair, parsing these influences was not the goal of the authors.)
Other relevant evidence comes from the study of childhood SES that performed transcriptional profiling of peripheral blood mononuclear cells of adults (Miller et al., 2009a). This study found that among participants reared in low-SES households, there was significant upregulation of genes with response elements for cyclic AMP response element binding (CREB) protein. Because one of CREB’s actions of is to convey adrenergic signals to the genome of white blood cells, it can be viewed as a rough marker of tissue exposure to SNS endproducts like epinephrine and norepinephrine. Accordingly, these findings suggest that the circulating immune cells of individuals raised in low-SES households have greater in vivo exposure to catecholamines, even in adulthood.
What consequences would increased catecholamine signaling have for inflammation? Recall from the section on “Tissue Remodeling” that SNS fibers innervate bone marrow, and via norepinephrine accelerate the departure of myeloid progenitors into circulation (Katayama et al., 2006). The monocytes that egress under these conditions have exaggerated pro-inflammatory cytokine responses to LPS and are relatively insensitive to inhibition by glucocorticoids (Avitsur et al., 2005; Engler et al., 2005). Monocytes continue to be sensitive to SNS products after entering circulation. In fact, norepinephrine upregulates the expression of a variety of pro-inflammatory genes in monocytes (Cole et al., 2010; Gruber-Olipitz et al., 2004), most of which code for proteins involved in activation and trafficking. Increased expression of these genes facilitates the migration of monocytes into tissues that have been injured or infected. The findings on cytokine activity are more complex. Exposure to SNS products causes IL-6 to be released in greater quantities (Mohamed-Ali et al., 2001), but has mixed effects on cells engaged with microbial stimuli. Some papers report that in cells cultured with LPS, co-incubation with catecholamines enhances inflammatory cytokine production (Grisanti et al., 2010; von Patay et al., 1998). However, others find the opposite effect (Rontgen et al., 2004). That said, the general patterns suggest that a bodily milieu rich in SNS endproducts would contribute to chronic inflammation, partly by selectively mobilizing aggressive monocytes from bone marrow into damaged tissue, and partly by shifting cellular activation patterns in these contexts (Kaplanski et al., 2003).
Cholinergic Activity
Childhood stress might also have ongoing effects on the immune milieu by shaping cholinergic discharge from the parasympathetic nervous system (PNS). Maltreatment has been linked to lower heart-rate variability (HRV), both at rest and during stress (Miskovic et al., 2009; Oosterman et al., 2010). HRV is viewed as an index of PNS regulation of cardiac rhythms via the vagus nerve, whose signals are propagated by acetylcholine. There is initial evidence that maltreatment-related alterations in HRV can persist into adulthood (Dale et al., 2009; Shenk et al., 2010). Reduced HRV has also been found in studies of low-SES adults (Hemingway et al., 2005; Lampert et al., 2005), but it is unclear whether childhood SES has a parallel association.
If these patterns are indicative of a more general reduction in cholinergic signaling, they could affect how inflammation is regulated. Tracey and colleagues have described a “cholinergic anti-inflammatory pathway” in rodents (Pavlov and Tracey, 2005), whereby acetylcholine inhibits production of inflammatory cytokines via ligation of the α7 subunit of nicotinic receptors. Studies have attempted to generalize these findings to humans by using HRV as a surrogate for cholinergic signaling. Resting HRV does associate inversely with CRP, IL-6, and other inflammatory markers (Haensel et al., 2008). Moreover, at least one study has linked higher vagal activity during paced respiration with lower stimulated production of IL-6 and TNF-α (Marsland et al., 2007). However, it remains unclear whether these associations reflect operation of a “cholinergic anti-inflammatory pathway” in humans, or are being driven by a different underlying mechanism altogether.
Oxytocin
Oxytocin (OT) is another molecule through which childhood stress could shape ongoing inflammatory dynamics, as well as some of their long-term disease consequences. OT is a hypothalamic neuropeptide implicated in a broad array of interpersonal processes, including affiliation, nurturant behavior, social cognition, emotion recognition, and feelings of love, trust, and security (Ross and Young, 2009). There is mounting evidence to suggest that OT levels in peripheral circulation rise when people experience feelings of warmth, trust, and security (Grewen et al., 2005; Light et al., 2005). These are precisely the states that our model suggests are missing in the internal lives of persons reared in stress. (Others have also speculated that oxytocin has a role in mediating the effects of childhood stress; see Cameron et al., 2005; Pollak, 2005).
Consistent with this line of reasoning, a handful of recent studies have found deficits in OT release and/or signaling among persons exposed to stress. For example, one recent project measured OT in the cerebrospinal fluid of adult women (Heim et al., 2009). Those with a history of childhood maltreatment had lower OT levels than non-exposed controls, particularly if they experienced emotional abuse. Another study measured the OT responses of five-year old children as they engaged in a brief physical contact task with their mothers (Fries et al., 2005a). Half of the participants had spent their first year of life in an orphanage, after which they were adopted into the family caring for them at the time of assessment. The others had been reared by their biologic mothers. Analyses revealed that following the physical contact period with mothers, orphanage-reared children had lower urinary OT than family-reared controls. The groups had similar “baseline” OT levels in daily life, as reflected in samples from overnight urine collections. These findings are provocative in suggesting that adversity in the very early years of life dampens the OT response to warm interactions. If such a tendency influences children’s proclivity for affiliative behavior, it could help explain the persistent social difficulties those reared in adversity experience.
More central to our model, reduced OT in lymphoid compartments could foster a milieu permissive of inflammation. OT is recognized as having anti-inflammatory properties. For example, a recent study treated adults with the bacterial component LPS and found that peripheral OT administration significantly ameliorated the magnitude of their in vivo inflammatory responses (Clodi et al., 2008). OT likewise reduced inflammation and retarded the progression of atherosclerosis in mice at risk for CHD due to social isolation (Nation et al., 2010; Szeto et al., 2008). There is also initial evidence that OT counteracts some of the pathogenic processes inflammation sets into motion. For example, studies in animal models have revealed that peripheral OT treatment reduces obesity, facilitates glucose control, and improves insulin sensitivity (Camerino, 2009), all of which are components of the metabolic syndrome that precedes and contributes to CHD.
Conclusions
As the model posits, there is mounting evidence that childhood stress has a durable influence on everyday patterns of endocrine and autonomic discharge. This results in altered quantities of cortisol, epinephrine, norepinephrine, and oxytocin in circulation. (And presuming that HRV accurately indexes PNS activity, stress affects bioavailable acetylcholine, too.) There is also convincing evidence that these molecules have regulatory influences on inflammation, mediated through effects on the distribution and activation of monocytes/macrophages. However, these strands of evidence have emerged from separate literatures, so it remains to be seen whether they are connected in a manner that the model specifies.
Inflammation and Disease
The model’s final aspect posits that chronic inflammation, acting in concert with the host’s genetics and history of exposures, drives forward pathogenic mechanisms that result in disease. Over the last decade evidence for this assertion has grown dramatically. Scientists now recognize excessive and persistent inflammation as a contributor to the metabolic syndrome, CHD and stroke, autoimmune conditions, some cancers, and premature aging (Chung et al., 2009).
The metabolic syndrome is a cluster of features that includes high blood pressure, impaired glucose control, abdominal adiposity, and lipid dysregulation (Program, 2002). Its presence markedly increases the risk of morbidity and mortality due to both diabetes mellitus and coronary disease (Isomaa et al., 2001; Ridker et al., 2003; Trevisan et al., 1998). While the root causes of the metabolic syndrome are obesity, inactivity, and genetics, recent evidence suggests that inflammation plays a key pathogenic role as well (Hotamisligil, 2006). Persons with high levels of CRP show increases over time in metabolic syndrome components (Dandona et al., 2005) and are more likely to progress from this condition into formal diseases like hypertension and Type 2 diabetes (Bertoni et al., 2010; Pradhan et al., 2001; Sesso et al., 2003; Wang et al., 2007). These effects are probably bidirectional in nature, with inflammation one feature of a complex and cyclical process that leads to the metabolic syndrome and its components.
There is increasing recognition that atherosclerosis, the pathological condition underlying CHD and many ischemic strokes, is a chronic inflammatory response to arterial injuries (Libby and Theroux, 2005; Ross, 1999). Inflammation contributes to each stage of the atherosclerotic process, from the growth of fatty streaks to the formation of full-blown plaques, and from the erosion and rupture of plaques to the formation of thrombi that occlude arteries and ultimately trigger CVD events (Libby et al., 2002). Because the inflammatory response is orchestrated by cytokines, studies have examined whether their presence forecasts disease. This work has shown that high levels of circulating inflammatory molecules, particularly IL-6 and CRP, confer risk for adverse outcomes through each stage of atherosclerosis. For instance, these markers have been prospectively associated with more rapid thickening of the carotid arteries; increased risk for myocardial infarction and cerebral vascular accidents in healthy persons; elevated rates of mortality in patients with acute coronary syndromes; and greater risk for all-cause mortality (Blake and Ridker, 2003; Cesari et al., 2003; Hashimoto et al., 2001; Kuo et al., 2005; Lindmark et al., 2001; Pradhan et al., 2002; Ridker et al., 1997; Ridker et al., 2000a; Ridker et al., 2000b; Willerson and Ridker, 2004).
Chronic inflammation contributes to the development and expression of other aging-related diseases. Autoimmune conditions like multiple sclerosis and rheumatoid arthritis arise when immune cells attack the body’s own tissues, after having lost tolerance to them or mistaking them for those of invaders. T-and B-cells have long been assigned blame for these conditions. However, it is becoming increasingly clear that chronic inflammation driven by macrophages and neutrophils also contributes to maintaining disease activity (Choy and Panayi, 2001; McGonagle and McDermott, 2006). Cancer is a diverse set of diseases that involve abnormal cell proliferation, and each has a distinct mechanism of pathogenesis. But mounting evidence indicates that inflammation plays a contributory role in many forms of cancer. Following a carcinogenic event that gives rise to an abnormally proliferating cell mass, inflammation can accelerate the tumor’s progression and metastasis to other tissues. Much of inflammation’s influence seems to be due to its tendency to foster a local environment that is favorable for tumor growth and spread. It helps the tumor cell divide rapidly, gain access to a blood supply, subvert detection by the immune system, and migrate to new tissues (Coussens and Werb, 2002; Mantovani et al., 2008). Finally, inflammation is increasingly recognized as a major cause of premature aging. Evidence suggests that it promotes a “frailty syndrome” marked by bone softening, loss of muscle mass, strength and function, and a decline in cognitive functions (Chung et al., 2009; Ershler and Keller, 2000).
Limitations of the Model
Having outlined evidence for the model, we now consider its weaknesses. One limitation is that although the model suggests that childhood stress calibrates monocytes/macrophages during a sensitive period, it fails to specify when this window opens and closes. The extant research does not provide much guidance in this regard. The SES literature typically indexes exposure with indicators like parental education or occupation. Because these markers of status generally remain stable across childhood, they cannot be used to differentiate the impact of early vs. later exposures. The experience of maltreatment also tends to be fairly stable, precluding researchers from identifying sensitive periods. That said, two recent studies have begun to shed light on issues of timing. In both cases, the studies indexed SES by having respondents report on whether their parents owned vs. rented their homes each year, and found evidence to suggest that years 2–3 of life were a sensitive period for stress-related calibration of immune responses (Cohen et al., 2004; Miller & Chen, 2007). These findings provide some initial evidence that the toddler years are a sensitive period for stress-related influences on the immune response. Substantiating these findings should be an important priority for future research, as should examining how well they generalize to other processes depicted in the model.
Another weakness of the model is that it depicts unidirectional relations among its components. The ANS and HPA axis engage in much crosstalk, and both readily exchange signals with inflammatory cells (Sternberg, 2006). Pro-inflammatory cytokines released peripherally can act on the CNS, and thereby evoke significant changes in behavior, as well as alterations in cognitive and affective functioning (Dantzer et al., 2008). Revisions of the model will need to feature this crosstalk, and specify its role in linking early stress and later disease. Relatedly, there may be value in considering a multi-system approach here. Some authors have argued that stress contributes to disease by creating “imbalances” amongst systems, rather than affecting any singular biological process (McEwen & Stellar, 1993; Thayer & Sternberg, 2006). Our model takes the opposite perspective, treating inflammation as a mechanistic superhighway. We see value in this approach because it calls attention to a pathogenic mechanism that is both affected by early stress and common across multiple diseases of aging. However, other biological mediators almost certainly come into play during the evolution of disease, and it will be important for later versions of the model to specify the degree to which they are necessary and/or sufficient.
Notably, the model does not acknowledge a host of physical exposures that could mediate the effects of early-life stress. Being raised in a low-SES family boosts the chances a child will be exposed to pollutants, toxicants, and infections with long-term health consequences (Evans, 2004; Wright and Subramanian, 2007), and at least in America, receive suboptimal access to medical care. Maltreated children are likely to have these exposures as well, because their parents are unlikely to be proactive about health promotion. Thus, to fully account for the effects of early stress, future work must consider social and physical “pollutants.” Work like this has begun and shows that these exposures can have synergistic influences on disease risks (Chen et al., 2008; Clougherty et al., 2007; Shankardass et al., 2009). More work on how social and physical exposures intersect, and interact, in shaping health outcomes should be an important priority in future research.
The model also fails to consider hereditary influences. There is allelic variation in many of the genes that orchestrate inflammation, regulate autonomic and endocrine discharge, and affect corticolimbic and corticostriatal signaling (Cole et al., 2010; DeRijk and de, 2005; McCaffery et al., 2006). There is also growing evidence that such variation explains variability in how early stress affects mental health (Belsky and Pluess, 2009; Binder et al., 2008; Bradley et al., 2008; Caspi et al., 2002; Gillespie et al., 2009). As this work extends to processes more relevant to physical health (Poole et al., 2006; Wang et al., 2009), the influences of genetic variation will need to be added to the model. But at present such moderating influences are outside the scope of our framework.
Some readers may wonder why the model does not assign a more prominent role to psychopathology. Of particular relevance in this regard is depression. Early stressors like maltreatment increase vulnerability to major depression, as well as sub-syndromal affective difficulties (Heim and Nemeroff, 2001). Moreover, depression is sometimes accompanied by processes featured in our model, like chronic inflammation, excessive HPA discharge, and social difficulties (Coyne, 1976; Glassman and Miller, 2007; Howren et al., 2009; Stetler and Miller, in press). Despite this overlap, we do not view depression as a necessary step on the causal pathway from early stress to later disease. There is much evidence that even without lowering mood, early stress can trigger the model’s behavioral and biological features. For example, one study found that familial harshness presaged trajectories of inflammatory responding (Miller and Chen, 2010), and did so in a manner that was completely independent of depressive symptoms. Similarly, work from the Dunedin birth-cohort found that at age 32 clinical depression was associated with greater inflammation. However, further analysis revealed that this association disappeared when childhood maltreatment was entered into equations. The authors then stratified the sample according to maltreatment (presence vs. absence) and depression (presence/absence). Participants who were maltreated and depressed had higher inflammation, relative to non-exposed controls. There was also heightened inflammation among participants exposed to maltreatment alone. However, levels of inflammation in participants who were depressed but had not been maltreated were statistically indistinguishable from controls (Danese et al., 2008). These patterns were echoed in the 40-year study of medical students from Johns Hopkins (Kittleson et al., 2006), which found that low childhood SES presaged CHD risk at age 50, net of depressive symptoms. In other studies like ACE, the effects of childhood adversity were attenuated, but not eliminated, by controls for depressed mood (Dong et al., 2004). Together, these findings suggest that childhood stress brings about inflammation, and perhaps disease, through mechanisms largely independent of depression.
Of course, other mood states and/or psychiatric conditions could be important mediators here. Consistent with this view, several papers have suggested a role for broader clusters of negative emotions (Lehman et al., 2005; Lehman et al., 2009). However, structural equation models have revealed that such clusters provide little incremental explanatory power beyond a model that does not include them (Lehman et al., 2009). In other papers, negative emotions have formed part of a broader “Psychosocial Functioning” construct that includes social contacts, so it is difficult to ascertain their specific contribution (Lehman et al., 2005; Taylor et al., 2006b). In the broader literature, there is fairly limited evidence for mood as a mediator of the health effects of SES (Matthews and Gallo, 2011) All of that said, a fairly narrow range of affective mediators has been considered to date, and the field would benefit from a more thorough look at other candidate states (e.g., shame, anxiety) and disorders (e.g., post-traumatic stress, antisocial personality).
Finally, the model does not address the issue of resilience, or why some individuals exposed to early stress remain healthy. Data show that even among children with lengthy and severe maltreatment, only a fraction go on to develop chronic disease. In the ACE cohort, for example, only 20% of those in the most profound adversity category went on to develop CHD as adults (Dong et al., 2004). Of course, this value could be low because not all respondents were in the age range in which CHD manifests, and the condition has a fairly low base rate. But even under conditions where all participants are exposed to a known disease-causing agent, the same pattern of resilience emerges. For instance, in the study where adults were exposed to viruses that cause the common cold, participants from low-SES backgrounds were more likely to become sick (Cohen et al., 2004). But even among those from the lowest SES category, fewer than 50% actually manifested diagnosable symptoms of disease, suggesting that resilience was the normative outcome.
Thus, a crucial task for the next wave of research in this area will be to specify why some individuals succumb but others are protected from the health consequences of early stress. There are hints from several recent articles that maternal nurturance may be an especially influential source of resilience, capable of offsetting some of the risky hormonal, metabolic, inflammatory, and cardiovascular profiles that tend to develop in persons exposed to childhood adversity (Chen et al., in press; Evans et al., 2007; Fisher et al., 2000; Luecken and Appelhans, 2006; Miller et al., 2011). Certain genetic variants also seem to confer protection against stress as well (Belsky and Pluess, 2009; Binder et al., 2008; Bradley et al., 2008; Caspi et al., 2002; Gillespie et al., 2009). As work like this matures our model will need to be refined, so that it offers pathways to both resilience and vulnerability (Chen and Miller, 2011; Cicchetti and Blender, 2006).
Apart from the limitations of the model itself, it is important to recognize weaknesses in our assessment of it. Despite the fact that children are exposed to many different kinds of chronic stressors, our evaluation of the model was based only on studies of disadvantage and maltreatment. We made the assumption that these experiences shared enough common features that they could be aggregated under the rubric of chronic stress. There were many instances where this proved to be a tenable assumption – people who were exposed to either stressor early in life tended to become adults who were vigilant, mistrusting, and social isolated. They also tended to have poor health practices, high levels of inflammation, and be at risk for CHD. But there were cases where the consequences of these experiences diverged, particularly in studies of HPA axis activity. It will be important in future research to more thoroughly evaluate the similarities and differences between these stressors, and simultaneously identify the biobehavioral residue of other adversities that children face more routinely but have not been the subject of much research (e.g., chronic parental conflict, severe illness in the family). Once more data like these become available, they can be used to evaluate the model more rigorously, and guide any necessary revisions to it.
Conclusions
Despite these weaknesses, the model has some important strengths. At the outset we suggested that to be successful, the model would need to specify mechanisms and explain incubation. We believe that it does so, at least in broad strokes. It suggests a cascade of events through which early stress “gets under the skin” at the level of tissues and organs. These events culminate in mild but persistent inflammation, which in concert with the host’s genetic makeup give rise to adult chronic diseases. It also suggests a plausible mechanistic resolution to the “incubation” problem, highlighting the ability of epigenetic processes, post-translational modifications, and tissue remodeling to bring about lasting changes in the operation of monocytes/macrophages. In addressing these issues, the model builds upon existing theories, and moves us closer to a mechanistic understanding of the effects of childhood stress.
However, we are still far away from a thorough understanding of this phenomenon. In the coming decade more research will need to be done, so that we can fill gaps in the model, discern how its elements relate to each other, and evaluate the chain of events it posits. This work will need to be done at multiple levels of analysis, ranging from the molecular and the physiological to the behavioral and the epidemiological, and then integrated into a model by scholars working at the crossroads of these disciplines. Studies of both animals and humans will be needed, and so will a diverse array of approaches, ranging from laboratory paradigms to longitudinal investigations in the field. Once data like these are in hand, we will be better positioned to construct detailed accounts of how childhood stress “gets under the skin,” and use this information to guide the development of interventions that ameliorate the deleterious health consequences of early stress.
Figure 1.
The Biological Embedding of Childhood Adversity Model. ACTH = adrenocorticotropic hormone; α7nAChr = α7 subunit of nicotinic acetylcholine receptor; β2AR = β2 adrenergic receptor; GR = glucocorticoid receptor; OR = oxytocin receptor.
Acknowledgments
Preparation of this article was supported by grants from the National Institute of Child Health and Human Development (HD058502) and the National Heart, Lung, and Blood Institute (HL073975). We are grateful to Steve Cole, Stan Floresco, and Pete Gianaros for critical feedback on the ideas presented in this manuscript.
Footnotes
Inflammation is the preliminary immunologic response to injuries and infections. It occurs when white blood cells accumulate at the site of damage, and attempt to eliminate the pathogen and/or repair any tissue that has been damaged. Some of the major cellular players in the inflammatory response are monocytes and macrophages. Monocytes are the immature form of these cells when they reside in the bloodstream. Once monocytes migrate into tissue, they differentiate into macrophages. The inflammatory response is essential for survival - without it, minor injuries or infections would become lethal. However, its duration and magnitude must be carefully regulated, otherwise the inflammation can become persistent, and contribute to emergence of multiple diseases of aging. For a nontechnical background on inflammation, readers should consult Sompayrac (2008).
Note that in this section, we limit discussion to molecules that are released into circulation, and once there could plausibly ligate receptors in/on monocytes/macrophages. For this reason, we do not cover studies of end-organ response, e.g., heart rate and blood pressure, which are sometimes used as surrogates for ANS activity. We also limit our focus here to studies of tonic activity, as these everyday patterns give a “readout” of the milieu in which immune cells typically operate. Reactivity is by definition a departure from the norm. For literature reviews of the evidence on adversity and reactivity, readers can seek out papers by Cameron et al. (2005), Luecken and Lemery (2004), and Lupien et al. (2009).
The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bul
Contributor Information
Gregory E. Miller, Department of Psychology, University of British Columbia
Edith Chen, Department of Psychology, University of British Columbia.
Karen J. Parker, Department of Psychiatry and Behavioral Sciences, Stanford University
References
- Ackerman SH, Hofer MA, Weiner H. Age at maternal separation and gastric erosion susceptibility in the rat. Psychosomatic Medicine. 1975;37(2):180–184. doi: 10.1097/00006842-197503000-00007. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1079604. [DOI] [PubMed] [Google Scholar]
- Ackerman SH, Hofer MA, Weiner H. Early maternal separation increases gastric ulcer risk in rats by producing a latent thermoregulatory disturbance. Science. 1978;201(4353):373–376. doi: 10.1126/science.566471. [DOI] [PubMed] [Google Scholar]
- Adams BN. The family: A sociological interpretation. New York, NY: Harcourt Brace; 1998. [Google Scholar]
- Adams J, White M. Time perspective in socioeconomic inequalities in smoking and body mass index. Health Psychology. 2009;28(1):83–90. doi: 10.1037/0278-6133.28.1.83. [DOI] [PubMed] [Google Scholar]
- Ader R. Social factors affecting emotionality and resistance to disease in animals: III. Early weaning and susceptibility to gastric ulcers in the rat. A control for nutritional factors. Journal of Comparative and Physiological Psychology. 1962;55:600–602. doi: 10.1037/h0041164. [DOI] [PubMed] [Google Scholar]
- Ader R, Friedman SB. Social factors affecting emotionality and resistance to disease in animals. V: Early separation from the mother and response to a transplanted tumor in the rat. Psychosomatic Medicine. 1965;27:119–122. doi: 10.1097/00006842-196503000-00004. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14276917. [DOI] [PubMed] [Google Scholar]
- Ader R, Tatum R, Beels CC. Social factors affecting emotinality and resistance to disease in animals: I. Age of separation from the mother and susceptibility to gastric ulcers in the rat. Journal of Comparative and Physiological Psychology. 1960;53:446–454. doi: 10.1037/h0044570. [DOI] [PubMed] [Google Scholar]
- Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annual Review of Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Albelda R. Fallacies of welfare-to-work policies. Annals of the American Academy of Political and Social Science. 2001;577:66–78. doi: 10.1177/0002716201577001006. [DOI] [Google Scholar]
- Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, et al. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35(4):544–556. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Anda RF, Dong M, Brown DW, Felitti VJ, Giles WH, Perry GS, et al. The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health. 2009;9:106. doi: 10.1186/1471–2458-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arata CM, Langhinrichsen-Rohling J, Bowers D, O’Brien N. Differential correlates of multi-type maltreatment among urban youth. Child Abuse and Neglect. 2007;31(4):393–415. doi: 10.1016/j.chiabu.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain, Behavior, and Immunity. 2006;20(4):339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain, Behavior, and Immunity. 2005;19(4):311–317. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Seminars in Cancer Biology. 2006;16(1):80–88. doi: 10.1016/j.semcancer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Peterson BL, Dahlstrom WG, Siegler IC, Anderson NB, Williams RBJ. Hostility patterns and health implications: correlates of Cook-Medley Hostility Scale scores in a national survey. Health Psychology. 1991;10(1):18–24. doi: 10.1037/0278-6133.10.1.18. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal and Infant Origins of Adult Disease. London: British Medical Journal; 1992. [Google Scholar]
- Barnes PJ. Corticosteroid resistance in airway disease. Proc Am Thorac Soc. 2004;1(3):264–268. doi: 10.1513/pats.200402-014MS. [DOI] [PubMed] [Google Scholar]
- Baum A, Cohen L, Hall M. Control and intrusive memories as possible determinants of chronic stress. Psychosomatic Medicine. 1993;55:274–286. doi: 10.1097/00006842-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, et al. Inflammation and the incidence of Type 2 diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010;33:804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. Journal of the American College of Cardiology. 2003;41(4):37S–42S. doi: 10.1016/S0735-1097(02)02953–4. [DOI] [PubMed] [Google Scholar]
- Blane D. The life course, the social gradient, and health. In: Marmot M, RG W, editors. Social Determinants of Health. New York: Oxford University Press; 1999. pp. 65–80. [Google Scholar]
- Bowlby J. Attachment and loss: Attachment. 1. New York: Basic Books; 1969. [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, Coll CG. The home environments of children in the United States part I: variations by age, ethnicity, and poverty status. Child Development. 2001;72(6):1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: a reappraisal of retrospective reports. Psychological Bulletin. 1993;113(1):82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- Bunevicius R, Hinderliter AL, Light KC, Leserman J, Pedersen CA, Girdler SS. Histories of sexual abuse are associated with differential effects of clonidine on autonomic function in women with premenstrual dysphoric disorder. Biological Psychology. 2005;69(3):281–296. doi: 10.1016/j.biopsycho.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 2009;17(5):980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29(4–5):843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Caspi A, Bem DJ, Elder GH. Continuities and consequences of interactional styles across the life course. Journal of Personality. 1989;57:375–406. doi: 10.1111/j.1467-6494.1989.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Shaver PR. Handbook of Attachment: Theory, Research and Clinical Applications. 2. New York: Guilford; 2008. [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects two-year hormonal trajectories in children. Psychological Science. 2010a;21:31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosomatic Medicine. 2003;65(6):984–992. doi: 10.1097/01.PSY.0000097340.54195.3C. [DOI] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Development. 2004;75(4):1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA. Development of the cognitive appraisal and understanding of social events (CAUSE) videos. Health Psychology. 2003;22(1):106–110. doi: 10.1037/0278-6133.22.1.106. [DOI] [PubMed] [Google Scholar]
- Chen E, Schreier HM, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect. 2008;116(7):970–975. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE. “Shift-and-Persist” strategies: Why being low in socioeconomic status isn’t always bad for your health. 2011. Manuscript Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. doi: 10.1038/mp.2010.53. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117(0091-6749 Print):1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews K, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. American Journal of Respiratory and Critical Care Medicine. 2007;175(4):316–322. doi: 10.1164/rccm.200607-898OC. [DOI] [PubMed] [Google Scholar]
- Choy EHS, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. New England Journal of Medicine. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Clark DP, Russell LD. Molecular biology made simple and fun. 3. Saint Louis, MO: Cache River Press; 2005. [Google Scholar]
- Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, et al. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. American Journal of Physiology: Endocrinology and Metababolism. 2008;295(3):E686–91. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Prenatal origins of individual variation in behavior and immunity. Neuroscience and Biobehavioral Reviews. 2005;29(1):39–49. doi: 10.1016/j.neubiorev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Mother-infant interactions and the development of immunity from conception through weaning. In: Ader R, editor. Psychoneuroimmunology. 4. London: Elsevier Academic Press; 2007. pp. 455–474. [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine. 2004;66(1534–7796):553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Underwood LG. Strategies for measuring stress in studies of psychiatric and physical disorders. In: Cohen S, Kessler RC, Underwood LG, editors. Measuring stress: A guide for health and social scientists. New York: Oxford University Press; 1995. pp. 3–28. [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CS, Rose RM, Cacioppo JT. Social regulation of gene expression: Inflammation and the human transcriptional response to loneliness. Genome Biology. 2007;8:r89. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Conger RD, Elder GH. Families in troubled times: Adapting to change in rural America. New York, NY: Aldine de Gruyter; 1994. [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids, and the programming of adult disease. Frontiers in Behavioral Neuroscience. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC. Toward an interactional description of depression. Psychiatry. 1976;39(1):28–40. doi: 10.1080/00332747.1976.11023874. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1257353. [DOI] [PubMed] [Google Scholar]
- Crick NC, Dodge KA. A review and reformulation of social information processing mechanisms in children’s social adjustment. Psychological Bulletin. 1994;115:74–101. doi: 10.1037/0033-2909.115.1.74. [DOI] [Google Scholar]
- Crouch J, Hanson RF, Saunders BE, Kilpatrick DG, Resnick HS. Income, race/ethnicity, and exposure to violence: Results from the National Survey of Adolescents. Journal of Community Psychology. 2000;28:625–641. [Google Scholar]
- Dale LP, Carroll LE, Galen G, Hayes JA, Webb KW, Porges SW. Abuse history is related to autonomic regulation to mild exercise and psychological wellbeing. Appl Psychophysiol Biofeedback. 2009;34(4):299–308. doi: 10.1007/s10484-009-9111–4. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111(11):1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, et al. Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry. 2010;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatric and Adolescent Medicine. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum A, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. Developmental traumatology Part I: Biological stress systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Flora JA, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality and Individual Differences. 2007;42:111–121. doi: 10.1016/j.paid.2006.06.026. [DOI] [Google Scholar]
- DeRijk R, de KER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28(0969–711X Print):263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS. A biopsychosocial model of the development of chronic conduct problems in adolescence. Developmental Psychology. 2003;39(2):349–371. doi: 10.1037/0012-1649.39.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, Bates JE. Socialization mediators of the relation between socioeconomic status and child conduct problems. Child Development. 1994;65(2 Spec):649–665. doi: 10.2307/1131407. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Das R, Weidman JR, Jirtle RL. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatric Research. 2007;61(5 Pt 2):30R–37R. doi: 10.1203/pdr.0b013e31804575f7. [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Dube SR, Giles WH, Felitti VJ. The relationship of exposure to childhood sexual abuse to other forms of abuse, neglect, and household dysfunction during childhood. Child Abuse and Neglect. 2003;27(6):625–639. doi: 10.1016/S0145-2134(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clinical Science. 2007;113(5):219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Drukker M, Kaplan C, Feron F, van Os J. Children’s health-related quality of life, neighbourhood socio-economic deprivation and social capital. A contextual analysis. Social Science and Medicine. 2003;57(5):825–841. doi: 10.1016/S0277-9536(02)00453-7. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71(2):243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. Journal of Neuroimmunology. 2004;148(1–2):106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. Journal of Neuroimmunology. 2005;163(1–2):110–119. doi: 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Feldman PJ, Cohen S, Doyle W, Skoner D, Gwaltney JM., Jr The impact of personality on the reporting of unfounded symptoms and illness. Journal of Personality and Social Psychology. 1999;77:370–378. doi: 10.1037/0022-3514.77.2.370. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/S0749-3797(98)00017–8. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Frontiers in Neuroendocrinology. 2006a;27(2):180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. Journal of Neuroscience. 2006b;26(9):2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: impact on children’s behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Dopaminergic regulation of limbic-striatal interplay. Journal of Psychiatry and Neuroscience. 2007;32(6):400–411. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18043763. [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(0027-8424 Print):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005a;102(47):17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. Anew view on hypocortisolism. Psychoneuroendocrinology. 2005b;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosomatic Medicine. 2009;71(3):329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. New England Journal of Medicine. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Cidlowski JA. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life. 2009;61(10):979–986. doi: 10.1002/iub.245. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiologic Reviews. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Garfinkel I, McLanahan SS. Single mothers and their children. Washington DC: Urban Institute Press; 1986. [Google Scholar]
- Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110(12):1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, et al. Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience. 2008;3(2):91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB, Sheu LK, Kuan DCH, AE C, Hariri AR. Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex. doi: 10.1093/cercor/bhq160. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and Anxiety. 2009;26(11):984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Ferrie JE, Elovainio M, Pulkki-Raback L, Keltikangas-Jarvinen L, Eklund C, et al. When do social inequalities in C-reactive protein start? A life course perspective from conception to adulthood in the Cardiovascular Risk in Young Finns Study. International Journal of Epidemiology. 2008;37(2):290–298. doi: 10.1093/ije/dym244. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, et al. Biologicalcorrelates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosomatic Medicine. 2003;65(5):849–856. doi: 10.1097/01.PSY.0000088593.38201.CD. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Gottlieb-Stematsky TE. Human herpesvirus infections: Clinical aspects. New York: Marcel Dekker; 1982. [Google Scholar]
- Glassman AH, Miller GE. Where there is depression, there is inflammation sometimes! Biological Psychiatry. 2007;62(4):280–281. doi: 10.1016/j.biopsych.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Gluckman P, Hanson M. The conceptual basis for developmental origins of health and disease. In: Gluckman P, Hanson M, editors. Developmental Origins of Health and Disease. New York, NY: Cambridge University Press; 2006. pp. 33–50. [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey K. The ‘developmental origins’ hypothesis: Epidemiology. In: Gluckman P, Hanson M, editors. Developmental Origins of Health and Disease. New York, NY: Cambridge University Press; 2006. pp. 6–32. [Google Scholar]
- Goodman E, Adler NE, Kawachi I, Frazier AL, Huang B, Colditz GA. Adolescents’ perceptions of social status: Development and evaluation of a new indicator. Pediatrics. 2001;108(2):e31. doi: 10.1542/peds.108.2.e31. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain, Behavior, and Immunity. 2006;20(4):389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Graves PL, Wang NY, Mead LA, Johnson JV, Klag MJ. Youthful precursors of midlife social support. Journal of Personality and Social Psychology. 1998;74(5):1329–1336. doi: 10.1037/0022-3514.74.5.1329. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67(1534–7796):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, et al. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Molecular Immunology. 2010;47(6):1244–1254. doi: 10.1016/j.molimm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griskevicius V, Tybur JM, Delton AW, Robertson TE. The influence of mortality and socioeconomic status on risk and delayed rewards: A life history theory approach. Journal of Personality and Social Psychology. 2011 doi: 10.1037/a0022403. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21299312. [DOI] [PMC free article] [PubMed]
- Gruber-Olipitz M, Stevenson R, Olipitz W, Wagner E, Gesslbauer B, Kungl A, et al. Transcriptional pattern analysis of adrenergic immunoregulation in mice. Twelve hours norepinephrine treatment alters the expression of a set of genes involved in monocyte activation and leukocyte trafficking. Journal of Neuroimmunology. 2004;155(1–2):136–142. doi: 10.1016/j.jneuroim.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Raikkonen K. Modeling relationships among socioeconomic status, hostility, cardiovascular reactivity, and left ventricular mass in African American and White children. Health Psychology. 1999;18(2):140–150. doi: 10.1037/0278-6133.18.2.140. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Janlert U, Theorell T, Hammarstrom A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2009;35:613–623. doi: 10.1016/j.psyneuen.2009.09.019. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19879057. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. 2008;33(10):1305–1312. doi: 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallqvist J, Lynch J, Bartley M, Lang T, Blane D. Can we disentangle life course processes of accumulation, critical period and social mobility? An analysis of disadvantaged socio-economic positions and myocardial infarction in the Stockholm Heart Epidemiology Program. Social Science and Medicine. 2004;58(8):1555–1562. doi: 10.1016/S0277-9536(03)00344-7. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Hart CL, Hole DJ, Smith GD. Influence of socioeconomic circumstances in early and later life on stroke risk among men in a Scottish cohort study. Stroke. 2000;31(9):2093–2097. doi: 10.1161/01.str.31.9.2093. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10978035. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Kitagawa K, Hougaku H, Shimizu Y, Sakaguchi M, Nagai Y, et al. C-reactive protein is an independent predictor f the rate of increase in early carotid atherosclerosis. Circulation. 2001;104:63–67. doi: 10.1161/hc2601.091705. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry. 2009;14(10):954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer D. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/S0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111(23):3071–3077. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- Herbeck YE, Gulevich RG, Amelkina OA, Plyusnina IZ, Oskina IN. Conserved methylation of the glucocorticoid receptor gene exon 1(7) promoter in rats subjected to a maternal methyl-supplemented diet. International Journal of Developmental Neuroscience. 2010;28(1):9–12. doi: 10.1016/j.ijdevneu.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Depression and immunity: A meta-analytic review. Psychological Bulletin. 1993;113(3):472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896(1):85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hess JL, Denenberg VH, Zarrow MX, Pfeifer WD. Modification of the corticosterone response curve as a function of handling in infancy. Physiology & Behavior. 1969;4:109–111. doi: 10.1016/0031-9384(69)90023-7. [DOI] [Google Scholar]
- Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009;458(7237):422–429. doi: 10.1038/nature07958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: a psychobiologic perspective on bereavement. Psychosomatic Medicine. 1984;46(3):183–197. doi: 10.1097/00006842-198405000-00001. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6739679. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early social relationships: a psychobiologist’s view. Child Development. 1987;58(3):633–647. doi: 10.2307/1130203. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Jefferis BJ, Power C, Graham H, Manor O. Effects of childhood socioeconomic circumstances on persistent smoking. American Journal of Public Health. 2004;94(2):279–285. doi: 10.2105/AJPH.94.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63(6):733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends in Immunology. 2003;24(1):25–29. doi: 10.1016/S1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. Journal of Clinical Epidemiology. 2002;55(7):696–710. doi: 10.1016/S0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosomatic Medicine. 2006;68(3):500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Kennedy BP, Wilkinson RG. Crime: social disorganization and relative deprivation. Social Science and Medicine. 1999;48(6):719–731. doi: 10.1016/S0277-9536(98)00400-6. [DOI] [PubMed] [Google Scholar]
- Keinan-Boker L, Vin-Raviv N, Liphshitz I, Linn S, Barchana M. Cancer incidence in Israeli Jewish survivors of World War II. Journal of the National Cancer Institute. 2009;101(21):1489–1500. doi: 10.1093/jnci/djp327. [DOI] [PubMed] [Google Scholar]
- Keough KA, Zimbardo PG, Boyd JN. Who’s smoking, drinking, and using drugs? Time perspective as a predictor of substance use. Journal of Basic and Applied Social Psychology. 1999;21:149–164. doi: 10.1207/S15324834BA210207. [DOI] [Google Scholar]
- Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosomatic Medicine. 2010;72(4):365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62(12):1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiesler DJ. Contemporary interpersonal theory and research: Personality, psychopathology, and psychotherapy. New York: Wiley; 1996. [Google Scholar]
- Kittleson MM, Meoni LA, Wang NY, Chu AY, Ford DE, Klag MJ. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Archives of Internal Medicine. 2006;166(21):2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- Kohn ML. Social class and conformity. Chicago, IL: University of Chicago Press; 1977. [Google Scholar]
- Kop WJ, Cohen N. Psychological risk factors and immune system involvement in cardiovascular disease. In: Ader R, Felten D, Cohen N, editors. Psychoneuroimmunology. 3. New York: Academic Press; 2001. pp. 525–544. [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. Journal of Neuroscience. 2010;30(2):703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschinski C, Skripuletz T, Bedoui S, Raber K, Straub RH, Hoffmann T, et al. Postnatal life events affect the severity of asthmatic airway inflammation in the adult rat. Journal of Immunology. 2008;180(6):3919–3925. doi: 10.4049/jimmunol.180.6.3919. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18322200. [DOI] [PubMed] [Google Scholar]
- Kuh D, Hardy R, Langenberg C, Richards M, Wadsworth ME. Mortality in adults aged 26–54 years related to socioeconomic conditions in childhood and adulthood: post war birth cohort study. BMJ. 2002;325(7372):1076–1080. doi: 10.1136/bmj.325.7372.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y. A life-course approach to chronic disease epidemiology. Oxford: Oxford University Press; 2004. [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: Systematic review and meta-analysis. Lancet Neurology. 2005;4(6):371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race-and class-related disparities in health outcomes. American Heart Journal. 2005;150(1):153–160. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatric and Adolescent Medicine. 2002;156(8):824–830. doi: 10.1001/archpedi.156.8.824. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12144375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Smith GD, Rumley A, Lowe GD, Ebrahim S. Associations of fibrinogen and C-reactive protein with prevalent and incident coronary heart disease are attenuated by adjustment for confounding factors. British Women’s Heart and Health Study. Thrombosis and Haemostasis. 2005;93(5):955–963. doi: 10.1160/TH04-12-0805. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15886815. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychology. 2009;28(3):338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine. 2005;67(6):846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Leinonen M, Saikku P. Evidence for infectious agents in cardiovascular disease and atherosclerosis. Lancet Infectious Disease. 2002;2(1):11–17. doi: 10.1016/S1473-3099(01)00168-2. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Eiden RD. Marital and family processes in the context of alcohol use and alcohol disorders. Annual Review of Clinical Psychology. 2007;3:285–310. doi: 10.1146/annurev.clinpsy.3.022806.091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nature Reviews Neuroscience. 2009;10(1):37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychological Bulletin. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32(7):824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biological Psychology. 2005;69(1):5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease. Journal of the American Medical Association. 2001;286:2107–2156. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Ljung R, Hallqvist J. Accumulation of adverse socioeconomic position over the entire life course and the risk of myocardial infarction among men and women: results from the Stockholm Heart Epidemiology Program (SHEEP) Journal of Epidemiology and Community Health. 2006;60(12):1080–1084. doi: 10.1136/jech.2006.047670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Berkman LF, Gruenewald TL, Seeman TE. Relation of social integration to inflammatory marker concentrations in men and women 70 to 79 years. American Journal of Cardiology. 2006a;97(7):1010–1016. doi: 10.1016/j.amjcard.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Pilote L, Lynch JW, Richard H, Almeida ND, Benjamin EJ, et al. Life course socioeconomic position is associated with inflammatory markers: the Framingham Offspring Study. Social Science and Medicine. 2010;71(1):187–195. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Sullivan LM, D’Agostino RBS, Larson MG, Berkman LF, Benjamin EJ. Social networks and inflammatory markers in the Framingham Heart Study. Journal of Biosocial Science. 2006b;38(6):835–842. doi: 10.1017/S0021932005001203. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Appelhans BM. Early parental loss and salivary cortisol in young adulthood: the moderating role of family environment. Development and Psychopathology. 2006;18(1):295–308. doi: 10.1017/S0954579406060160. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Review. 2004;24(2):171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annual Review of Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA. Socioeconomic position. In: Berkman LF, Kawachi I, editors. Social Epidemiology. Oxford: Oxford University Press; 2000. pp. 13–35. [Google Scholar]
- Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Social Science and Medicine. 1997;44(6):809–819. doi: 10.1016/S0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress innoculation, arousal regulation, and resilience. Frontiers in Behavioral Neuroscience. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marmot M, Bosma H, Hemmingway H, Brunner E, Stansfiled S. Contribution of job control and other risk factors to social variations in coronary heart disease incidence. Lancet. 1997;350:235–239. doi: 10.1016/S0140-6736(97)04244-X. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosomatic Medicine. 2007;69(8):709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain, Behavior, and Immunity. 2008;22(5):753–761. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA. Psychological perspectives on the development of coronary heart disease. American Psychologist. 2005;60(8):783–796. doi: 10.1037/0003-066X.60.8.783. [DOI] [PubMed] [Google Scholar]
- Maulik PK, Darmstadt GL. Community-based interventions to optimize early childhood development in low resource settings. Journal of Perinatology. 2009;29(8):531–542. doi: 10.1038/jp.2009.42. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosomatic Medicine. 2006;68(1534–7796 Electronic):187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. American Journal of Human Biology. 2005;17(1):81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. doi: 10.1001/archinte.153.18.2093. [DOI] [PubMed] [Google Scholar]
- McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Medicine. 2006;3(8):e297. doi: 10.1371/journal.pmed.0030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC. The impact of economic hardship on black families and children: psychological distress, parenting, and socioemotional development. Child Development. 1990;61(2):311–346. doi: 10.2307/1131096. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037/0003-066X.53.2.185. [DOI] [PubMed] [Google Scholar]
- McLoyd VC, Jayaratne TE, Ceballo R, Borquez J. Unemployment and work interruption among African American single mothers: effects on parenting and adolescent socioemotional functioning. Child Development. 1994;65(2 Spec):562–589. doi: 10.2307/1131402. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Research. 1985;354(2):301–304. doi: 10.1016/0165-3806(85)90183-x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=4052820. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of pro-inflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok A, Walker H, Lim A, Nicholls EP, et al. Low early-life social class leaves a biological residue manifest by decreased glucocorticoid and increased pro-inflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009a;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman T. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. 2011. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69(5):402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009b;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro-and anti-inflammatory signaling pathways 6 months later. Psychosomatic Medicine. 2009;71(1):57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA, Georgiades K, Boyle M, MacMillan HL. Stability of resting frontal electroencephalogram (EEG) asymmetry and cardiac vagaltone in adolescent females exposed to child maltreatment. Developmental Psychobiology. 2009;51(6):474–487. doi: 10.1002/dev.20387. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, et al. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. Journal of Clinical Endocrinology and Metabolism. 2001;86(12):5864–5869. doi: 10.1210/jc.86.12.5864. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Katz DR, Miles JM, Yudkin JS, Klein S, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-‡ in vivo. Journal of Clinical Endocrinology & Metabolism. 1997;82:4196–4200. doi: 10.1210/jc.82.12.4196. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Pelletier D. A temporal framework for understanding the effects of stressful life events on inflammation in patients with multiple sclerosis. Brain, Behavior, and Immunity. 2006;20(1):27–36. doi: 10.1016/j.bbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Moser D, Molitor A, Kumsta R, Tatschner T, Riederer P, Meyer J. The glucocorticoid receptor gene exon 1-F promoter is not methylated at the NGFI-A binding site in human hippocampus. World Journal of Biological Psychiatry. 2007;8(4):262–268. doi: 10.1080/15622970701429862. [DOI] [PubMed] [Google Scholar]
- Nation DA, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, et al. Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated ApoE−/− mice. Psychosomatic Medicine. 2010;72(4):376–382. doi: 10.1097/PSY.0b013e3181d74c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Program NCE. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002 Retrieved from http://www.nhlbi.nih.gov/guidelines/cholesterol/___. [PubMed]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Ohgane J, Yagi S, Shiota K. Epigenetics: the DNA methylation profile of tissue-dependent and differentially methylated regions in cells. Placenta. 2008;29:S29–35. doi: 10.1016/j.placenta.2007.09.011. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18031808. [DOI] [PubMed] [Google Scholar]
- Oosterman M, De Schipper JC, Fisher P, Dozier M, Schuengel C. Autonomic reactivity in relation to attachment and early adversity among foster children. Development and Psychopathology. 2010;22(1):109–118. doi: 10.1017/S0954579409990290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, Petersen L, Prescott E, Teasdale TW, Sorensen TI. Genetic and environmental influences on the relation between parental social class and mortality. International Journal of Epidemiology. 2006;35(5):1272–1277. doi: 10.1093/ije/dyl045. [DOI] [PubMed] [Google Scholar]
- Osterman P. Welfare participation in a full employment economy: The impact of neighborhood. Social Problems. 1991;38(4):475–491. doi: 10.1525/sp.1991.38.4.03a00050. [DOI] [Google Scholar]
- Overstreet S, Braun S. Exposure to community violence and post-traumatic stress symptoms: mediating factors. American Journal of Orthopsychiatry. 2000;70(2):263–271. doi: 10.1037/h0087828. [DOI] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity. 2005;19(6):493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. Introduction and overview. In: Pennebaker JW, editor. The psychology of physical symptoms. New York: Springer-Verlag; 1983. pp. 1–18. [Google Scholar]
- Pensola TH, Martikainen P. Cumulative social class and mortality from various causes of adult men. Journal of Epidemiology and Community Health. 2003;57(9):745–751. doi: 10.1136/jech.57.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, Manuck SB. Parental education is related to C-reactive protein among female middle-aged community volunteers. Brain, Behavior, and Immunity. 2009;23(5):677–683. doi: 10.1016/j.bbi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD. Early adversity and mechanisms of plasticity: integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology. 2005;17(3):735–752. doi: 10.1017/S0954579405050352. [DOI] [PubMed] [Google Scholar]
- Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science. 2008;17:370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38(5):784–791. doi: 10.1037/0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112(3):323–338. doi: 10.1037/0021-843X.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Vardi S, Putzer Bechner AM, Curtin JJ. Physically abused children’s regulation of attention in response to hostility. Child Development. 2005;76(5):968–977. doi: 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology. 2007;22(1):55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JC, Snieder H, Davis HC, Treiber FA. Anger suppression and adiposity modulate association between ADRB2 haplotype and cardiovascular stress reactivity. Psychosomatic Medicine. 2006;68(1534–7796 Electronic):207–212. doi: 10.1097/01.psy.0000204925.18143.4f. [DOI] [PubMed] [Google Scholar]
- Portal-Celhay C, Perez-Perez GI. Immune responses to Helicobacter pylori colonization: mechanisms and clinical outcomes. Clinical Science. 2006;110(3):305–314. doi: 10.1042/CS20050232. [DOI] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, et al. Association between children’s experience of socioeconomic disadvantage and adult health: A life-course study. Lancet. 2002;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Graham H, Due P, Hallqvist J, Joung I, Kuh D, et al. The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: an international comparison. International Journal of Epidemiology. 2005a;34(2):335–344. doi: 10.1093/ije/dyh394. [DOI] [PubMed] [Google Scholar]
- Power C, Hypponen E, Smith GD. Socioeconomic position in childhood and early adult life and risk of mortality: A prospective study of the mothers of the 1958 British Birth Cohort. American Journal of Public Health. 2005b;95(8):1396–1402. doi: 10.2105/AJPH.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin-6, and risk of developing Type 2 diabetes mellitus. Journal of the American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. Journal of the American Medical Association. 2002;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Prescott SL. The development of respiratory inflammation in children. Paediatric Respiratory Review. 2006;7(2):89–96. doi: 10.1016/j.prrv.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Presser HB, Cox AG. The work wchedules of low-educated American women and welfare reform. Monthly Labor Review. 1997;120(4) Retrieved from http://www.questia.com/PM.qst?a=o&se=gglsc&d=5000466401.
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Reis HT, Collins WA, Berscheid E. The relationship context of human behavior and development. Psychological Bulletin. 2000;126(6):844–872. doi: 10.1037/0033-2909.126.6.844. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. doi: 10.1037/0033-2909.128.2.330. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun HJ, Todd TJ, Kawachi I, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. American Journal of Preventive Medicine. 2010;39(6):529–536. doi: 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EJ. Inherited epigenetic variation -revisiting soft inheritance. Nature Reviews Genetics. 2006;8:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(1524–4539):363–369. doi: 10.1161/01.CIR.0000053730.47739.3C. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events. Circulation. 2003;107:391–397. doi: 10.1161/01.CIR.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109(16):1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. New England Journal of Medicine. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000a;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000b;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. Journal of Epidemiology and Community Health. 2010;64(5):413–418. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RN, Bryan A. Relationships Between Future Orientation, Impulsive Sensation Seeking, and Risk Behavior Among Adjudicated Adolescents. Journal of Adolescent Research. 2004;19(4):428–445. doi: 10.1177/0743558403258860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annual Review of Neuroscience. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rontgen P, Sablotzki A, Simm A, Silber RE, Czeslick E. Effect of catecholamines on intracellular cytokine synthesis in human monocytes. European Cytokine Network. 2004;15(1):14–23. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15217748. [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis -An inflammatory disease. New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Scherwitz L, Perkins L, Chesney M, Hughes G. Cook-Medley Hostility scale and subsets: relationship to demographic and psychosocial characteristics in young adults in the CARDIA study. Psychosomatic Medicine. 1991;53(1):36–49. doi: 10.1097/00006842-199101000-00004. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2011649. [DOI] [PubMed] [Google Scholar]
- Schreier HM, Chen E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain, Behavior, and Immunity. 2010;24(8):1324–1331. doi: 10.1016/j.bbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Scott R, Tzelepis A. Exposure to violence among inner-city youth. Journal of Adolescent Health. 1993;14:214–219. doi: 10.1016/1054-139X(93)90008-D. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science and Medicine. 2004;58(10):1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC. Resources, stress, and immunity: An ecological perspective on human psychoneuroimmunology. Annals of Behavioral Medicine. 2010;40:114–125. doi: 10.1007/s12160-010-9195-3. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290(22):2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk CE, Noll JG, Putnam FW, Trickett PK. A prospective examination of the role of childhood sexual abuse and physiological asymmetry in the development of psychopathology. Child Abuse and Neglect. 2010;34(10):752–761. doi: 10.1016/j.chiabu.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(8):2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Skolnick NJ, Ackerman SH, Hofer MA, Weiner H. Vertical transmission of acquired ulcer susceptibility in the rat. Science. 1980;208(4448):1161–1163. doi: 10.1126/science.7189606. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. Journal of Neuroscience. 2007;27(33):8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, et al. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic Medicine. 2010;72(7):694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Hart C, Blane D, Hole D. Adverse socioeconomic conditions in childhood and cause specific adult mortality: prospective observational study. BMJ. 1998;316(7145):1631–1635. doi: 10.1136/bmj.316.7145.1631. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9603744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey B. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac LM. How the immune system works. Malden, MA: Blackwell; 2008. [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nature Reviews Immunology. 2006;6(4):318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine. doi: 10.1097/PSY.0b013e31820ad12b. (in press) Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21257974. [DOI] [PubMed]
- Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29(9):1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain, Behavior, and Immunity. 2002;16(6):675–684. doi: 10.1016/S0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine and Tobacco Research. 2008;10(10):1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Swinny JD, O’Farrell E, Bingham BC, Piel DA, Valentino RJ, Beck SG. Neonatal rearing conditions distinctly shape locus coeruleus neuronal activity, dendritic arborization, and sensitivity to corticotrophin-releasing factor. International Journal of Neuropsychopharmacology. 2009:1–11. doi: 10.1017/S146114570999037X. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19653930. [DOI] [PMC free article] [PubMed]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, et al. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. American Journal of Physiology: Endocrinology and Metababolism. 2008;295(6):E1495–501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environmental and Molecular Mutagenesis. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- Tabassum F, Kumari M, Rumley A, Lowe G, Power C, Strachan D. Effects of socioeconomic position on inflammatory and hemostatic markers: a life-course analysis in the 1958 British birth cohort. American Journal of Epidemiology. 2008;167:1332–1341. doi: 10.1093/aje/kwn055. [DOI] [PubMed] [Google Scholar]
- Taylor J, Spencer N, Baldwin N. Social, economic, and political context of parenting. Archives of Disease in Childhood. 2000;82(2):113–120. doi: 10.1136/adc.82.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006a;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006b;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Annals of the New York Academy of Sciences. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Tobin AB, Butcher AJ, Kong KC. Location, location, location.site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29(8):413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: A population-based study. American Journal of Epidemiology. 1998;148(10):958–966. doi: 10.1093/oxfordjournals.aje.a009572. Retrieved from http://aje.oupjournals.org/cgi/content/abstract/148/10/958. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA. What are the costs of marital conflict and dissolution to children’s physical health? Clinical Child and Family Psychology Review. 2004;7(1):29–57. doi: 10.1023/B:CCFP.0000020191.73542.b0. [DOI] [PubMed] [Google Scholar]
- van der Vegt EJ, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults A study of international adoptees. Psychoneuroendocrinology. 2009;34(5):660–669. doi: 10.1016/j.psyneuen.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Ndlovu MN, Hoya-Arias R, Dijsselbloem N, Gerlo S, Haegeman G. Keeping up NF-kappaB appearances: epigenetic control of immunity or inflammation triggered epigenetics. Biochemical Pharmacology. 2006;72(9):1114–1131. doi: 10.1016/j.bcp.2006.07.012. [DOI] [PubMed] [Google Scholar]
- von Patay B, Loppnow H, Feindt J, Kurz B, Mentlein R. Catecholamines and lipopolysaccharide synergistically induce the release of interleukin-6 from thymic epithelial cells. Journal of Neuroimmunology. 1998;86(2):182–189. doi: 10.1016/S0165-5728(98)00051-4. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49(3):432–438. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- Wang X, Ding X, Su S, Li Z, Riese H, Thayer JF, et al. Genetic influences on heart rate variability at rest and during stress. Psychophysiology. 2009;46(3):458–465. doi: 10.1111/j.1469-8986.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Steptoe A. Socioeconomic differences in attitudes and beliefs about healthy lifestyles. Journal of Epidemiology and Community Health. 2003;57(6):440–443. doi: 10.1136/jech.57.6.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine. 2009;71(8):805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- White JL, Moffitt TE, Caspi A, Bartusch DJ, Needles DJ, Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. Journal of Abnormal Psychology. 1994;103(2):192–205. doi: 10.1037/0021-843X.103.2.192. [DOI] [PubMed] [Google Scholar]
- Whitelaw E, Garrick D. Epigenetic mechanisms. In: Gluckman P, Hanson M, editors. Developmental Origins of Health and Disease. New York, NY: Cambridge University Press; 2006. pp. 62–74. [Google Scholar]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- Wills TA, Sandy JM, Yaeger AM. Time perspective and early-onset substance use: a model based on stress-coping theory. Psychology of Addictive Behaviors. 2001;15(2):118–125. doi: 10.1037/0893-164X.15.2.118. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Panter-Brick C. Homeless street children in Nepal: use of allostatic load to assess the burden of childhood adversity. Development and Psychopathology. 2008;20(1):233–255. doi: 10.1017/S0954579408000114. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Health effects of socially toxic neighborhoods: the violence and urban asthma paradigm. Clinics in Chest Medicine. 2006;27(3):413–21. v. doi: 10.1016/j.ccm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology. 2007;21(Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Perinatal stress and early life programming of lung structure and function. Biological Psychology. 2010;48–56:84. doi: 10.1016/j.biopsycho.2010.01.007. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20080145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(5 Suppl):757S–769S. doi: 10.1378/chest.07–1904. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood Innate and adaptive cytokine responses in an inner-city cohort. American Journal of Respiratory and Critical Care Medicine. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. Journal of Allergy and Clinical Immunology. 2004a;113(0091–6749 Print):1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, et al. Community violence and asthma morbidity: The Inner-City Asthma Study. American Journal of Public Health. 2004b;94(4):625–632. doi: 10.2105/AJPH.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress, and coronary heart disease: Is interleukin-6 the link? Atherosclerosis. 2001;148:209–214. doi: 10.1016/S0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, et al. Maternal programming of defensive responses through sustained effects on gene expression. Biological Psychology. 2006;73(1):72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]