Abstract
Purpose
To determine the utility of post contrast susceptibility-weighted magnetic resonance imaging (PCSWI) in the evaluation of vascular malformations of the brain (BVM).
Materials and Methods
We retrospectively evaluated PCSWI and digital subtraction angiography (DSA) data from 16 consecutive patients with known or suspected BVM, which had been entered into a prospectively maintained database during a 1-year period. There had been no intervening treatment or change in patients’ symptoms between the PCSWI and DSA studies. The utility of PCSWI in the detection of AVS was compared to that of routine non-contrast susceptibility weighted imaging (SWI), time of flight Magnetic Resonance angiography (TOFMRA) and contrast enhanced Magnetic Resonance angiography (CEMRA) using DSA results as the reference standard. The presence of AVS in PCSWI or SWI sequences was defined by the presence of abnormal signal hyperintensity in the venous structures adjacent to the BVM.
Results
A total of 17 BVMs were identified by DSA (9 newly diagnosed arteriovenous malformations, 3 dural arteriovenous fistulas, 4 treated arteriovenous malformations with residual AVS and 1 complex developmental venous anomaly). PCSWI was 100% sensitive and 100% specific with 100% positive predictive value (PPV) and 100% negative predictive value (NPV) for the detection of AVS in these BVMs. The PCSWI/SWI signal intensity ratio in the most prominent early draining venous structure was 1.2 ± 0.32.
Conclusion
PCSWI appears to be superior to SWI, TOFMRA and CEMRA in detecting AVS in BVMs and may be useful in the initial diagnosis and follow-up of patients with BVMs.
Keywords: Susceptibility-weighted imaging (SWI), Arteriovenous shunting, Arteriovenous malformation (AVM), Developmental venous anomalies (DVA)
INTRODUCTION
Susceptibility weighted imaging (SWI) is a magnetic resonance imaging (MRI) technique that combines both phase and magnitude signal to produce high resolution images of the cerebral venous system.1 In SWI images, veins appear hypointense due to the presence of deoxyhemoglobin and the arteries are hyperintense due to time-of-flight effects and lack of T2* effects.1,2 Therefore, using SWI, it is possible to simultaneously and distinctly evaluate both the arterial and venous systems of the brain.
Abnormal hyperintense signal within the veins draining high-flow vascular malformations of the brain (BVM) can be seen on SWI images due to arterialized blood flow from arteriovenous shunting (AVS). We have previously shown that this signal can be used to classify BVMs as those with and without AVS with a high degree of accuracy when the results from SWI are compared to those from DSA 3. We hypothesize that the accuracy of the SWI sequence in the detection of AVS can be further improved by performing post contrast SWI studies (PCSWI) after the intravenous administration of gadolinium contrast agents. On the post-contrast SWI (PCSWI) studies, the normal arteries become even brighter when compared to non-contrast SWI studies secondary to a time of flight effect, and the normal veins become darker when compared to non-contrast SWI studies secondary to a more pronounced T2* effect. Therefore, post-contrast studies are likely to be even more sensitive to the presence of AVS in high flow BVMs.
MATERIALS AND METHODS
Patient Selection
The study was approved by our hospital’s institutional review board and conducted in compliance with the Health Insurance Portability and Accountability Act. We retrospectively analysed the data from both magnetic resonance imaging studies as well as digital subtraction angiography studies which had been entered into a prospectively maintained quality assurance database at our institution on a series of twenty consecutive patients with known or suspected BVMs. All sixteen patients had undergone both a brain MRI study, which included PCSWI as well as a DSA study for the evaluation of a known or suspected BVM, at our institution between January 1st, 2010 and December 31st, 2010. Patient exclusion criteria were (1) a time interval between the MRI and DSA examinations greater than 365 days, or (2) endovascular or surgical treatment for the BVM between the MRI and DSA examinations, (3) contraindications to MRI examinations per hospital protocol including ferromagnetic metallic implants such as pacemakers, severe claustrophobia, (4) decreased renal function with GFR < 60 ml/1.73 m2 within a period of 6 months prior to the study (5) allergy to gadolinium contrast agents, or (6) changes in patient symptoms between MRI and DSA studies. Both the MRI with PCSWI and DSA examinations were performed as part of standard of care procedures, and the decision to perform these examinations was at the discretion of the clinical providers.
Image Acquisition
All MRI studies were performed on the 3-Tesla scanners at our institution (Trio 3-Tesla, Siemens AG, Munich, Germany) and included the standard, FDA approved SWI sequence. The SWI scanning parameters were: flip angle, 15 degrees; TE, 20 msec; TR, 27 msec; slice thickness, 2.0 mm; and in-plane resolution of 0.9 × 0.9 mm. TOF MRA and CEMRA were performed on all patients using standard vendor provided sequences (Siemens inc, Erlnagen, Germany). All TOFMRA studies were done using the following imaging parameters. Acquisition mode: 3D MOTSA, Echo time: 4.12 ms, Repetition time: 22 msec, Slice thickness: 0.8 mm, Acquisition matrix: 512 × 226, Flip angle 20. The CEMRA studies included both an arterial phase and a venous phase acquisition using vendor provided sequences with bolus timing methods. The PCSWI sequence was performed immediately after the end of the venous phase of the CEMRA sequence and is otherwise identical to the routine pre-contrast SWI sequence in all respects. The post contrast sequences in all patients were performed after the intravenous administration of 0.1 mmol/kg of Optimark (Gadoversatamide, Mallinckrodt Inc, Hazelwood, MO).
Catheter angiography was performed using a dedicated biplanar neuroangiographic unit (Axiom Artis, Siemens AG, Munich, Germany) with transfemoral arterial access and intravenous conscious sedation. All catheter angiograms included biplanar intracranial images after selective catheterization and contrast injection (Optiray 320, Covidien, Hazelwood, MO) of the vessels of interest.
Image Analysis
Two experienced neuroradiologists, blinded to the results of DSA, TOFMRA, CEMRA and clinical characteristics, independently reviewed the SWI and PCSWI sequences after multiplanar reformatting of the original trans-axial slices. Images were reformatted and reviewed using the Emageon Ultravisual Viewer (Amicas, Inc., Boston, MA) embedded within our hospital’s clinical information system (Clinical Desktop, BJC Healthcare, St. Louis, MO), to assess for the presence of AVS, as determined by the presence of signal hyperintensity within at least one venous structure draining the BVM being evaluated or within the nidus of the vascular malformation. When such abnormal hyperintensity was identified, the signal intensity within the most prominent hyperintense draining venous structure was measured on both the SWI and PCSWI studies by drawing a region of interest over this structure. All differences in reader interpretation were resolved by consensus using a panel including an additional board certified neuroradiologist.
Subsequently, the DSA examinations were reviewed in conjunction with an experienced interventional neuroradiologist to correlate the presence of SWI signal hyperintensity within a given venous structure draining the BVM with the presence of AVS within the same venous structure in the catheter angiogram. On the catheter angiograms, vascular malformations which were identified during the arterial phase of imaging and which had an associated early draining vein were identified as high flow malformations with AVS whereas malformations which were visible only during the venous phase of imaging and without an associated early draining vein during the arterial phase of imaging were identified as low flow vascular malformations without AVS.
The TOFMRA and CEMRA studies were interpreted independently by a board-certified neuroradiologist. Arteriovenous shunting was determined to be present on these studies when a nidus and/or abnormally enlarged draining veins, which were fed by an arterial branch were clearly identified.
Medical Record Review
Medical records were reviewed for patient age, sex, time interval between the MRI and DSA examinations, hemorrhage on initial head CT images, and prior surgical or endovascular treatments for BVMs.
Statistical Analysis
Statistical analysis was performed utilizing the MedCalc 11.1 software package (MedCalc Software, Mariakerke, Belgium). Inter-observer agreement for the presence of AVS on PCSWI was determined with the kappa statistic. Standard statistical parameters of PCSWI, SWI, TOFMRA and CEMRA for the prediction of AVS in BVMs were calculated utilizing DSA as the reference standard.
RESULTS
The mean age of the 16 patients (9 male, 7 female) who underwent MRI with PCSWI and DSA for evaluation of a known or suspected BVM was 47 ± 16.65 years. The mean time interval between the MRI and DSA examinations was 29 ± 52.4 days (median 4 days, range 0–182 days).
Results of DSA Examinations
A total of 17 BVMs were identified in the 16 patients included in our cohort. Of these, 9 were newly diagnosed arteriovenous malformations (52.9%), three were dural arteriovenous fistulas (17.6%), four were previously-treated arteriovenous malformations with residual AVS (23.5%) and one was a complex developmental venous anomaly without AVS (5.9%).
Accuracy of PCSWI for the Detection of AVS in BVMs
PCSWI was 100% sensitive and 100% specific with 100% positive predictive value (PPV) and 100% negative predictive value (NPV) for the detection of AVS in these patients with known or suspected BVMs. In contrast, SWI was only 80% sensitive and 100% specific with a 40% NPV and 100% PPV for the detection of AVS in BVMs. TOFMRA was 67% sensitive and 100% specific with an NPV of 29% and a PPV of 100%. CEMRA was 87% sensitive, 50% specific with an NPV of 33% and a PPV of 93% (Table 1). The PCSWI/SWI signal intensity ratio in the most prominent early draining venous structure in patients with AVS was 1.2 ± 0.32.
Table 1.
Table showing the performance of PCSWI, SWI, TOFMRA and CEMRA techniques in the detection of arteriovenous shunting in the 17 BVMs included in our study.
| Technique | Sensitivity | Specificity | PPV* | NPV** |
|---|---|---|---|---|
| PCSWI | 100 | 100 | 100 | 100 |
| SWI | 80 | 100 | 100 | 40 |
| TOFMRA | 67 | 100 | 100 | 29 |
| CEMRA | 87 | 50 | 93 | 33.3 |
PPV: Positive predictive value.
NPV: Negative predictive value.
DISCUSSION
Currently, the majority of patients with known or suspected BVMs undergo non-invasive evaluation with conventional MRI or MRA, which can reliably differentiate between a typical non-treated AVM and a typical DVA with its pathognomonic “medusa-head” appearance from radially-oriented small veins converging to a central draining vein. However, the performance of conventional MRI and MRA examinations in the follow-up of patients with treated AVMs and DAVFs is often suboptimal.4,5,6,7 Indeed, conventional MRA studies may continue to show dilated draining veins or enlarged arteries in previously treated AVMs even in the absence of AVS, since the vessel caliber changes often take time to reverse after elimination of AVS.8 Likewise, there is often contrast enhancement in the region of the treated AVM nidus secondary to reactive gliosis9.
Additionally, it is often difficult to differentiate between an atypical DVA with complex vascular anatomy and a high-flow BVM with these techniques 10–13. Likewise, these studies are less reliable in the presence of ICH. 4,5. The recent development of time-resolved MRA offers a valuable new tool in the evaluation of BVMs but diagnostic time-resolved MRAs with high temporal resolution are often technically challenging to obtain.8,14 Hence invasive evaluation with DSA is often required in patients in whom differentiating between atypical DVAs and AVMs/AVFs is necessary, and in those patients presenting with ICH and a suspected BVM.
We had previously shown that SWI offers unique advantages in the detection of AVS 3. This property of the SWI sequence arises from the intrinsic contrast between hyperintense rapidly-flowing oxygenated arterial blood and hypointense slowly-flowing deoxygenated venous blood on this sequence15, 16. This unique contrast between arteries and veins is further improved when the SWI sequence is performed after intravenous contrast administration (Figure 1). More importantly, this improvement in contrast is achieved without the need for accurate timing of image acquisition or technically-demanding dynamic acquisition techniques. Unlike routine rigorously timed CEMRA studies, the PCSWI studies in this study were performed after the venous phase of the CEMRA studies regardless of inter-individual differences in timing of the PCSWI study from the administration of the contrast bolus dose. This phenomenon is likely to be particularly advantageous in the pediatric population since cumbersome bolus timing methods are of limited utility in children given the limitations on contrast dose that can be used in pediatric patients17. Our PCSWI studies also retain high spatial resolution unlike many of the dynamic post-contrast MRA sequences.
Figure 1.

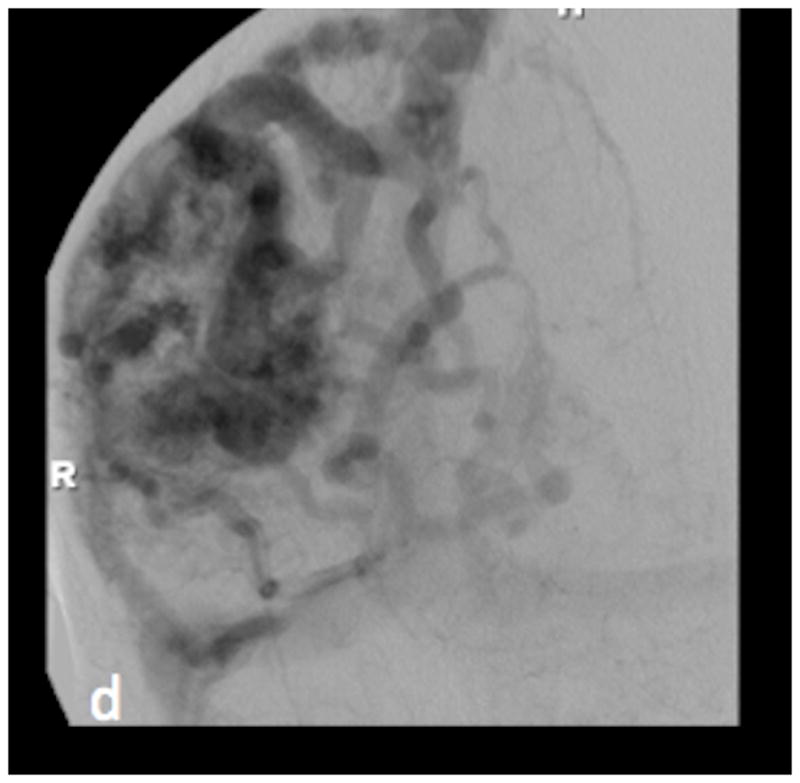
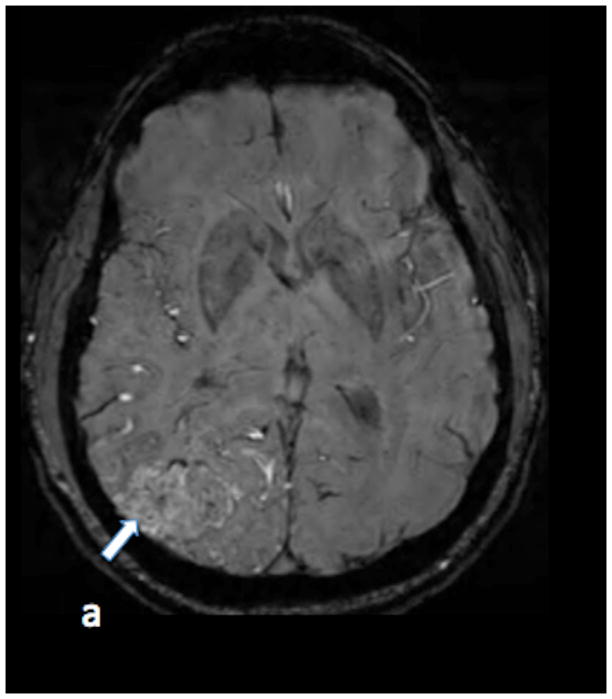
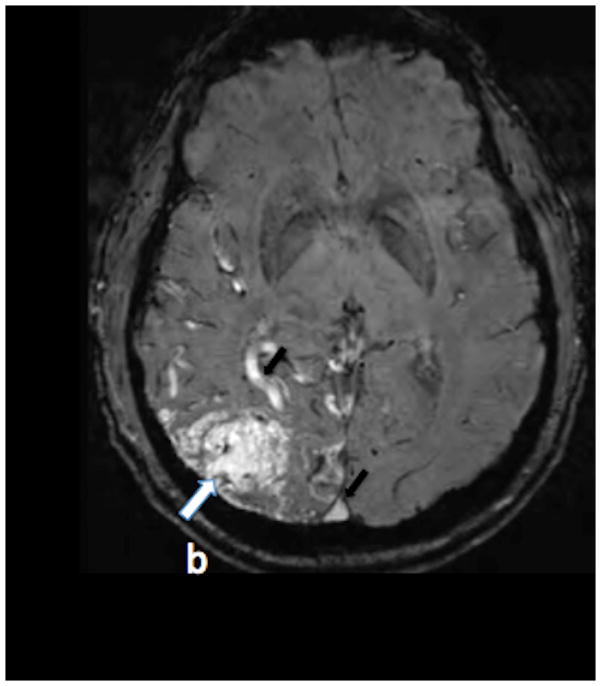
SWI (figure 1a) and PCSWI (figure 1b) studies performed on a 56 y/o male patient who presented with headaches show a large right parietal arteriovenous malformation nidus (white arrows on the SWI and PCSWI images). The PCSWI images show additional enlarged deep draining veins and shunting to the superior saggital sinus (black arrows on figure 1b). 3D volume rendered PCSWI image (figure 1c) further illustrates the large AVM and its draining pattern, which can be seen to closely correspond to the image from an oblique projection right internal carotid DSA study (figure 1d).
In addition to our recently published study, Fujiyama et al. utilized SWI to perform quantitative oxygenation measurements in the anterior spinal veins to detect changes in blood oxygenation within these veins after the endovascular treatment of spinal DAVFs.18 Tsui et al. described faint SWI signal hyperintensity within a venous varix in a patient with a cerebral DAVF.19 Saini et al. also described other SWI findings in a patient with a DAVF but did not describe abnormal hyperintensity in a venous structure.20 George et al also recently described the utility of SWI in the evaluation of brain arteriovenous malformations and used the magnitude images from the SWI studies to differentiate between the components of AVMs 21. However, our study is the first to utilize PCSWI in the evaluation of brain AVMs and to directly compare these results with the results from SWI, TOFMRA and CEMRA studies. In our current study, we found the sensitivity of SWI to be lower than we had previously reported 3, this may partly be accounted for by the smaller number of patients included in the current study. It is also likely that the lower sensitivity of the SWI sequence in this study arises from the inclusion of smaller AVMs and dural AVFs in the current study. Ultimately, the true sensitivity of precontrast SWI studies is likely to be more accurately evaluated with a prospective study. Moreover, even in those patients with positive SWI or CEMRA studies, the PCSWI studies were more useful in depicting crucial AVS patterns such as retrograde flow in superior ophthalmic veins or additional deep draining veins in cerebral parenchymal AVMs (Figure 2). PCSWI was also superior to CEMRA in demonstrating very small vascular malformations with AVS. This included two AVMs that were demonstrated in a patient with a family history of hereditary hemorrhagic telangiectasia (Figure 3) and a small dural AVF (Figure 4) in a patient with headaches that was only demonstrated on the PCSWI and DSA studies.
Figure 2.
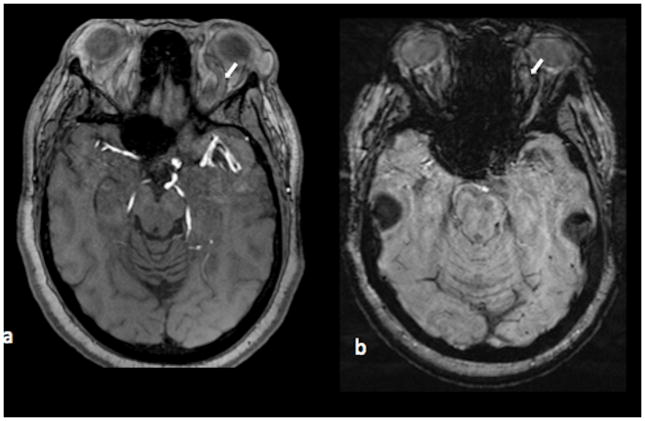
Axial TOF (figure 2a) and SWI (figure 2b) images through the brain at the level of the left orbit show an enlarged left superior ophthalmic vein without evidence for arteriovenous shunting (white arrows). Corresponding axial PCSWI imageand 3D volume rendered PCSWI image (figure 2c) show abnormal hyperintensity in the enlarged left superior ophthalmic vein (white arrow in the axial image) secondary to AVS and retrograde filling of the left superior ophthalmic vein from a large left frontal AVM. The PCSWI findings correspond to the images from a lateral projection DSA study after a left common carotid artery injection (figure 2d)
Figure 3.



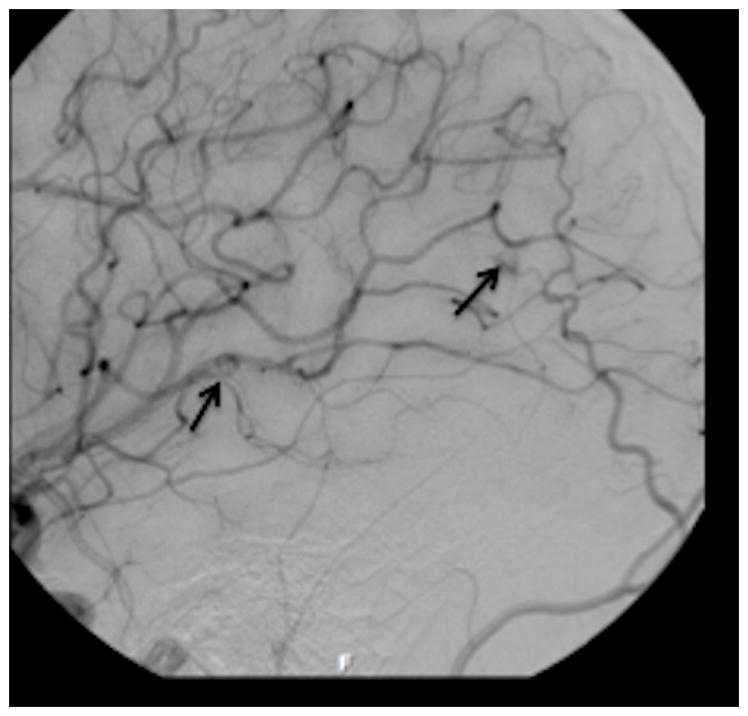
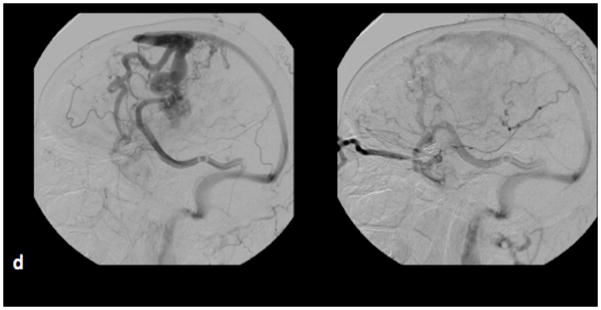
Axial PCSWI images in a 19 y/o patient with known hereditary hemorrhagic telangiectasia showing small hypervascular tangle of vessels in the right temporal and occipital regions (white arrows in figure 3 c and figure 3 d). Coronal source image from a contrast enhanced MRA study shows a small vascular malformation (white arrow, figure 3 c). No other malformation was identified on the CEMRA study. A magnified oblique projection from a right internal carotid DSA study on the same patient shows two small AVMs (black arrows) corresponding to the abnormalities on the PCSWI images.
Figure 4.


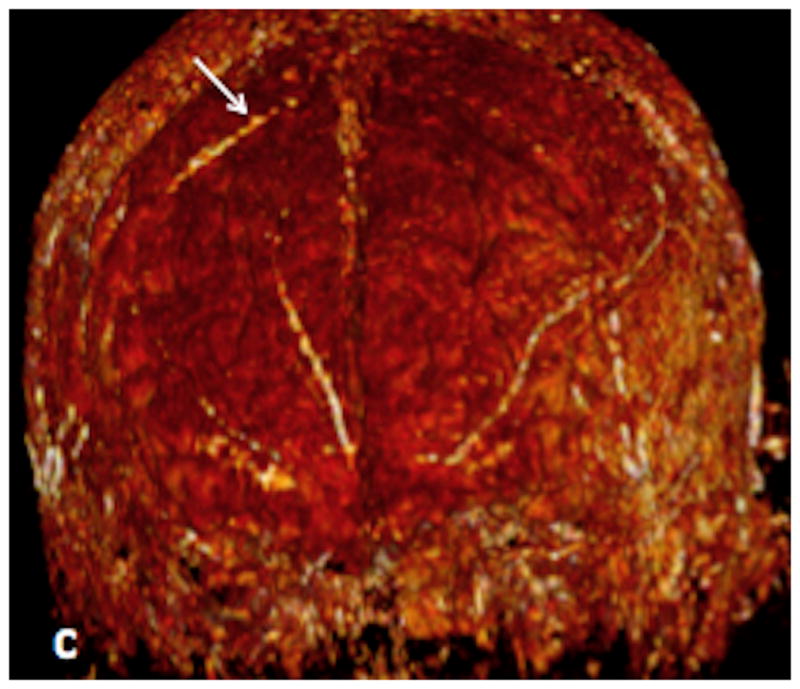

Axial (figure 4a) and coronal (figure 4b) Maximal intensity projection images from a PCSWI study in a 51 year old male show a small nidus (white arrow in a) and an abnormally hyperintense draining vein (white arrow in b) situated over the convexity of the right frontal lobe. 3D volume rendered PCSWI image (figure 4c) again illustrates the abnormal hyperintense vein on the surface of the brain (white arrow). AP projection from a right common carotid angiogram performed on the same patient shows a small dural arteriovenous fistula (black arrow) over the right frontal convexity, which is fed by the right middle meningeal artery and drains medially onto a small vein over the surface of the right frontal lobe.
These preliminary results suggest that PCSWI can reliably detect AVS in both de-novo and previously-treated high-flow BVMs. However, there are several limitations to our study. First, our study includes only a small number of patients, this makes assessment of the true diagnostic accuracy of this technique difficult. Second, our study does not elucidate the degree of AVS that is required before PCSWI signal hyperintensity can be identified in a venous structure draining a BVM, which may be particularly crucial in the follow-up of patients with treated AVMs/AVFs with small residual AVS. Third, the negative predictive value of PCSWI needs to be studied further given that only one lesion without AVS on DSA was included in our study. A prospective multi-center comparative study including a larger number of patients is likely to be useful in addressing these issues.
CONCLUSION
The presence of PCSWI signal hyperintensity within the venous structures draining a BVM is an accurate indicator of AVS from an underlying high-flow vascular malformation. Although a larger prospective study is needed, our results suggest that PCSWI appears to be superior to SWI, TOFMRA and CEMRA for the detection of AVS in BVMs. Hence, this novel sequence may be useful in the initial diagnosis and follow-up of patients with BVMs.
Acknowledgments
FUNDING
Dr. Benzinger received support from the Bracco/American Roentgen Ray Society (ARRS) Scholar Award, the National Multiple Sclerosis Society (NMSS) RG 4190A2/1, and the NIH R01 1RO1NS066905-01 and NIH/NIA AG003991-27 for this research.
Footnotes
CONFLICTS OF INTEREST
Dr. Benzinger has previously served on the Siemens speakers’ bureau where she received support for travel (no fee). Dr Moran serves as consultant for eV3 and Codman Neurovascular. He also serves in the speakers’ bureau for these companies. No conflict of interest is identified pertaining to this research project. Specifically, the authors do not know of any potential conflicts of interests pertaining to this research project with regards to relationships with pharmaceutical companies, device manufacturers or other corporations whose services or products are directly related to the subject matter of this article.
References
- 1.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YCN. Susceptibility-weighted imaging: Technical Aspects and Clinical Applications, Part 1. Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes SRS, Haacke EM. Susceptibility-weighted imaging: Clinical Angiographic Applications. Magn Reson Imaging Clin N Am. 2009;17:47–61. doi: 10.1016/j.mric.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagadeesan BD, Delgado Almandoz JE, Moran CJ, Benzinger TLS. Accuracy of Susceptibility-Weighted Imaging for the Detection of Arteriovenous Shunting in Vascular Malformations of the Brain. Stroke. 2011;42:87–92. doi: 10.1161/STROKEAHA.110.584862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KE, Choi CG, Choi JW, Choi BS, Lee DH, Kim SJ, et al. Detection of residual brain arteriovenous malformations after radiosurgery: diagnostic accuracy of contrast-enhanced three-dimensional time of flight MR angiography at 3.0 tesla. Korean J Radiol. 2009;10:333–9. doi: 10.3348/kjr.2009.10.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unlu E, Temizoz O, Albayram S, Genchellac H, Hamamcioglu MK, Kurt I, et al. Contrast-enhanced MR 3D angiography in the assessment of brain AVMs. Eur J Radiol. 2006;60:367–78. doi: 10.1016/j.ejrad.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Cashen TA, Carr JC, Shin W, Walker MT, Futterer SF, Shaibani A, et al. Intracranial time-resolved contrast-enhanced MR angiography at 3T. Am J Neuroradiol. 2006;27:822–829. [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M, Ide M, Jimbo M, Takakura K, Lindquist C, Steiner L. Neuroimaging studies of postobliteration nidus changes in cerebral arteriovenous malformations treated by gamma knife radiosurgery. Surg Neurol. 1996;45:110 – 122. doi: 10.1016/s0090-3019(96)80003-6. [DOI] [PubMed] [Google Scholar]
- 8.Hadizadeh DR, von Falkenhausen M, Gieseke J, Meyer B, Urbach H, Hoogeveen R, et al. Cerebral arteriovenous malformation: Spetzler-Martin classification at subsecond-temporal-resolution four-dimensional MR angiography compared with that at DSA. Radiology. 2008;246:205–213. doi: 10.1148/radiol.2453061684. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Ide M, Jimbo M, Takakura K, Lindquist C, Steiner L. Neuroimaging studies of postobliteration nidus changes in cerebral arteriovenous malformations treated by gamma knife radiosurgery. Surg Neurol. 1996;45:110 – 122. doi: 10.1016/s0090-3019(96)80003-6. [DOI] [PubMed] [Google Scholar]
- 10.Seiz M, Brockmann MA, Schneider UC, Woitzik J, Scharf J. Combination of supratentorial vrnous anomaly and infratentorial developmental venous anomalies mimicking AV-malformation: a case report. Zentralbl Neurochir. 2007;68:217–219. doi: 10.1055/s-2007-985854. [DOI] [PubMed] [Google Scholar]
- 11.Komiyama M, Yamanaka K, Iwai Y, Yasui T. Venous angiomas with arteriovenous shunts: report of three cases and review of the literature. Neurosurgery. 1999;44:1328–1334. doi: 10.1097/00006123-199906000-00100. discussion 1334–1335. [DOI] [PubMed] [Google Scholar]
- 12.Boukobza M, Enjolras O, Guichard JP, Gelbert F, Herbreteau D, Reizine D, et al. Cerebral developmental venous anomalies associated with head and neck venous malformations. Am J Neuroradiol. 1996;17:987–994. [PMC free article] [PubMed] [Google Scholar]
- 13.Aksoy FG, Gomori JM, Tuchner Z. Association of intracerebral venous angioma and true arteriovenous malformation: a rare, distinct entity. Neuroradiology. 2000;42:455–457. doi: 10.1007/s002340000307. [DOI] [PubMed] [Google Scholar]
- 14.Eddleman CS, Jeong HJ, Hurley MC, Zuehlsdorff S, Dabus G, Getch CG, et al. 4D radial acquisition contrast-enhanced MR angiography and intracranial arteriovenous malformations: quickly approaching digital subtraction angiography. Stroke. 2009;40:2749–53. doi: 10.1161/STROKEAHA.108.546663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du YP, Jin Z. Simultaneous acquisition of MR angiography and venography (MRAV) Magn Reson Med. 2008;59:954–958. doi: 10.1002/mrm.21581. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 17.Muthupillai R, Vick GW, Flamm SD, Chung T. Time-Resolved Contrast-Enhanced Magnetic Resonance Angiography in Pediatric Patients Using Sensitivity Encoding. J Magn Reson Imaging. 2003;17:559 – 564. doi: 10.1002/jmri.10292. [DOI] [PubMed] [Google Scholar]
- 18.Fujima N, Kudo K, Terae S, Hida K, Ishizaka K, Zaitsu Y, et al. Spinal Arteriovenous Malformation: Evaluation of Change in Venous Oxygenation with Susceptibility-Weighted MR Imaging after Treatment. Radiology. 2010;254:891–9. doi: 10.1148/radiol.09090286. [DOI] [PubMed] [Google Scholar]
- 19.Tsui YK, Tsai FY, Hasso AN, Greensite F, Nguyen BV. Susceptibility-weighted imaging for differential diagnosis of cerebral vascular pathology: a pictorial review. J Neurol Sci. 2009;287:7–16. doi: 10.1016/j.jns.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Saini J, Thomas B, Bodhey NK, Periakaruppan A, Babulal JM. Susceptibility-weighted imaging in cranial dural arteriovenous fistulas. Am J Neuroradiol. 2009;30:E6. doi: 10.3174/ajnr.A1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George U, Jolappara M, Kesavadas C, Gupta AK. Susceptibility-weighted imaging in the evaluation of brain arteriovenous malformations. Neurol India. 2010;58:608–1. doi: 10.4103/0028-3886.68668. [DOI] [PubMed] [Google Scholar]