Abstract
Objectives
The goal of this study was to investigate the effect of visual acuity on the anticipatory (APAs) and compensatory (CPAs) components of postural control.
Methods
Ten individuals participated in the experiments involving perturbations induced by a pendulum while their visual acuity was altered. The different visual acuity conditions were no glasses, blurred vision induced by wearing glasses with positive or negative lenses, and no vision. EMG activity of trunk and leg muscles and ground reaction forces were recorded during the typical anticipatory and compensatory periods.
Results
In the no vision condition the subjects did not generate APAs, which resulted in the largest displacements of the center of pressure (COP) after the perturbation (p<0.01). In all other visual conditions APAs were present showing a distal to proximal order of muscle activation. The subjects wearing positive glasses showed earlier and larger anticipatory EMGs than while wearing negative glasses or no glasses at all.
Conclusions
The study outcome revealed that changes in visual acuity induced by wearing differently powered eye glasses alter the generation APAs and as a consequence, affect the compensatory components of postural control.
Significance
The observed changes in APAs and CPAs in conditions with blurred vision induced by positive and negative glasses suggest the importance of using glasses with an appropriate power. This outcome should be taken into consideration in balance rehabilitation of individuals wearing glasses.
Keywords: Vision, Posture, Anticipatory, Compensatory
INTRODUCTION
Posture stability depends not only on the functionality of the visual system but also on the distance (Le and Kapoula 2006) and illumination of the visual targets (Straube et al. 1990). Moreover, past posturo-graphic experiments have revealed increased postural instability in conditions with reduced visual acuity induced by semitransparent foils (Paulus et al. 1984) and increased postural sway area in the elderly with poor near vision acuity (Lichtenstein et al. 1988). It was also reported that if poor visual acuity and contrast sensitivity are combined with insufficient proprioceptive information associated with standing on a compliant surface, postural sway escalates, thus increasing the risk of falls (Lord and Webster 1990; Lord et al. 1991; Elliott and Chapman 2010). Additionally, it was demonstrated that motion in the peripheral visual field and changes in stereo acuity and depth perception that result in blurring vision can affect control of posture (Previc and Neel 1995) and might increase risk for falls (Dhital et al. 2010). Furthermore, poor vision is an established risk factor for falls in the elderly (Harwood 2001; Lord 2006). It was also reported that older people who wear glasses with outdated prescriptions or no glasses at all are more prone to falls (Jack et al. 1995). Moreover, the rate of falls in the elderly who use multifocal glasses is increased and the incidence of falls in this population could be minimized by using single lens glasses (Haran et al. 2010).
Human beings frequently experience either internal or external perturbations challenging their stability. Reaching for a book or lifting a leg during gait initiation are examples of internal perturbations that we experience daily, whereas losing balance while standing on a moving bus or being hit by another person while walking in a crowded train station are examples of external perturbations. To minimize the loss of balance, the central nervous system (CNS) uses two types of adjustments in the activation of the trunk and leg muscles. The first type of correction that occurs prior to the forthcoming perturbation is based on predictions of the effect of the perturbation, and is called anticipatory postural adjustments (APAs) or feedforward postural control (Belen’kii et al. 1967; Massion 1992). Multiple studies documented that the magnitude of APAs are dependent on many factors including, but not limited to, direction (Aruin and Latash 1995b); (Santos and Aruin 2008) and magnitude of the perturbation (Aruin and Latash 1996; Nouillot et al. 2000), body stability (Nouillot et al. 1992; Aruin et al. 1998; Nouillot et al. 2000), characteristics of motor action used to induce the perturbation (Aruin and Latash 1995b; Aruin 2003; Shiratori and Aruin 2007), body configuration (van der Fits et al. 1998; Aruin 2003) and fear of falling (Adkin et al. 2002).
The second type of adjustment in the activity of postural muscles is observed after the body perturbation has already occurred. These alterations in muscle activity, termed compensatory postural adjustments (CPAs), are initiated by sensory feedback signals (Park et al. 2004; Alexandrov et al. 2005), and serve to restore the body’s position after a perturbation takes place (Macpherson et al. 1989; Maki and McIlroy 1996; Henry et al. 1998b). Past literature suggests that feedback postural control (CPAs) depends upon many factors including, but not limited to, direction and magnitude of perturbation, dimensions of base of support (Horak and Nashner 1986; Henry et al. 1998a; Dimitrova et al. 2004; Jones et al. 2008), predictability of perturbation characteristics (Burleigh and Horak 1996), instructions given to the subject (McIlroy and Maki 1993), and the implementation of a secondary task (Bateni et al. 2004).
It was demonstrated recently that CPAs are reduced in the presence of APAs and that they are increased when no APAs are generated (Santos et al. 2010a). The described relationship between APAs and CPAs was established in experiments where subjects were perturbed by a pendulum impact in conditions with either full vision (APAs are generated) or while being blindfolded (no APAs are generated). While these extreme visual conditions demonstrate the importance of visual information in generation of feedforward postural adjustments, it is unknown how other visual conditions affect the interplay between APAs and CPAs. Moreover, to our knowledge no data exists on the effect of eyeglasses in generation of APAs and CPAs.
Since changes in visual acuity affect body sway (Uchiyama and Demura 2008); (Schmid et al. 2008), one can expect that blurred vision will affect both the feedforward and feedback components of postural control. We hypothesized that blurred vision induced by wearing differently powered eye glasses will influence the generation of APAs. We also hypothesized that blurred vision will affect the relationship between APAs and CPAs. To test these hypotheses, healthy subjects were exposed to similar body perturbations while being blindfolded, wearing improper eyeglasses, or no glasses at all.
METHODS
Subjects
Ten subjects (3 males and 7 females) without any known neurological or musculoskeletal disorders participated in the experiment. The mean age of the subjects was 25.2±2.5 years; mean body mass 62.6±13 kg; and mean height 1.67±0.08m. All of the subjects had normal vision. They all signed a written informed consent approved by the Institution Review Board of the University of Illinois at Chicago.
Procedure
The subjects were instructed to maintain an upright stance with their feet shoulder width apart while standing barefoot on the force platform. They were positioned in front of an aluminum pendulum attached to the ceiling. An additional load (mass = 5% of subject’s body weight) was fixed to the pendulum at its lower end. The width of the padded hitting surface of the pendulum was adjusted to match the subject’s shoulder width. The pendulum was positioned at an initial angle of 30 degrees to the vertical (distance of 0.6 m from the body) and released by an experimenter. Perturbations consisted of unidirectional forces applied by the pendulum on the shoulders of the subjects. The subjects were instructed to look straight towards a target attached to the pendulum at eye level and maintain their balance after the perturbation. The four experimental conditions were: (1) eyes open with no eye glasses (no glasses), (2) eyes open with eye glasses of +10 Dioptre (positive), (3) eyes open with eye glasses of −6.0 Dioptre (negative), and (4) eyes closed (no vision) (Fig. 1). Wearing positive glasses (convex lens) forms the image in front of the retina, causing the image to appear blurred; looking through negative glasses (concave lens) forms the image behind the retina causing the object to appear clear at first but become blurrier as it gets closer (Maheshwari and Williams 2010). As such, the positive powered eye glasses blurred and magnified the image of the pendulum, and the negative powered eye glasses blurred and diminished the size of the pendulum as seen by the subject. In the no-vision condition, the subjects did not see a pendulum at all and were therefore not aware of the moment of its release. The magnitude and direction of the pendulum perturbations were kept constant throughout the study.
Fig. 1.
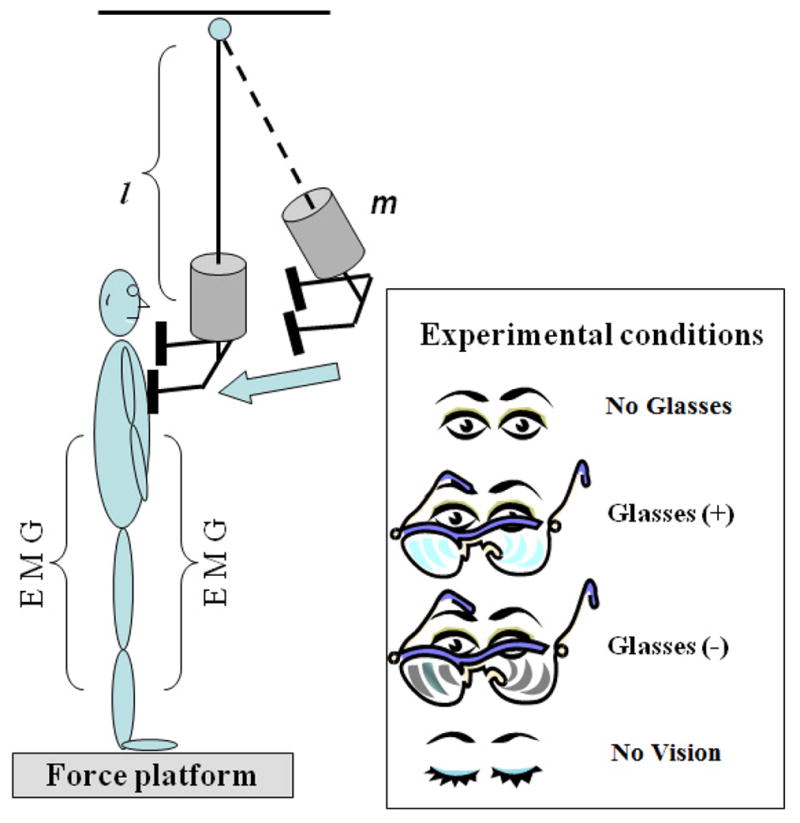
Schematic diagram of the experimental setup. The subjects stood with their arms at their sides, and the pendulum impact was applied to their shoulders while visual conditions were manipulated. l is the length and m is an additional mass (5% of subject’s body weight).
In addition, no advance warning of the impending perturbation was provided; the subjects wore wireless headphones playing music throughout all of the conditions to mask any kind of auditory information which may alert them about the moment of release of the pendulum. For safety purposes during all of the experimental conditions the participants were provided with a harness with two straps attached to the ceiling. The subjects performed two to three practice trials in each experimental condition prior to the start of data collection. Five trials each of 5 s in duration were performed in each experimental condition and the order of the experiments was randomized across each subject.
Instrumentation
Electrical activity of muscles (EMGs) were recorded unilaterally (right side) from the following muscles: tibialis anterior (TA), soleus (Sol), biceps femoris (BF), rectus femoris (RF), gluteus medius (GMed), rectus abdominis (RA), external oblique (EO) and erector spinae (ES). The selected trunk and leg muscles are involved in control of vertical posture while dealing with symmetrical perturbations induced in the sagittal plane and were previously used to study anticipatory and compensatory control of posture (e.g. Latash et al. 1995; Aruin and Latash 1996). After the skin area was cleaned with alcohol wipes, disposable electrodes (Red Dot 3M) were attached to the muscle belly of each of the above mentioned muscles, based on recommendations reported in the literature (Basmajian 1980). After similar skin preparations, a ground electrode was attached to the anterior aspect of the leg over the tibial bone. The EMG signals were collected, filtered and amplified (10–500 Hz, gain 2000) with a commercially available EMG system (Myopac, RUN Technologies, USA).
Ground reaction forces and moments of forces were recorded using a force platform (Model OR-5, AMTI, USA). An accelerometer (Model 208CO3, PCB Piezotronics Inc, USA) was attached to the subject’s proximal clavicle; its signal was used to record the moment of pendulum impact. The forces, moments of forces, EMG and accelerometer signals were digitized with a 16 bit resolution at 1000 Hz by means of a customized LabVIEW 8.6.1 software (National Instruments, Austin TX, USA).
Data processing
The data were analyzed off-line using the MATLAB (Math Works, Natick, MA) program. First, the accelerometer signal was corrected for offset, and ‘time-zero’ (T0=0, the moment of pendulum impact) was acquired by a computer algorithm as a point in time at which the signal exceeded 5% of the maximum acceleration. This value was confirmed through visual inspection by an experienced researcher. Then, all trials were aligned to T0. EMG signals were then rectified and filtered with a 50 Hz low-pass, 2nd order, zero-lag Butterworth filter, while the reaction forces and moments were filtered with a 20 Hz low-pass, 2nd order, zero-lag Butterworth filter. Data in the range from −1000 ms (before T0) to +1000 ms (after T0) were selected for further analysis. Processed trials within each condition were averaged for each subject. Integrals of EMG, muscle latencies, and center of pressure displacements were calculated.
i) Integrals of the EMG
The average EMG signals for each muscle and each subject were integrated (IntEMGi) with 150 ms time windows for a total of 6 time windows representing the −400 ms to +500 ms of the data. Each of the time windows were further corrected by the averaged 150 ms baseline activity time window of the corresponding EMG integral from −1000 ms to −850 ms in relation to T0.
| (1) |
In equation 1, IntEMGi is the integral of EMG activity of muscles inside each 150 ms interval which was corrected by the baseline activity. Then the integrals of EMG for each muscle for each subject were normalized to peak activity across all of the conditions (equation 2).
| (2) |
Due to the normalization, all of the IEMGNORM values were within the range from +1 to −1. Four epochs were selected (each of 150 ms in duration) in relation to T0. The four epochs were: (1) from −250 ms to −100 ms (anticipatory, APA1); (2) from −100 ms to +50 ms (anticipatory, APA2); (3) from +50 ms to +200 ms (compensatory reactions, CPA1); and (4) +200 ms to +350 ms (late compensatory reactions, CPA2) (Santos et al. 2010b; Santos et al. 2010a).
ii) Muscle latencies
Muscle latencies were detected in a time window from −250 ms to +250 ms in relation to T0 by a combination of a computer algorithm and visual inspection of the individual trials for each muscle. To identify the baseline, mean and standard deviation (SD) of the EMG signal were calculated from −500 ms to −400 ms before T0. The latency for a specific muscle was defined as the instant lasting for at least 50 ms when its EMG amplitude was greater (activation) or smaller (inhibition) than the mean plus 2 SD of the baseline. The latencies of the muscles were categorized as: (1) Distal muscles (SOL and TA), (2) Intermediate muscles (RF, BF, and GMED), and (3) Proximal muscles (EO, RA and ES) (Aruin and Latash 1995a).
iii) COP calculation
Time-varying COPAP displacement was calculated using the following approximation (Winter et al. 1996):
| (3) |
Where Mx is the moment in the sagittal plane, Fz and Fy are the vertical and anterior-posterior components of the ground reaction force, and dz is the distance from the origin of the platform to the surface (0.038 m). Since the perturbations were induced symmetrically, only COP displacements in the anterior-posterior direction (Y-axis according to our experimental set-up) will be reported. The COPAP signals were averaged with 50 ms time windows for a total of 20 time windows representing the −450 ms to +550 ms of the data and corrected by its respective baseline (averaged COP baseline activity from −950 ms to −800 ms in relation to T0). The COP data windows were shifted 50 ms forward to account for the electro-mechanical delay (Cavanagh and Komi 1979; Howatson et al. 2009). We calculated the peak magnitude of the COP, the time of the peak magnitude, and the magnitude of COPAP at the moment of perturbation (T0).
Statistics
Multiple repeated measures ANOVAs were performed with two within subject factors: visual condition (No glasses, Negative, Positive and No vision) and epochs (APA1, APA2, CPA1 and CPA2). Subsequent multiple repeated measures ANOVAs were performed with three visual conditions and four epochs. The vision conditions were a) normal-negative, b) normal-positive, and c) positive-negative. The dependent variables were IEMGNORMs, latency of trunk and leg muscles, peak magnitude of the COPAP, time of the peak magnitude, and magnitude of COPAP at the moment of perturbation (T0). A post hoc analysis with Bonferroni correction was further done to compare between conditions, epochs and their interactions. In case the Mauchly’s test of sphericity was not met, the Greenhouse-Geisser correction was used. For all tests, statistical significance was set at p < 0.05. Statistical analysis was performed in SPSS 17 for Windows 7 (SPSS Inc., Chicago, USA).
RESULTS
EMG Profiles
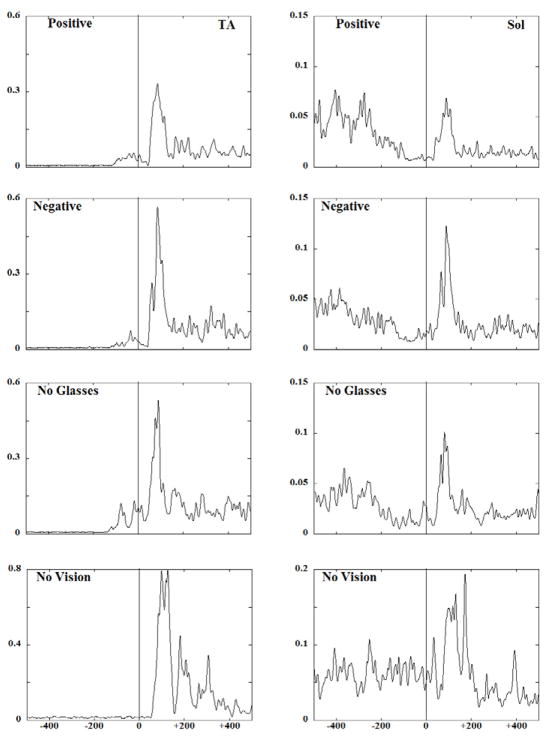
Fig. 2 shows EMG traces obtained from the anterior (TA) and the posterior (Sol) muscles of a representative subject performing the experimental task under four different visual conditions. Anticipatory activity seen as bursts (TA) and inhibition (Sol) of the background EMG activity was present in the three conditions with vision available. Quite to the contrary, anticipatory activity was negligible in the no vision condition. As a result, larger compensatory EMG activity was observed in conditions with no vision as compared to other visual conditions.
Fig. 2.
EMG patterns (averaged across 5 trials) for a representative subject for the anterior (TA) and the posterior (SOL) muscles of the right side are presented across all the visual acuity conditions
Integrals of EMG activity
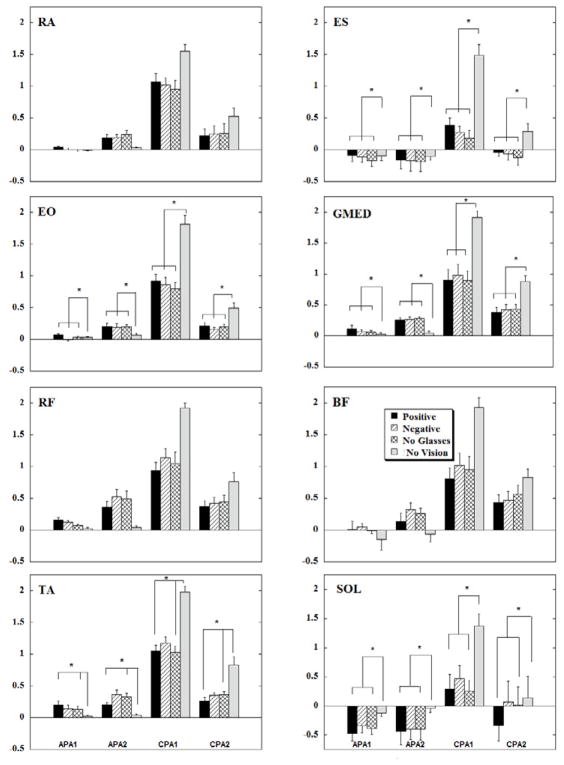
Figure 3 shows anticipatory IEMGNORM of the trunk and leg muscles averaged across subjects. In general, the anticipatory integrals of EMG (APA1 and APA2) are seen in all conditions with vision available. On the contrary, anticipatory integrals of EMG for the no vision condition were negligible in the three anterior muscles (RA, RF, and TA). Moreover, the magnitudes of the CPA integrals, especially the CPA1, were the largest in the no vision condition. Comparatively, the existence of anticipatory activity of the anterior muscles in the conditions with no glasses and with differently powered glasses was associated with smaller integrals of EMG during the CPA1 and CPA2 intervals as compared to the no vision condition. Table 1 shows the repeated measures ANOVA results for conditions, epochs and their interaction. The statistical analysis revealed that there was a significant effect of the conditions, epochs and their interactions. Post hoc analysis showed the no vision condition to be significantly different from conditions with no glasses and conditions in which subjects wore positive eye glasses in the TA (p<0.05). Post hoc analysis also showed that the APA1 and APA2 were significantly different from CPA1 across all anterior muscles (p<0.01). Moreover, the IEMGNORM for TA showed a trend in which the CPA1 magnitude was the highest followed by CPA2, APA2 and APA1.
Fig 3.
Mean EMG integrals during the four visual acuity conditions across ten subjects. Each column represents the IntEMGi for 150 ms epochs (APA1, APA2, CPA1 and CPA2) with its standard error bars. * indicates statistical significance between visual conditions at p<0.05.
Table 1.
Repeated measures ANOVA of IEMGNORM for conditions, epochs and their interaction. The results are shown for the four experimental conditions: no glasses, positive and negative glasses and no vision and four epochs: APA1, APA2, CPA1 and CPA2.
| Muscles | Conditions | Epochs | Conditions X Epochs | |||
|---|---|---|---|---|---|---|
| F (3,27) | p | F (3,27) | p | F (9,81) | p | |
| RA | 3.49 | 0.03 | 49.19 | <0.0001 | 7.75 | <0.0001 |
| RF | 4.92 | 0.007 | 99.63 | <0.0001 | 25.87 | <0.0001 |
| TA | 10.55 | <0.0001 | 163.90 | <0.0001 | 19.75 | <0.0001 |
| ES | 21.13 | <0.0001 | 35.94 | <0.0001 | 15.69 | <0.0001 |
| BF | 3.82 | 0.06 | 35.84 | <0.0001 | 25.26 | <0.0001 |
| SOL | 10.41 | <0.0001 | 13.25 | <0.0001 | 3.89 | <0.0001 |
| EO | 16.82 | <0.0001 | 177.35 | <0.0001 | 19.33 | <0.0001 |
| GMED | 14.14 | <0.0001 | 79.52 | <0.0001 | 23.62 | <0.0001 |
The posterior muscles (ES and Sol, with the exception of BF), showed inhibition during the APA1 and APA2 epochs in all conditions involving vision (Fig. 3). ES, BF, and Sol IEMGNORM calculated during the APA epochs were negligible in the no vision condition, but were, however, very large during the CPA1 epoch across all muscles in the no vision condition. The results of statistical analysis revealed that the epochs and their interactions were significant across all muscles. Additionally, the effect of conditions was significantly different for the ES and SOL muscles. Post hoc analysis showed the no vision condition to be significantly different from other visual acuity conditions in ES (p<0.01). Moreover, IEMGNORM in the SOL during the no vision condition were significantly different from integrals calculated in conditions with no glasses and in conditions wearing positive eye glasses (p<0.05). The APA1 and APA2 epochs were significantly different from the CPA1 epoch in all posterior muscles (p<0.01).
The anticipatory integrals of EMGs for the lateral muscles (EO, GMed) were also observed in conditions with vision available, and the APA integrals were negligible in the no vision conditions (Fig. 3). The absence of APAs in the no vision conditions resulted in large IEMGNORM during the CPA epochs. Statistical analysis revealed significant effects of conditions, epochs and their interaction. Post hoc analysis showed that the no vision condition to be significantly different from other visual acuity conditions for both the EO and GMed muscles (p<0.05). The integrals for the APA 1 and APA 2 epochs were significantly different from IEMGNORM during the CPA 1 and CPA 2 epochs in both of these muscles (p<0.01). Post hoc analysis of the epochs for the EO and GMed showed a trend in which CPA1 magnitude was the highest followed by CPA2, APA2 and APA1.
When statistical analysis was performed comparing the three visual conditions, the results revealed a significant effect for BF: main effect of vision (F2,18=3.19; p=0.058) and main effect of epochs (F3,27=15.67; p<0.01) but no interaction. Pair wise analysis of IEMGNORM between conditions with positive and negative glasses showed that the difference was approaching the level of statistical significance (p=0.058). Post hoc analysis of the epochs for all the studied muscles showed that CPA1 epoch was significantly greater than APA2 epoch.
Additional statistical analyses were performed to assess the effect of blurred vision. Thus, there was a significant main effect of condition (positive and negative glasses) in BF (F 1, 9=8.09, p=0.02), Sol (F 1, 9=7.13, p=0.026), RF (F 1,9=5.23 p=0.048) and it was close to the level of statistical significance in EO (F 1,9=4.06 ,p=0.07). There were conditions-epochs interactions observed in RF (positive-negative glasses, F3, 27=3.94 p=0.019 and no glasses-positive glasses F3, 27=3.51, p=0.029). The visual conditions (no glasses-positive glasses) × epochs interactions revealed that positive glasses resulted in increased magnitudes of RF IEMGNORM in the APA1 whereas it decreased during the CPA1. In addition, visual conditions (negative glasses-positive glasses) × epochs interactions revealed lesser IEMGNORM in APA1 in conditions with negative glasses which resulted in larger IEMGNORM in CPA1 epoch.
Onsets of EMG activity
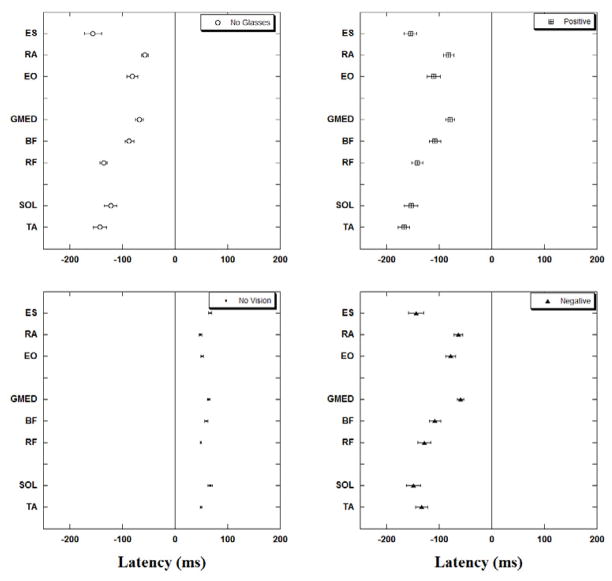
The onsets of EMG activity of all studied muscles for each of the four experimental conditions are presented in Fig. 4. In all conditions with vision available (no glasses, positive and negative glasses) a distal to proximal pattern of activation or inhibition of muscles (with the exception of ES) could be noticed. Thus, in the no glasses condition the distal muscles showed anticipatory activation (e.g. TA at −142.1±12.3 ms) followed by the activation of intermediate muscles (e.g. RF at −135.4±7.2 ms). The proximal group of muscles were the last to activate (e.g. RA at −56.8±6.7 ms). When the subjects wore positive glasses, a similar distal to proximal pattern was seen (TA at −166.7±10.6 ms, RF at −141.6±10.4 ms, and RA at −81.3±10.3 ms). This pattern was also preserved in conditions with negative glasses (TA at −133.2±11.6 ms, RF at −127.4±12.2 ms, and RA at −63.03±8.1 ms). In the no vision condition, muscles were active only after the perturbation (e.g. TA at 49.9±1.6, RF at 49.4±1.6, and RA at 48.8±2.2) and did not follow any specific pattern of activation or inhibition.
Fig. 4.
Muscle latencies for the four experimental conditions. Note that for the no vision conditions the onset of activity for all studied muscles is after the perturbation (T0). The onsets of activity of muscles in conditions with no glasses, positive and negative eyeglasses are before the perturbation impact (T0). Latencies are grouped for proximal, intermediate and distal muscles.
In general, in conditions with positive glasses, latencies of all muscles were the earliest when compared to other experimental conditions. The latencies recorded in conditions with negative glasses and no glasses at all followed the latencies in the conditions with positive glasses. This could be best observed in Sol, BF, RA, and ES (negative glasses) and in TA, RF, GMed and EO (no glasses).
The results of statistical analysis comparing the four experimental conditions are presented in Table 2. Pair-wise analysis revealed that significantly different results for all the muscles were only found for the no vision condition when compared with all other visual conditions (P<0.05) as well as between the positive and no glasses conditions (p<0.05).
Table 2.
Repeated measures ANOVA of latencies for all the muscles. The results are shown for the four experimental conditions: no glasses, positive and negative glasses and no vision.
| Muscles | RA | RF | TA | ES | BF | Sol | EO | GMED |
|---|---|---|---|---|---|---|---|---|
| F3,120 | 60.07 | 123.52 | 333.62 | 83.89 | 89.38 | 103.06 | 82.09 | 110.95 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Additional statistical analysis comparing the latencies in the three visual conditions (no glasses, positive and negative glasses) revealed a main effect of conditions for TA (F2,78=3.85, p=0.03), Sol (F2,78=3.05, p=0.05), and EO (F2,78=4.46, p=0.02). Pair wise comparison revealed differences between the positive and negative glasses conditions in TA (p=0.02) while it approached the level of significance in EO (p=0.05). In addition, differences between the positive and no glasses conditions were near the level of statistical significance for EO (p=0.05) and Sol (p=0.07) muscles.
Changes in the COP displacement
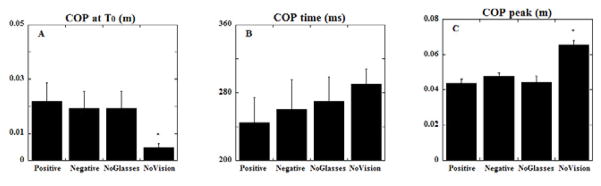
The COP displacements were all in the backward direction irrespective of the visual condition. The statistical analysis comparing all the four conditions showed main effect for conditions in displacement of COP (F 3,27= 24.28, p<0.0001) and for the peak COP displacement after perturbation (F 3,27=24.43, p<0.0001). The displacement of COP at the moment of perturbation (T0) in the no vision condition was the smallest (p<0.01) (Fig 5a). Although not statistically significant, the time at which COP displacement reached its peak after the perturbation was the shortest in experiments with positive glasses. It was slightly longer in conditions with negative glasses and no glasses. In the no vision condition the time at which COP displacement reached its peak was the longest among all four conditions (Fig 5b). The peak COP displacement (measured after the perturbation) was significantly higher (p<0.01) for the no vision condition when compared to the other conditions in which vision was available (Fig 5c).
Fig. 5.
The magnitude of COP displacement at T0 (A), the time of the COP peak (B) and the magnitude of peak COP displacement (C) are shown as mean with standard errors. COP positive values correspond to displacements in the direction opposite to the perturbation. Note that, COP displacements in conditions of standing with no vision are larger than in conditions with no glasses and when wearing positive or negative eye glasses.
However, when statistical analysis was performed for the three vision conditions, no statistical significant results were found.
DISCUSSION
The current study was designed to investigate how the changes in visual acuity, induced by wearing differently powered glasses, influence APAs and CPAs in terms of muscular activity and COP displacements. The outcome of the experiments demonstrated that the subjects were able to generate APAs in conditions with blurred vision, thus, partially disputing the hypotheses that blurred vision induced by wearing differently powered eye glasses will affect the generation of APAs. The second hypothesis, that blurred vision influences the relationship between APAs and CPAs, however, was supported as we observed vision condition-related changes in CPAs.
1. Role of full vision
The role of vision in minimizing body sway has been reported earlier (Paulus et al. 1984; Wade and Jones 1997). However, the investigation of the effect of vision on generation of anticipatory postural adjustments is scarce. This is partially due to the fact that APAs were mostly studied using self-initiated body perturbation such as fast arm (Belenkiy et al. 1967; Friedli et al. 1984) or leg (Nardone and Schieppati 1988) movements. Since the parameters of the self-induced body perturbation became available to the subject’s CNS even with no vision, APAs could be expected even in conditions with no vision. Moreover, previously used approaches included voluntary movements, such as arm lifts (used to study APAs and CPAs) or application of unexpected external perturbations, such as movement of the platform on which the subjects stood (to study CPAs) (Hughey and Fung 2005; Gage et al. 2007). However, due to the difference in nature of the perturbations applied, the control processes differed and the outcomes could not be compared. The pendulum-impact paradigm that we used in the current study allows us to apply the same magnitude of whole-body external perturbation (the nature of which does not change during the experiments) to human upright posture, thereby allowing both APAs and CPAs and their interaction to be studied.
The results of the current study reveal that in conditions with vision available, strong APAs (seen as bursts of activity in the majority of muscles and inhibition of activity in ES and Sol) were observed. However, when vision was not available, no APAs were called into play since no information on the moment of the pendulum release was available. Hence, large compensatory postural adjustments were used in order to restore balance (Fig. 3). This result is in accordance with the recent literature that reported a lack of APAs and large CPAs in blindfolded conditions (Santos et al. 2010a). Thus, the outcome of the current study further supports previous data on the importance of full vision in the generation of anticipatory postural adjustments and subsequent minimization of compensatory corrections.
2. Role of blurred vision
It has been described in the literature that blurred vision affects how humans control their posture. For example, it was reported that blurred vision was responsible for the increased postural instability in healthy subjects when somatosensory and vestibular inputs were disrupted (Anand et al. 2002). Moreover, there was a 25% increase in postural instability in individuals with myopia when they did not wear glasses (Paulus et al. 1984). Visual loss can also be associated with age related macular degeneration (Szabo et al. 2008), or due to diseases such as Glaucoma, Cataract, Diabetes Mellitus (Dhital et al. 2010) and multiple sclerosis (Regan et al. 1981). It was also reported that subjects took 11% longer to execute a stepping task when their vision was blurred by cataract simulation (Heasley et al. 2004).
Blurred vision in the current study was induced by wearing eyeglasses with positive or negative lenses which affected the perception of the pendulum impact. When compared to the perception of the pendulum in the no glasses condition, negative glasses caused the pendulum to appear clear but farther away at the moment of its release, while positive glasses caused the pendulum to appear blurred and closer at the moment of its release.
The observed differences in latencies of anticipatory EMGs as well as IEMGNORM for certain conditions (i.e., negative glasses-positive glasses) were associated with differences between eyeglass types. For example, it appears as though positive lenses create a perception that the pendulum is going to hit the body sooner than in conditions with no glasses. This perception might result in the CNS selecting a strategy of an earlier and larger anticipatory activation of certain postural muscles to prevent body destabilization. On the other hand, negative lenses cause the object to be perceived as positioned farther away, thus delaying and reducing the anticipatory preparation needed to handle the forthcoming perturbation. Indeed, the latency analysis revealed that anticipatory activation of muscles was seen early in conditions with positive glasses as compared to negative glasses or no glasses conditions. Hence, we can assume that the glasses-related differences in perception of the pendulum affect the timing and magnitude of anticipatory muscle activation. Furthermore, we observed that the vision-related changes in APAs translated to differences in the magnitude and timing of CPAs. Thus, peak COPAP displacement was the earliest for the positive glasses condition, followed by conditions with negative glasses, while the no vision condition showed peak displacement the latest. The magnitude of the CPA, measured as the peak COPAP displacement, was the largest for the no vision condition. This result is in agreement with the literature that shows that while standing quietly, COPAP displacements are significantly higher when the visual target is positioned at a far distance (200 cm), compared to the target located at a near distance (40 cm) (Le and Kapoula 2006).
3. Relationship between APAs and CPAs
The existence of a relationship between the anticipatory and the compensatory components of postural control has been described in experiments with full vision and when visual information about the upcoming body perturbation was not available (Santos et al. 2010b; Santos et al. 2010a). However, there are conditions when vision is partially obstructed or diminished due to, for example, dim light or health issues. In addition, studies have shown that using inappropriate eye glasses, especially in older people, creates a significant risk factor for falls (Lord 2006). Therefore, it is important to gain knowledge on whether changes in the generation of APAs due to modified vision translate into changes of the compensatory component of postural control.
The results of this experiment suggest that modifying vision conditions by using differently powered glasses alters the generation of APAs, and as a result, affects CPAs. Thus, anticipatory IEMGNORM in RF, GMed, and TA were larger in the positive glasses condition when compared to the negative glasses condition. This resulted in small IEMGNORM in conditions with positive glasses during the compensatory phase. Such an eyeglasses-related alteration in the electrical activity of muscles was accompanied by small changes in the magnitude of the COP peak displacement during the CPA periods: COP peaks were larger while wearing negatively powered glasses and smaller while wearing positively powered glasses. Thus, it seems as though the existence of APAs in conditions with differently powered glasses was a primary reason for the diminishing CPAs. Moreover, information about the forthcoming perturbation was available to the CNS even in conditions with altered vision (wearing positive and negative glasses). As a result, APAs were generated and subsequent CPAs were smaller compared to conditions with no vision. This suggests that 1) Even with altered vision the CNS could successfully utilize feed forward postural control, and 2) CPAs are still influenced by APAs even when vision is modified with differently powered glasses. Indeed, earlier and larger anticipatory activation of certain postural muscles in the condition with positive glasses resulted in lesser magnitudes of compensatory corrections as compared to the condition with negative glasses.
Conclusion
The study outcome revealed that vision plays an important role in the generation of anticipatory and compensatory corrections while maintaining posture. It was also demonstrated that changes in visual acuity induced by differently powered glasses might affect the way anticipatory postural control is generated, and as a consequence, affect the compensatory components of postural control. Hence, the role of properly selected glasses in postural control should not be underestimated.
HIGHLIGHTS.
The effects of visual acuity in control of posture were examined.
Blurred vision induced by positive and negative glasses affects feedforward and feedback postural control
The role of properly selected glasses in postural control is highlighted
Acknowledgments
This work was supported in part by NIH grant HD-51628 and NIDRR grant H133P060003. We thank Maria Reyes Lopez Cerezo for the help in the data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkin AL, Frank JS, Carpenter MG, Peysar GW. Fear of falling modifies anticipatory postural control. Exp Brain Res. 2002;143:160–170. doi: 10.1007/s00221-001-0974-8. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biol Cybern. 2005;93:309–322. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand V, Buckley J, Scally A, Elliott DB. The effect of refractive blur on postural stability. Ophthalmic Physiol Opt. 2002;22:528–534. doi: 10.1046/j.1475-1313.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- Aruin AS. The effect of changes in the body configuration on anticipatory postural adjustments. Motor Control. 2003;7:264–277. doi: 10.1123/mcj.7.3.264. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Forrest WR, Latash ML. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol. 1998;109:350–359. doi: 10.1016/s0924-980x(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res. 1995a;103:323–332. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res. 1995b;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalogr Clin Neurophysiol. 1996;101:497–503. doi: 10.1016/s0013-4694(96)95219-4. [DOI] [PubMed] [Google Scholar]
- Basmajian JV. Electromyography--dynamic gross anatomy: a review. Am J Anat. 1980;159:245–260. doi: 10.1002/aja.1001590302. [DOI] [PubMed] [Google Scholar]
- Bateni H, Zecevic A, McIlroy WE, Maki BE. Resolving conflicts in task demands during balance recovery: does holding an object inhibit compensatory grasping? Exp Brain Res. 2004;157:49–58. doi: 10.1007/s00221-003-1815-8. [DOI] [PubMed] [Google Scholar]
- Belen’kii VE, Gurfinkel VS, Pal’tsev EI. Control elements of voluntary movements. Biofizika. 1967;12:135–141. [PubMed] [Google Scholar]
- Belenkiy V, Gurfinkel V, Pal’tsev Y. Elements of control of voluntary movements. Biofizika. 1967;10:135–141. [PubMed] [Google Scholar]
- Burleigh A, Horak F. Influence of instruction, prediction, and afferent sensory information on the postural organization of step initiation. J Neurophysiol. 1996;75:1619–1628. doi: 10.1152/jn.1996.75.4.1619. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Dhital A, Pey T, Stanford MR. Visual loss and falls: a review. Eye (Lond) 2010;24:1437–1446. doi: 10.1038/eye.2010.60. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J Neurophysiol. 2004;91:489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- Elliott DB, Chapman GJ. Adaptive gait changes due to spectacle magnification and dioptric blur in older people. Invest Ophthalmol Vis Sci. 2010;51:718–722. doi: 10.1167/iovs.09-4250. [DOI] [PubMed] [Google Scholar]
- Friedli WG, Hallett M, Simon SR. Postural adjustments associated with rapid voluntary arm movements 1. Electromyographic data. J Neurol Neurosurg Psychiatry. 1984;47:611–622. doi: 10.1136/jnnp.47.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage WH, Zabjek KF, Hill SW, McIlroy WE. Parallels in control of voluntary and perturbation-evoked reach-to-grasp movements: EMG and kinematics. Exp Brain Res. 2007;181:627–637. doi: 10.1007/s00221-007-0959-3. [DOI] [PubMed] [Google Scholar]
- Haran MJ, Cameron ID, Ivers RQ, Simpson JM, Lee BB, Tanzer M, et al. Effect on falls of providing single lens distance vision glasses to multifocal glasses wearers: VISIBLE randomised controlled trial. BMJ. 2010;340:c2265. doi: 10.1136/bmj.c2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood RH. Visual problems and falls. Age Ageing. 2001;30(Suppl 4):13–18. doi: 10.1093/ageing/30.suppl_4.13. [DOI] [PubMed] [Google Scholar]
- Heasley K, Buckley JG, Scally A, Twigg P, Elliott DB. Stepping up to a new level: effects of blurring vision in the elderly. Invest Ophthalmol Vis Sci. 2004;45:2122–2128. doi: 10.1167/iovs.03-1199. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. Control of stance during lateral and anterior/posterior surface translations. IEEE Trans Rehabil Eng. 1998a;6:32–42. doi: 10.1109/86.662618. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol. 1998b;80:1939–1950. doi: 10.1152/jn.1998.80.4.1939. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- Howatson G, Glaister M, Brouner J, van Someren KA. The reliability of electromechanical delay and torque during isometric and concentric isokinetic contractions. J Electromyogr Kinesiol. 2009;19:975–979. doi: 10.1016/j.jelekin.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Hughey LK, Fung J. Postural responses triggered by multidirectional leg lifts and surface tilts. Exp Brain Res. 2005;165:152–166. doi: 10.1007/s00221-005-2295-9. [DOI] [PubMed] [Google Scholar]
- Jack CI, Smith T, Neoh C, Lye M, McGalliard JN. Prevalence of low vision in elderly patients admitted to an acute geriatric unit in Liverpool: elderly people who fall are more likely to have low vision. Gerontology. 1995;41:280–285. doi: 10.1159/000213695. [DOI] [PubMed] [Google Scholar]
- Jones SL, Henry SM, Raasch CC, Hitt JR, Bunn JY. Responses to multi-directional surface translations involve redistribution of proximal versus distal strategies to maintain upright posture. Exp Brain Res. 2008;187:407–417. doi: 10.1007/s00221-008-1312-1. [DOI] [PubMed] [Google Scholar]
- Latash ML, Aruin AS, Neyman I, Nicholas JJ. Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1995;58:326–334. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Kapoula Z. Distance impairs postural stability only under binocular viewing. Vision Res. 2006;46:3586–3593. doi: 10.1016/j.visres.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Lichtenstein MJ, Shields SL, Shiavi RG, Burger MC. Clinical determinants of biomechanics platform measures of balance in aged women. J Am Geriatr Soc. 1988;36:996–1002. doi: 10.1111/j.1532-5415.1988.tb04365.x. [DOI] [PubMed] [Google Scholar]
- Lord SR. Visual risk factors for falls in older people. Age Ageing. 2006;35(Suppl 2):ii42–ii45. doi: 10.1093/ageing/afl085. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Visual acuity and contrast sensitivity in relation to falls in an elderly population. Age Ageing. 1991;20:175–181. doi: 10.1093/ageing/20.3.175. [DOI] [PubMed] [Google Scholar]
- Lord SR, Webster IW. Visual field dependence in elderly fallers and non-fallers. Int J Aging Hum Dev. 1990;31:267–277. doi: 10.2190/38MH-2EF1-E36Q-75T2. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Horak FB, Dunbar DC, Dow RS. Stance dependence of automatic postural adjustments in humans. Exp Brain Res. 1989;78:557–566. doi: 10.1007/BF00230243. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Williams DR. Learning Optics using Vision. Vol. 2010 2010. [Google Scholar]
- Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med. 1996;12:635–658. [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Changes in early ‘automatic’ postural responses associated with the prior-planning and execution of a compensatory step. Brain Res. 1993;631:203–211. doi: 10.1016/0006-8993(93)91536-2. [DOI] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Postural adjustments associated with voluntary contraction of leg muscles in standing man. Exp Brain Res. 1988;69:469–480. doi: 10.1007/BF00247301. [DOI] [PubMed] [Google Scholar]
- Nouillot P, Bouisset S, Do MC. Do fast voluntary movements necessitate anticipatory postural adjustments even if equilibrium is unstable? Neurosci Lett. 1992;147:1–4. doi: 10.1016/0304-3940(92)90760-5. [DOI] [PubMed] [Google Scholar]
- Nouillot P, Do MC, Bouisset S. Are there anticipatory segmental adjustments associated with lower limb flexions when balance is poor in humans? Neurosci Lett. 2000;279:77–80. doi: 10.1016/s0304-3940(99)00947-7. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res. 2004;154:417–427. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Paulus WM, Straube A, Brandt T. Visual stabilization of posture. Physiological stimulus characteristics and clinical aspects. Brain. 1984;107 ( Pt 4):1143–1163. doi: 10.1093/brain/107.4.1143. [DOI] [PubMed] [Google Scholar]
- Previc FH, Neel RL. The effects of visual surround eccentricity and size on manual and postural control. J Vestib Res. 1995;5:399–404. [PubMed] [Google Scholar]
- Regan D, Raymond J, Ginsburg AP, Murray TJ. Contrast sensitivity, visual acuity and the discrimination of Snellen letters in multiple sclerosis. Brain. 1981;104:333–350. doi: 10.1093/brain/104.2.333. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Role of lateral muscles and body orientation in feedforward postural control. Exp Brain Res. 2008;184:547–559. doi: 10.1007/s00221-007-1123-9. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010a;20:388–397. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol. 2010b;20:398–405. doi: 10.1016/j.jelekin.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Casabianca L, Bottaro A, Schieppati M. Graded changes in balancing behavior as a function of visual acuity. Neuroscience. 2008;153:1079–1091. doi: 10.1016/j.neuroscience.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Aruin A. Modulation of anticipatory postural adjustments associated with unloading perturbation: effect of characteristics of a motor action. Exp Brain Res. 2007;178:206–215. doi: 10.1007/s00221-006-0725-y. [DOI] [PubMed] [Google Scholar]
- Straube A, Paulus W, Brandt T. Influence of visual blur on object-motion detection, self-motion detection and postural balance. Behav Brain Res. 1990;40:1–6. doi: 10.1016/0166-4328(90)90037-f. [DOI] [PubMed] [Google Scholar]
- Szabo SM, Janssen PA, Khan K, Potter MJ, Lord SR. Older women with age-related macular degeneration have a greater risk of falls: a physiological profile assessment study. J Am Geriatr Soc. 2008;56:800–807. doi: 10.1111/j.1532-5415.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Demura S. Low visual acuity is associated with the decrease in postural sway. Tohoku J Exp Med. 2008;216:277–285. doi: 10.1620/tjem.216.277. [DOI] [PubMed] [Google Scholar]
- van der Fits IB, Klip AW, van Eykern LA, Hadders-Algra M. Postural adjustments accompanying fast pointing movements in standing, sitting and lying adults. Exp Brain Res. 1998;120:202–216. doi: 10.1007/s002210050394. [DOI] [PubMed] [Google Scholar]
- Wade MG, Jones G. The role of vision and spatial orientation in the maintenance of posture. Phys Ther. 1997;77:619–628. doi: 10.1093/ptj/77.6.619. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]