Abstract
Impaired ovarian function alters lipid metabolism, ultimately resulting in increased visceral fat mass. Currently, we have a poor understanding of alterations in signaling events regulating lipolysis after ovarian function declines. The purpose of this study was to determine if cellular mechanisms regulating lipolysis are altered in mice after ovariectomy (OVX) and if OVX mice exhibit impaired lipolytic signaling when stimulated by acute exercise. SHAM and OVX mice were divided into two groups: control (SHAM cont; OVX cont) or acute treadmill exercise (SHAM ex; OVX ex). The omental/mesenteric (O/M) fat mass of all OVX mice was significantly greater than the SHAM mice. Serum glycerol and blood glucose levels were significantly elevated in OVX cont compared to SHAM cont. Treadmill exercise increased serum glycerol levels only in SHAM mice, with no exercise-induced change detected in OVX mice. NEFA levels were significantly elevated by acute exercise in the SHAM and OVX groups. In O/M fat from both OVX groups there were significant increases in cytosolic ATGL and PLIN2 in the fat cake fraction with concurrent reductions in PLIN1 in the fat cake compared to SHAM. Further, exercise induced significant increases in HSL Ser660 phosphorylation in SHAM mice, but not OVX mice. This suggests that reduced ovarian function has significant effects on critical lipolytic cell signaling mechanisms in O/M adipose tissue.
Keywords: Lipid, Adipose, Adipose Triglyceride Lipase, Lipolysis, PLIN, Female sex steroids
INTRODUCTION
The prevalence of obesity-related conditions, such as the metabolic syndrome, has increased dramatically in recent years. Android, or centripetal (i.e. visceral) obesity, is of particular concern due to its high correlation with the development of obesity-related conditions. While expansion of subcutaneous adipose tissue depots may be metabolically favorable [Kim et al., 2007], studies have linked visceral fat accumulation to increased incidence rates of type 2 diabetes, cardiovascular disease, and risk of myocardial infarction in both men and women [Yusuf et al., 2005]. In females, fat storage in subcutaneous adipose tissue depots is favored over visceral fat storage when ovarian function is normal [Nedungadi and Clegg, 2009]. However, an increase in visceral fat mass is observed when female sex steroid function is disrupted, such as following menopause, hysterectomy, or breast cancer treatment [Howard et al., 2005; Schneider et al., 2006]. For example, post-menopausal women have a 60% chance of developing metabolic syndrome, while in age-matched pre-menopausal women the risk is only 20–30% [Park et al., 2003]. However, little is known regarding the mechanisms by which ovarian hormones impact the metabolic function of adipose tissue.
Adipose tissue mass is controlled by a balance between storage of triglyceride (TAG) and breakdown of TAG via lipolysis. When lipolysis is activated, such as during an acute bout of moderate intensity exercise or during starvation, free fatty acids (FFAs), the breakdown products of TAG, become key energy substrates for peripheral tissues. However, in certain circumstances it is possible to detect high levels of circulating FFAs at rest and it has been suggested that the FFAs may contribute to increases in ectopic fat storage. For example, we and others have shown that ovarian hormone status in females impacts basal lipolytic function, with a reduction in circulating female sex steroids resulting in elevated levels of circulating glycerol [D’Eon et al., 2005; Wohlers and Spangenburg]. Further, we have previously demonstrated an increase in unstimulated glycerol release from isolated adipose tissue of OVX mice compared to SHAM mice [Wohlers and Spangenburg]. In addition, other studies have also reported an increase in circulating glycerol in sedentary female mice following ovariectomy [D’Eon et al., 2005]. One would expect that metabolic abnormalities in adipose tissue during diminished female sex steroid function could increase the risk for type 2 diabetes or cardiovascular disease [Yusuf et al., 2005]. Indeed, adipocytes isolated from OVX animals exhibit impaired in vitro lipolytic responses to catecholamine stimulation, which are reversed with 17β-estradiol supplementation [D’Eon et al., 2005]. Together, these findings support a role for female sex steroids in the metabolic regulation of adipose tissue.
Complete catabolic breakdown of TAG is enzymatically regulated. First, adipose triglyceride lipase (ATGL), also known as desnutrin and encoded by the gene PNPLA2, catalyzes the conversion of TAG to diacylglycerol (DAG), releasing one FA [Villena et al., 2004; Zimmermann et al., 2004]. Next, hormone sensitive lipase (HSL) cleaves off a second FA, resulting in the breakdown of DAG to monoacylglycerol (MAG). The last step in lipolysis is performed by monoglycerol lipase (MGL), which separates the final FA from the glycerol backbone [Fredrikson et al., 1986]. The acute regulatory mechanism of ATGL is still relatively unknown, however interaction with a protein known as comparative gene identification-58 (CGI-58), which is encoded by the gene abhydrolase domain containing 5 (ABHD5), is critical for activation of ATGL [Lass et al., 2006]. Unlike ATGL, the regulation of HSL has been well elucidated. Multiple phosphorylation sites on HSL exist which serve either to activate or inhibit HSL activity. Further, complete activation of HSL is dependent upon translocation from the cytosol to the lipid droplet (LD), which is the site of TAG storage. Beta adrenergic stimulation results in protein kinase A (PKA)-induced phosphorylation of HSL at serine 563, 659, and 660 (in rodents) residues, all of which activate HSL, promote translocation of HSL, and enhance lipolysis by 2–3 fold [Anthonsen et al., 1998]. In isolated rat adipocytes, mutational analysis has determined that phosphorylation of Ser659 and Ser660 is necessary for lipolytic activation in response to isoproternol stimulation [Anthonsen et al., 1998]. In addition, the ability of HSL to interact with the LD is dependent upon PKA-phosphorylation of perilipin (PLIN1), which undergoes a conformational change leaving the LD exposed for access by HSL [Miyoshi et al., 2006]. PLIN1 is a member of the PLIN gene family and is found to exclusively localize to surfaces of intracellular lipid storage droplets. In this manner, PLIN1 serves two roles: first to protect the LD from unstimulated lipolytic breakdown, and secondly to enhance stimulated lipolysis [Shen et al., 2009]. Further, PLIN1 interacts with the ATGL co-activator CGI-58, releasing CGI-58 to bind to ATGL when lipolytic activity is stimulated [Subramanian et al., 2004]. In the PLIN1 null mouse, adipose tissue mass is severely reduced due to constitutively active lipolytic function, demonstrating the importance of PLIN1 in lipolytic regulation [Saha et al., 2004].
Thus, although numerous seminal investigations have shed valuable light defining how these lipases are critical in the regulation of both basal and stimulated lipolysis, we still have a poor understanding of how these mechanisms are activated in various conditions in which metabolic function of adipose tissue is disrupted. Using the ovariectomized (OVX) mouse model we previously found sedentary animals exhibit increased circulating levels of glycerol compared to SHAM mice, which suggests female sex steroids may affect protein regulators of lipolytic function [Wohlers and Spangenburg]. Unfortunately, the study left unanswered questions that are critically important to defining the regulation of cellular lipolytic responses in the OVX animals. Specifically, we did not determine in vivo if stimulated glycerol release would be impaired in the OVX mice, nor could we account for the cellular location of these lipolytic proteins. Thus, the purpose of the present study was to determine if physiological activation (i.e. acute exercise bout) of lipolysis would be reduced in the OVX model due to low PLIN1 content. We hypothesized that PLIN1 content would be reduced in OVX compared to SHAM animals resulting in a lower corresponding release of glycerol in response to acute exercise in the OVX mice. Further, a second purpose was to determine if impairments in stimulated glycerol release in the OVX model were due to alterations in the cellular location of critical lipolytic proteins or due to changes in the composition of PLIN proteins found in the fat cake fraction. For this aim, we hypothesized that OVX mice would exhibit altered localization events of key lipolytic proteins and/or potential changes in PLIN protein content compared to the SHAM mice.
METHODS
ANIMALS
Twenty-seven 8-week-old C57/BL6 (Harlan) female mice were divided into two groups, SHAM and surgical ovariectomy (OVX). The OVX mice (n=14) underwent a bi-lateral ovariectomy, a frequently used method to disrupt the female sex steroid signaling axis [Keck et al., 2007]. Our lab has previously shown that this model decreases the levels of circulating estrogens by 70% within 48h [Sitnick et al., 2006] and we also find substantial reductions in uterine weight (~80%; unpublished data) in this model. Further, previous research has shown that surgical removal of the ovaries from mice does not result in changes in feeding patterns [Brown, 2008; Rogers et al., 2009; Witte et al., 2010]. The SHAM group (n=13) was subjected to a SHAM surgery where they were anesthetized, but did not undergo ovariectomy. Mice were given ad libitum access to water and standard rodent chow (Purina Laboratory Rodent Diet 5001: 23% protein, 4.5% fat, 6% fiber) and were housed in a temperature-controlled room on a 12 h light/dark cycle. Mice were kept in standard cages for 8 weeks, at which point an acute bout of exercise was performed by half of the mice in each group (n=14). Mice did not undergo exercise training; however, an acclimation period was included prior to acute exercise testing in order to familiarize the animals with the treadmill. Both groups underwent treadmill acclimation in order to keep the environmental stimuli as equal as possible across groups in the period prior to testing and sacrifice. To acclimate the mice to the treadmill, mice were exposed to treadmill running for 6 days, beginning with 10 mins at 10m/min and 5° incline, and gradually increasing the running duration and speed each day until by day 6 they were running for 35 minutes (5 mins at 25m/min followed by 20 mins at 28m/min, then 5 mins at 20m/min). For the acute about of exercise, mice ran on the treadmill at 26 m/min with a 5° incline until refusal to run, which was determined by continuous sitting (>1 min) on the shock pad. There was no difference in total run time between the SHAM and OVX animals. Other studies of exercise-induced lipolysis have employed treadmill running protocols which closely resemble the protocol employed in this investigation [Schoiswohl et al., 2010]. Our treadmill running protocol was selected based on the ability to induce lipolysis in adipose tissue and ensuring that both groups were able to complete the bout of exercise. Immediately following the acute exercise bout, animals were sacrificed, tissues were collected and snap frozen in liquid nitrogen, and then stored at −80° C. The control animals (n=13) were exposed to the initial acclimation period to ensure the same exposure to the treadmill. On the day of the acute treadmill test the control groups were not subjected to treadmill running but were still placed in the treadmill in a stationary fashion with an active shock pad for a duration equivalent to the exercise groups. All aspects of this study were approved by the University of Maryland Institutional Animal Care & Use Committee (IACUC) Review Board.
ISOLATION OF PROTEIN FROM ADIPOSE TISSUE
The omental/mesenteric fat was homogenized on ice using a protocol to separate the cytosolic and fat cake (i.e. proteins associated with the LDs) fractions using previously described methods with some slight modifications [Clifford et al., 2000; Egan et al., 1992]. It should be noted that this procedure was used to first demonstrate translocation of HSL from the cytosolic region to the LD [Egan et al., 1992]. Fat was homogenized in Mueller buffer (50 mM HEPES (pH 7.4), 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7H2O, 100 mM β-glycerophosphate, 25 mM NaF, 5 mM Na3VO4, and 1 protease inhibitor tablet/10 ml (Roche, Indianapolis, IN)), then centrifuged for 15 minutes at 4°C and 13,000g. The supernatant (cytosolic fraction) between the fat cake and the pellet was removed using a syringe and placed into a separate tube. A second buffer (50 mM HEPES (pH 7.4), 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7H2O, 100 mM β-glycerophosphate, 25 mM NaF, 5 mM Na3VO4, 0.05g SDS, 0.01% Triton X, and 1 protease inhibitor tablet/10 ml) was added to the pellet, which was heavily vortexed and then centrifuged for 15 minutes at 4°C and 13,000g. The middle layer (fat cake fraction) was again drawn off and placed in a fresh tube. Previous investigations have eloquently shown that this fractionation technique can be used to determine the cellular location of critical regulators of lipolytic function [Clifford et al., 2000; Egan et al., 1992]. Specifically, the fat cake fraction contains proteins which are localized to the surface of lipid storage droplets, while the cytosolic fraction contains the proteins located in the cytosol [Greenberg et al., 1991]. Protein concentrations were determined on the cytosolic and fat cake fractions using the BCA protein assay (Pierce Rockford, Ill.). To confirm proper isolation of the fractions, western blotting for ERK1/2 (cytosolic specific [Zheng and Guan, 1994]) and PLIN1 (LD specific [Greenberg et al., 1991]) was performed. We found ERK1/2 protein content was contained in the cytosolic fraction and PLIN1 protein content was strictly observed in the fat cake fraction (see Figure 1).
Figure 1.
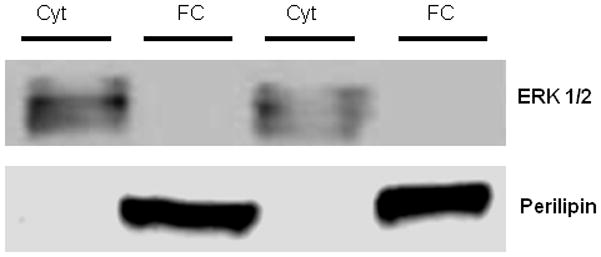
Determination of appropriate isolation of cytosolic and fat cake fractions from O/M adipose tissue. The expression of total ERK1/2, a cytosolic protein, appears only in the cytosolic fraction (Cyt), while PLIN1, which is known to be localized to the LD, appears only in the fat cake (FC) fraction.
WESTERN BLOTTING OF ADIPOSE TISSUE
Equal amounts of total protein (50 μg) were resolved on 7.5% SDS–PAGE gels and transferred to PVDF membranes as previously described [Spangenburg et al., 2008; Wohlers and Spangenburg,; Wohlers et al., 2009]. Blots were visualized with Ponceau S (Sigma Chemical, St. Louis, MO) to confirm equal loading of the lanes (data not shown) and then blocked with 3% nonfat dry milk in Tris-buffered saline with 0.1% Tween (TBS-T). In addition, in a secondary fashion, gels were run and stained with Coomassie blue to demonstrate equal loading of the samples (see Figure 2). Membranes were probed with antibodies for ATGL (Cell Signaling; 1:1,000), PLIN1 (perilipin) (Cell Signaling; 1:500), CGI-58 (kind gift from Dr. Dawn Brasaemle, Rutgers University; 1:25,000), or PLIN2 (ADRP) (Novus Biologicals; 1:1000) in a buffer of 1–5% BSA in TBS-T on a rocker at 4°C overnight. Following incubation with the primary antibody, membranes were washed in TBS-T (3 × 5 min) and then incubated for 1 hr with horseradish peroxidase (HRP)-conjugated rabbit secondary antibody (1:1,000) in 3% nonfat dry milk in TBS-T. Next, membranes were washed in TBS-T (1 × 10 min, 3 × 5 min), followed by enhanced chemiluminescence reagent (ECL) (Pierce, Rockford, IL). Membranes were visualized with a chemiluminescence imager (Syngene, Frederick, MD) and quantified with Image J software (NIH, Bethesda, MD).
Figure 2.

Figure 2(A–B). ATGL protein content is upregulated in the cytosolic fraction from O/M adipose tissue, but not the fat cake fraction, in OVX animals compared to SHAM animals.
(A) ATGL protein content in different fractions isolated from O/M adipose tissue from control and acute exercised SHAM and OVX mice. Abbreviations for x-axis are as followed and consistently displayed on all of the remaining figures: SC C= sham control cytosolic fraction, SC FC = sham control fat cake fraction, SE C = sham exercised cytosolic fraction, SE FC = sham exercised fat cake fraction, OC C = OVX control cytosolic fraction, OC FC = OVX control fat cake fraction, OE C = OVX exercised cytosolic fraction, OE FC = OVX exercised fat cake fraction. A representative ATGL blot is shown below the graph. In addition, in this figure a coomassie stained gel is depicted to demonstrate equal loading of the samples from each group. N=5/group
*Indicates significantly different from SC C (P<0.05).
(B) ATGL percent localization to the cytosolic and fat cake fractions. Within each bar, the grey area indicates ATGL localization to the fat cake fraction and the black area indicates localization to the cytosolic fraction. Abbreviations for x-axis are as followed and consistently displayed on all of the remaining figures: SC = SHAM control, SE = SHAM exercised, OC = OVX control, OE = OVX exercised.
BLOOD AND SERUM MEASURES
Non-esterified fatty acids (NEFA’s) were measured in serum using a colorimetric assay (Wako Diagnostics, Richmond, VA). Glycerol levels were determined in serum using a free glycerol determination kit (Sigma–Aldrich, St. Louis, MO). Glucose levels in blood were measured using a glucometer (LifeScan, Milpitas, CA). Insulin levels were measured in serum using an ELISA kit (Millipore, Billerica, MA).
STATISTICAL ANALYSIS
All data are expressed as means ± SEM. Statistical significance was determined using t-tests with Sigma Stat statistical analysis software (Systat Software, Inc., San Jose, CA). T-tests were chosen for statistical analysis due to the a priori derived specific and directional hypotheses. Specifically, the purpose of these experiments was to determine if known lipolytic mechanisms in adipose tissue failed to respond to a bout of exercise in the OVX mice. A P-value of ≤ 0.05 was considered significant.
RESULTS
ANATOMICAL CHARACTERISTICS
No significant differences in body weights were detected between the SHAM and OVX groups. Previously, we have found a small significant difference of ~9% and here we saw a non-significant difference of ~4% [Wohlers and Spangenburg]. However, O/M fat mass was significantly greater in both the control OVX (referred to as cont in tables/figures) and acute exercised OVX (referred to as ex in tables/figures) groups compared to the control SHAM and acute exercised SHAM groups. When fat mass was normalized to body weight there were significant elevations in both groups of OVX mice compared to the SHAM mice. No significant differences in other tissue weights (i.e. heart, liver, skeletal muscle) were detected (data not shown). No significant difference in total run time was observed between the exercised OVX and SHAM groups (Table 1A).
Table 1.
Table 1 (A/B).OVX mice exhibit significant differences in anatomic and metabolic parameters when compared to the SHAM mice. (N=6–7/group)
| A | Body Weight (BW) | O/M Fat Mass (FM) | FM/BW | Run Time (min) |
|---|---|---|---|---|
| SHAM cont | 22.92±0.50 | 0.278±0.017 | 0.012±0.0008 | |
| SHAM ex | 22.47±0.69 | 0.264±0.024 | 0.012±0.0008 | 36.5±4.3 |
| OVX cont | 23.42±0.52 | 0.710±0.061* | 0.030±0.002* | |
| OVX ex | 23.60±0.50 | 0.632±0.084* | 0.026±0.003* | 38.8±9.99 |
| B | Glycerol (mg/ml) | NEFA (mmol/L) | Glucose (mg/dl) | Insulin (ng/ml) |
|---|---|---|---|---|
| SHAM cont | 309.22±14.02 | 0.664 ±0.066 | 137.17±3.36 | 0.627±0.230 |
| SHAM ex | 424.34±43.80* | 0.887±0.068* | 111.29±7.92* | 0.634±0.244 |
| OVX cont | 390.69±37.33* | 0.513±0.085 | 152.57±7.30* | 0.460±0.219 |
| OVX ex | 417.88±21.07* | 1.003±0.101$ | 100.71±7.84$ | 0.390±0.089 |
Indicates significantly different from SHAM cont
Indicates significantly different from OVX cont (P<0.05).
BLOOD/SERUM ANALYSES
In line with our previously published data [Wohlers and Spangenburg], serum glycerol levels were significantly elevated in control OVX mice compared to control SHAM mice (Table 1B). Here, an acute bout of exercise was performed to physiologically stimulate lipolysis in the SHAM and OVX mice, with increases in serum glycerol and NEFA acting as surrogate indicators of lipolytic activation. Glycerol levels were significantly elevated in control OVX mice compared to control SHAM mice. When compared to the control group, there was a non-significant 14% (28% when normalized to O/M fat mass) increase in glycerol levels in response to exercise in the OVX animals and a significant 34% (41% when normalized to O/M fat mass) increase in the SHAM animals. No significant difference in serum NEFA levels was detected between control SHAM and OVX mice (Table 1B). In contrast, the acute exercise bout resulted in significant elevations in serum NEFA levels in both SHAM and OVX groups when compared to their control counterparts, with no differences detected across groups in response to exercise. When compared to the control group, there was a 95% (119% when normalized to O/M fat mass) increase in NEFA levels in response to exercise in the OVX animals and a 34% (41% when normalized to O/M fat mass) increase in the SHAM animals. The finding of an exercise-induced increase in NEFA levels without an increase in glycerol levels in the OVX mice was surprising; however, this may be the result of a reduction in futile cycling. Blood glucose levels were significantly elevated in the control OVX mice compared to the control SHAM mice (Table 1B). The acute bout of exercise resulted in significant reductions in blood glucose in both the SHAM and OVX mice, with no difference observed between the two groups. No differences in insulin levels were detected between any groups (Table 1B).
PROTEIN CONTENT IN O/M ADIPOSE TISSUE
ATGL
In a previous study, we detected elevations in ATGL protein content in total cell lysates isolated from the visceral adipose tissue of OVX animals. The elevation in ATGL coincided with elevated levels of circulating glycerol [Wohlers and Spangenburg]. Further, recent data have suggested that ATGL is a critical mediator of exercise-induced lipolysis [Huijsman et al., 2009]. Thus we sought to expand our previous findings to determine if ATGL distribution between the cytosolic and fat cake fraction was altered in the OVX mice and to investigate whether an acute bout of exercise would alter the location of ATGL in SHAM or OVX mice. In agreement with our previous data, ATGL protein content was significantly elevated in the cytosolic fraction in both groups of OVX mice compared to control SHAM mice. The exercised SHAM mice had significantly greater cytosolic ATGL protein content than the control SHAM mice, however, no effect of acute exercise on ATGL protein content was observed in OVX mice (Figure 2A). In addition, to determine if localization of ATGL was altered between groups we calculated the percent of total ATGL that was present in each fraction. However, we did not find any significant differences between groups (Figure 2B).
CGI-58
CGI-58 is a known regulator of ATGL through direct protein interaction, enhancing activation of ATGL [Lass et al., 2006]. Therefore, we measured CGI-58 protein content to determine whether the capacity for ATGL activation is altered by acute exercise or by ovariectomy. As expected, CGI-58 protein content was primarily found in the fat cake fraction compared to the cytosolic fraction in all groups, with no between group differences detected. Thus, loss of ovarian function or performance of an acute exercise bout did not alter CGI-58 protein content in O/M adipose tissue (Figure 3A). In addition, to determine if localization of CGI-58 was altered between groups we calculated the percent of total CGI-58 that was present in each fraction. However, we did not find any significant differences between groups (Figure 3B).
Figure 3.
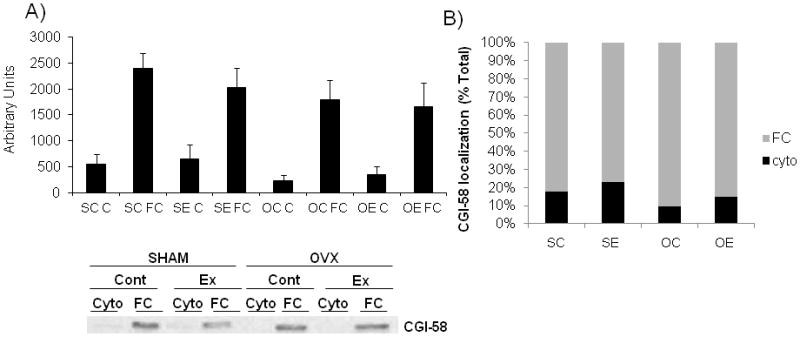
Figure 3(A–B). CGI-58 protein is mostly contained in the FC fraction and does not differ between SHAM and OVX animals.
(A) CGI-58 protein content in O/M adipose tissue from control and acute exercised SHAM and OVX groups. A representative CGI-58 blot is shown below the graph. N=5/group
(B) CGI-58 percent localization to the cytosolic and fat cake fractions. Within each bar, the grey area indicates CGI-58 localization to the fat cake fraction and the black area indicates localization to the cytosolic fraction.
HSL Ser660
In the mouse, induction of HSL-mediated lipolysis is dependent upon phosphorylation of Ser660, thus to determine if HSL was associated with the increased levels of circulating glycerol we used a phosphorylation site specific antibody for HSL. In O/M adipose tissue, we found elevated levels of HSL Ser660 phosphorylation in the cytosolic fraction of exercised SHAM mice compared to control SHAM mice (Figure 4A). In the SHAM mice, the increase in HSL660 phosphorylation in the fat cake fraction of exercised mice did not reach statistical significance (p=0.08). However, we found no changes in HSL Ser660 phosphorylation in the control OVX mice nor did we observe a response to acute exercise (Figure 4A).
Figure 4.

Phosphorylation of HSL (Ser660) in the cytosolic fractions from O/M adipose tissue is upregulated in response to acute exercise only in the SHAM animals and not the OVX animals. A representative HSL Ser 660 blot is shown below the graph. N=4/group
*Indicates significantly different from SC C (P<0.05).
PLIN1 (Perilipin)
PLIN1 is exclusively localized to the lipid storage droplet and plays an important role in the regulation of basal and stimulated lipolysis [Granneman et al., 2009]. We have previously shown PLIN1 protein content in a total cell lysate to be reduced in O/M adipose tissue of mice following ovariectomy [Wohlers and Spangenburg]. In the present study, we sought to determine if PLIN1 protein content/localization in the fat cake fraction is altered in the OVX mice or by an acute exercise bout. Since PLIN1 was not detected in the cytosolic fraction (Figure 1) only the fat cake fraction is shown. Confirming our previous findings, PLIN1 protein content in the fat cake fraction was significantly reduced in both OVX groups compared to the SHAM mice. The acute exercise bout did not result in alterations in PLIN1 protein content in SHAM or OVX mice (Figure 5).
Figure 5.

PLIN1 (perilipin) protein content in O/M adipose tissue is significantly reduced in OVX animals compared to SHAM animals. Since PLIN1 is localized to the LD and was not detected in the cytosolic fraction, only the fat cake fraction is shown. A representative PLIN1 blot is shown below the graph. N=5/group
*Indicates significantly different from SC FC and SE FC (P<0.05).
PLIN2 (also known as adipose differentiation-related protein (ADRP/ADFP)
Genetic ablation of PLIN1 results in a significant upregulation of PLIN2 protein content on the LD [Tansey et al., 2001]. However, the upregulation of PLIN2 does not confer the LD the same protection from lipolytic degradation, nor does PLIN2 act as an effective means for enhancing stimulated lipolysis [Tansey et al., 2001]. In our model, we observed both an elevation in resting circulating glycerol and a decrease in PLIN1 protein content in OVX mice, therefore we hypothesized that PLIN2 may be replacing PLIN1 on the LD. In both OVX groups, there was a significant increase in PLIN2 protein content in the fat cake fraction compared to the SHAM groups. No effect of acute exercise on PLIN2 protein content was observed in SHAM or OVX groups (Figure 6).
Figure 6.
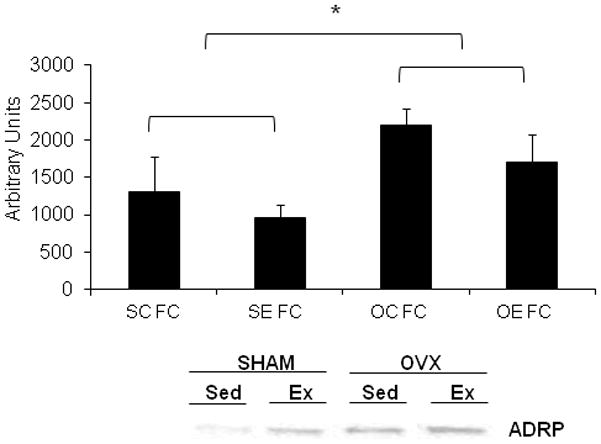
PLIN2 (ADRP) protein content is significantly elevated in O/M adipose tissue of OVX animals compared to SHAM animals. Since PLIN2 is localized to the LD and was not detected in the cytosolic fraction, only the fat cake fraction is shown. A representative PLIN2 blot is shown below the graph. N=5/group
*Indicates significantly different from SC FC and SE FC (P<0.05).
DISCUSSION
Here we demonstrate that in the OVX mice there are significant alterations in critical cellular regulators of lipolytic function in O/M adipose tissue when compared to SHAM mice. As with our own and others previous findings [D’Eon et al., 2005; Wohlers and Spangenburg], we observed an increased in O/M fat mass in the OVX mice. The elevated fat mass in the OVX mice was coupled with increased circulating glycerol and glucose levels under control conditions, with no differences in insulin levels observed. An acute bout of exercise resulted in significantly higher glycerol levels in exercised SHAM mice compared to control SHAM mice, however, no significant effect of exercise on glycerol levels was observed in OVX mice. This suggests that the OVX mice cannot further enhance the release of glycerol from the adipose tissue during this exercise bout. In both SHAM and OVX mice, an acute bout of exercise resulted in quantitatively similar reductions in blood glucose levels compared to control counterparts, indicating an equivalent response from the two different groups of mice. Cytosolic ATGL protein content was elevated in control and exercised OVX mice compared to control SHAM mice. However, there was no increase in ATGL protein content in the fat cake fraction in response to exercise in either group. There was significantly more of the phosphorylated form of HSL (Ser660) in the cytosolic fraction of exercised SHAM mice compared to control SHAM mice. However, no increase in HSL Ser660 phosphorylation was observed in OVX mice in response to acute exercise. Lastly, PLIN1 protein content in the fat cake fraction was reduced, while PLIN2 protein content in the fat cake fraction was elevated, in OVX mice compared to SHAM mice. These data demonstrate that signaling mechanisms that regulate basal and stimulated lipolytic function are impaired in OVX mice compared to SHAM mice, suggesting a critical role for female sex steroids in the regulation of lipolytic function.
We, and multiple other investigators, have previously reported increases in visceral fat mass coupled with elevations in basal circulating glycerol, and glucose levels in OVX mice [D’Eon et al., 2005; Wohlers and Spangenburg]. These metabolic changes qualitatively resemble changes reported in post-menopausal women who are not taking hormone replacement therapy [Panotopoulos et al., 1997]. The elevation in circulating glycerol levels in the control OVX mice suggests that lipolysis is elevated even under resting conditions. A similar finding has been confirmed in adipocytes from humans [Nicklas et al., 1996]. In addition, multiple other studies have reported estrogens to reduce adipose tissue lipolytic function in females [Tchernof et al., 2004; Van Pelt et al., 2006]. In particular, a reduction in ovarian hormones results in increased basal lipolytic rate in adipocytes isolated from human omental adipose tissue [Tchernof et al., 2004]. The acute bout of exercise resulted in a smaller, non-significant increase in glycerol levels in the OVX mice compared to the SHAM mice. Further, NEFA levels were elevated in both the OVX and SHAM mice in response to acute exercise, with a greater percent increase compared to the control group in the OVX mice. Due to the observed alterations in serum glycerol and NEFA levels, we explored potential alterations in localization of key lipolytic proteins in the SHAM and OVX mice.
In adipose tissue, 95% of all lipolytic activity is controlled by the lipases HSL and ATGL [Schweiger et al., 2006]. When ATGL is genetically ablated in the mouse model, total TAG hydrolase activity in white adipose tissue is reduced by 82% [Haemmerle et al., 2006], while in contrast overexpression of ATGL in adipocytes results in increased basal and beta-adrenergic stimulated lipolysis [Zimmermann et al., 2004]. In our study we found increased ATGL protein content in the cytosolic fraction of O/M adipose tissue from OVX mice, which may explain the elevated basal glycerol levels in the OVX mice. While the increase in glycerol release without an increase in HSL phosphorylation may seem counterintuitive, multiple studies have demonstrated that glycerol release can occur when ATGL is overexpressed [Zimmermann et al., 2004], or when HSL expression is absent. Specifically, Rydén et al. found inhibition of HSL attenuates, but does not prevent, increases in glycerol release from stimulated adipocytes [Ryden et al., 2007]. In addition, unstimulated glycerol release is also elevated under conditions of PLIN1 ablation [Zhai et al., 2010], suggesting that the combination of increased ATGL and decreased PLIN1 is a potential contributor to the increased serum glycerol values in the control OVX animals. During the acute exercise bout, we did not find a large increase in serum glycerol values in the OVX animals compared to the SHAM animals. This could potentially be explained by the low levels of HSL phosphorylation in the OVX mice coupled with the low PLIN1 content since both HSL and PLIN1 work synergistically to increase lipolytic rate. Alternatively, the reduced glycerol response in the OVX animals during the exercise bout may have been a result of increased glycerol kinase activity, which would prevent the release of glycerol into circulation. Glycerol kinase was originally thought to be absent from adipose tissue, however multiple studies have demonstrated an increase in glycerol kinase to occur in the obese state in animal models as well as in humans [Chakrabarty et al., 1984; Stern et al., 1983; Taylor et al., 1979]. Albeit, at this time there is no evidence to indicate if glycerol kinase is elevated in the OVX model and this needs to be tested. Finally, ATGL also has acylglycerol transacylase activity [Jenkins et al., 2004], and can play a role in the formation of TAG and DAG, thus contributing to futile cycling between TAG storage and breakdown. It is possible that we are detecting indices of futile cycling in the OVX mice. Potentially, this may have been observed when NEFA levels increased in the OVX mice in response to acute exercise without a substantial increase in serum glycerol levels. This suggests that higher serum NEFA values may not be derived from an increase in lipolytic rate. Thus, perhaps the exercise bout in the OVX mice reduced the acylglycerol transacylase activity of ATGL thereby releasing fatty acids that may have undergone re-esterification under the control conditions. While we do not have a measure to directly indicate futile cycling, our findings may suggest that under conditions where adipose tissue lipolysis is stimulated (i.e. exercise), there is a decrease in re-esterification in OVX mice and thus a reduction in futile cycling. The dual role of ATGL in lipolysis and TAG formation may explain how the OVX animals demonstrated increases in fat mass in spite of what appears to be an elevation in basal lipolytic rate. We are currently conducting further detailed studies in order to elucidate the effects of reduced female sex steroids and acute exercise on ATGL function as well as the potential role of futile cycling in the regulation of adipose tissue function by female sex steroids.
The increase in ATGL in the OVX mice is in line with observations in humans [Steinberg et al., 2007]. At the mRNA level, they reported an increase in ATGL in visceral fat from obese post-menopausal females and obese males. Further, they did not detect a change in mRNA or protein content of CGI-58, an activator of ATGL, which is in agreement with our findings [Steinberg et al., 2007]. We did not detect significant changes in ATGL protein content in the fat cake fraction in either SHAM or OVX mice in response to the acute bout of exercise. However, this is in agreement with other observations which have also shown that ATGL localization to the LD is unaffected by isoproternol stimulation, with the greatest proportion of ATGL protein residing in the cytoplasm [Zimmermann et al., 2004]. Thus, at this time it is clear that our understanding of ATGL function needs to be further clarified in future studies.
HSL phosphorylation at the Ser660 site, the primary regulatory site for activation [Anthonsen et al., 1998], results in HSL translocation from the cytosol to the LD where it may contribute to lipolytic breakdown of stored TAG [Watt et al., 2006]. We found elevated levels of the phosphorylated form of HSL (Ser660) in the cytosolic fraction (p=0.08 in the fat cake fraction) from SHAM mice in response to the acute exercise bout. In contrast, no significant change in HSL Ser660 phosphorylation was detected in OVX mice in response to acute exercise. No difference in HSL phosphorylation (Ser660) was observed between SHAM and OVX mice under basal conditions, suggesting that HSL is not responsible for the elevated serum glycerol levels in the OVX mice. However, the failure to increase HSL Ser660 phosphorylation may partially explain the inability of the OVX mice to significantly increase glycerol release in response to an acute bout of exercise. In adipocytes isolated from OVX mice and OVX mice supplemented with 17β-estradiol, D’Eon et al. showed no difference in total HSL between groups and suggested that changes in HSL content were not responsible for the increased catecholamine stimulated lipolysis in the mice supplemented with 17β-estradiol [D’Eon et al., 2005]. However, they did not measure HSL phosphorylation status or localization of HSL, which is critical to examining HSL activity. Our observation in OVX mice of an inability to increase HSL Ser660 phosphorylation in response to an acute bout of exercise suggests that dysfunction of upstream regulators of HSL play a role in altered lipolytic activation in adipose tissue when female sex steroids are reduced.
Lipolytic regulation is multi-faceted with important regulatory steps defined by proteins that coat the LD’s within adipocytes. PLIN1 is the primary LD associated protein involved in the regulation of adipocyte lipolysis [Shen et al., 2009]. In the fat cake fraction isolated from the OVX mice, we found significant reductions in PLIN1 or Perilipin and elevations in PLIN2 or ADRP protein content in O/M adipose tissue compared to the SHAM mice. These changes in PLIN1 and PLIN2 protein content in the OVX mice would have substantial effects on the regulation of lipolytic function. For example, the reduction in PLIN1 protein content may explain the lack of HSL response in OVX mice following the acute exercise bout. In adipocytes, the presence of PLIN1 on the LD has been shown to be critical for HSL translocation to the LD in order to promote catecholamine stimulated lipolytic activity [Miyoshi et al., 2006]. Thus, in the OVX mice where PLIN1 content is significantly reduced, activation and translocation of HSL may be impaired as a result. Other studies have demonstrated an effect of female sex steroids on lipolytic function. For example, reduced adipocyte PLIN1 content in OVX mice corresponds to impaired ex vivo catecholamine activated lipolysis, suggesting reduced lipolytic function in adipose tissue following OVX [D’Eon et al., 2005]. Our data extend the findings of D’Eon et al by demonstrating that reduced female sex steroid levels also impair lipolysis when activated in vivo by a physiological stimuli (i.e. exercise). Although under most circumstances very little, if any, PLIN2 is found in mature adipocytes, there are suggestions that during states of increased FFA flux there is elevated content of PLIN2 associated with the LD’s [Bickel et al., 2009]. Further, alternative explanation for PLIN2 appearing in the fat cake may be that PLIN2 can displace PLIN1 under conditions of extended lipolytic activation resulting in PLIN2 associating with LD’s formed during re-esterification of the FAs [Gross et al., 2006]. Further, an increase in PLIN2 expression is observed in the PLIN1 knockout mouse, suggesting that PLIN2 may be upregulated in circumstances where PLIN1 is reduced [Tansey et al., 2001]. Even though PLIN2 is not typically found in mature adipocytes, due to the unique metabolic phenotype of the OVX mouse there appears to be a plausible explanation for the increased PLIN2 protein content.
LIMITATIONS
One limitation to our findings is that adipocyte lipolysis was not directly measured. However, circulating glycerol levels are often used as an indicator of lipolysis [Haemmerle et al., 2006; Huijsman et al., 2009; Hurley et al., 1986; Jocken et al., 2007; Lonnqvist et al., 1990; Schoiswohl et al., 2010], and coupled with the findings of D’Eon et al [D’Eon et al., 2005] and our own [Wohlers and Spangenburg] which demonstrate increased basal glycerol release from adipose tissue isolated from OVX animals, it does appear that unstimulated lipolysis appears to be elevated in the OVX animal. Another limitation to our study comes from the fractionation technique used to separate cytosolic and lipid droplet (fat cake) proteins. We chose to use this technique in hopes of capturing the localization of ATGL and HSL in our animals. Although isolation of the adipocytes from each group would have been ideal, staining of primary adipocytes is extremely difficult due to the large size of the LD, resulting in very little visible cytoplasmic volume [Malide, 2008]. In addition, we were concerned that the adipocyte isolation process might result in movement of the proteins independent of the effects induced by exercise. Finally, we measured our targets in protein isolated from whole adipose tissue rather than from isolated adipocytes. Since CGI-58 and PLIN2 have been detected in the stromal vascular fraction of adipose tissue, we cannot be completely certain that some of the protein signal was not coming from cells other than the adipocytes themselves.
As summarized in Figure 7, the data presented here provide novel evidence that a reduction in female sex steroids results in impairment of stimulated lipolytic signaling and alterations in PLIN protein content in O/M adipose tissue. Using a physiological stimulus (acute exercise) to activate lipolysis, we determined that exercise fails to further elevate circulating levels of glycerol, which appears to be a result of dysfunctional lipolytic signaling. These data suggest that significant reductions in female sex steroid levels not only alter lipid partitioning, but also compromise key lipolytic functions in the adipose tissue. Thus, it is imperative that we begin to critically examine the role of sex steroids in the regulation of adipose tissue dynamics to allow for the development of a treatment to prevent metabolic disorders in females experiencing reduced ovarian function.
Figure 7.
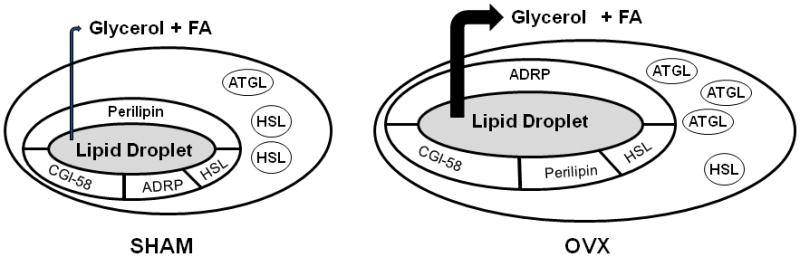
Summary of lipolytic protein content in adipose tissue from SHAM and OVX mice. OVX mice exhibit a reduction in perilipin protein content and an increase in ADRP protein content on the lipid droplet. Further, an increase in cytosolic ATGL protein content is observed in animals following ovariectomy.
Acknowledgments
The authors wish to thank Dr. Carole Sztalryd for helpful discussions and Dr. Dawn Brasaemle for the kind gift of the CGI-58 antibody. This work was supported by NIH AG000268 and UMD Kinesiology GRIF (LMW).
Abbreviations List
- ABHD5
Abhydrolase domain containing 5
- ADRP/ADFP
Adipose differentiation related protein
- ATGL
Adipose triglyceride lipase
- CGI-58
Comparative gene identification 58
- Cont
Control
- DAG
Diacylglycerol
- Ex
Exercised
- FFA
Free fatty acid
- FA
Fatty acid
- HSL
Hormone sensitive lipase
- LD
Lipid droplet
- MAG
Monoacylglycerol
- MGL
Monoglyceride lipase
- NEFA
Non-esterified fatty acid
- O/M
Omental/mesenteric
- OVX
Ovariectomy
- PKA
Protein kinase A
- PLIN
Perilipin
- TAG
triglyceride
References
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–21. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–40. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32:120–6. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- Chakrabarty K, Tauber JW, Sigel B, Bombeck CT, Jeffay H. Glycerokinase activity in human adipose tissue as related to obesity. Int J Obes. 1984;8:609–22. [PubMed] [Google Scholar]
- Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem. 2000;275:5011–5. doi: 10.1074/jbc.275.7.5011. [DOI] [PubMed] [Google Scholar]
- D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–91. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci U S A. 1992;89:8537–41. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson G, Tornqvist H, Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta. 1986;876:288–93. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–44. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–6. [PubMed] [Google Scholar]
- Gross DN, Miyoshi H, Hosaka T, Zhang HH, Pino EC, Souza S, Obin M, Greenberg AS, Pilch PF. Dynamics of lipid droplet-associated proteins during hormonally stimulated lipolysis in engineered adipocytes: stabilization and lipid droplet binding of adipocyte differentiation-related protein/adipophilin. Mol Endocrinol. 2006;20:459–66. doi: 10.1210/me.2005-0323. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Howard BV, Kuller L, Langer R, Manson JE, Allen C, Assaf A, Cochrane BB, Larson JC, Lasser N, Rainford M, Van Horn L, Stefanick ML, Trevisan M. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111:1462–70. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, Haemmerle G, Zechner R, Watt MJ. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab. 2009;297:E505–13. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- Hurley BF, Nemeth PM, Martin WH, 3rd, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60:562–7. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–75. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab. 2007;92:2292–9. doi: 10.1210/jc.2006-1318. [DOI] [PubMed] [Google Scholar]
- Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL. Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol. 2007;293:F193–9. doi: 10.1152/ajprenal.00022.2007. [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–19. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lonnqvist F, Nyberg B, Wahrenberg H, Arner P. Catecholamine-induced lipolysis in adipose tissue of the elderly. J Clin Invest. 1990;85:1614–21. doi: 10.1172/JCI114612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malide D. Application of immunocytochemistry and immunofluorescence techniques to adipose tissue and cell cultures. Methods Mol Biol. 2008;456:285–97. doi: 10.1007/978-1-59745-245-8_21. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem. 2006;281:15837–44. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2:321–7. doi: 10.1007/s12265-009-9101-1. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270:E72–8. doi: 10.1152/ajpendo.1996.270.1.E72. [DOI] [PubMed] [Google Scholar]
- Panotopoulos G, Raison J, Ruiz JC, Guy-Grand B, Basdevant A. Weight gain at the time of menopause. Hum Reprod. 1997;12(Suppl 1):126–33. doi: 10.1093/humrep/12.suppl_1.126. [DOI] [PubMed] [Google Scholar]
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–36. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–8. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden M, Jocken J, van Harmelen V, Dicker A, Hoffstedt J, Wiren M, Blomqvist L, Mairal A, Langin D, Blaak E, Arner P. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am J Physiol Endocrinol Metab. 2007;292:E1847–55. doi: 10.1152/ajpendo.00040.2007. [DOI] [PubMed] [Google Scholar]
- Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem. 2004;279:35150–8. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]
- Schneider JG, Tompkins C, Blumenthal RS, Mora S. The metabolic syndrome in women. Cardiol Rev. 2006;14:286–91. doi: 10.1097/01.crd.0000233757.15181.67. [DOI] [PubMed] [Google Scholar]
- Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, Zierler KA, Radner FP, Eichmann TO, Kienesberger PC, Eder S, Lass A, Haemmerle G, Alsted TJ, Kiens B, Hoefler G, Zechner R, Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res. 2010;51:490–9. doi: 10.1194/jlr.M001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–41. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res. 2009;50:2306–13. doi: 10.1194/jlr.M900176-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol. 2006;100:286–93. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586:283–91. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE, Watt MJ. Adipocyte triglyceride lipase expression in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E958–64. doi: 10.1152/ajpendo.00235.2007. [DOI] [PubMed] [Google Scholar]
- Stern JS, Hirsch J, Drewnowski A, Sullivan AC, Johnson PR, Cohn CK. Glycerol kinase activity in adipose tissue of obese rats and mice: effects of diet composition. J Nutr. 1983;113:714–20. doi: 10.1093/jn/113.3.714. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–71. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–9. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WM, Goldrick RB, Ishikawa T. Glycerokinase in rat and human adipose tissue: response to hormonal and dietary stimuli. Horm Metab Res. 1979;11:280–4. doi: 10.1055/s-0028-1092723. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, Rheaume C, Dupont P. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89:3425–30. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 2006;14:2163–72. doi: 10.1038/oby.2006.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–75. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–8. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010;166:520–8. doi: 10.1016/j.ygcen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlers LM, Spangenburg EE. 17beta-estradiol supplementation attenuates ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J Cell Biochem. 2010;110:420–7. doi: 10.1002/jcb.22553. [DOI] [PubMed] [Google Scholar]
- Wohlers LM, Sweeney SM, Ward CW, Lovering RM, Spangenburg EE. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J Cell Biochem. 2009;107:171–8. doi: 10.1002/jcb.22113. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- Zhai W, Xu C, Ling Y, Liu S, Deng J, Qi Y, Londos C, Xu G. Increased lipolysis in adipose tissues is associated with elevation of systemic free fatty acids and insulin resistance in perilipin null mice. Horm Metab Res. 2010;42:247–53. doi: 10.1055/s-0029-1243599. [DOI] [PubMed] [Google Scholar]
- Zheng CF, Guan KL. Cytoplasmic localization of the mitogen-activated protein kinase activator MEK. J Biol Chem. 1994;269:19947–52. [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]


