Abstract
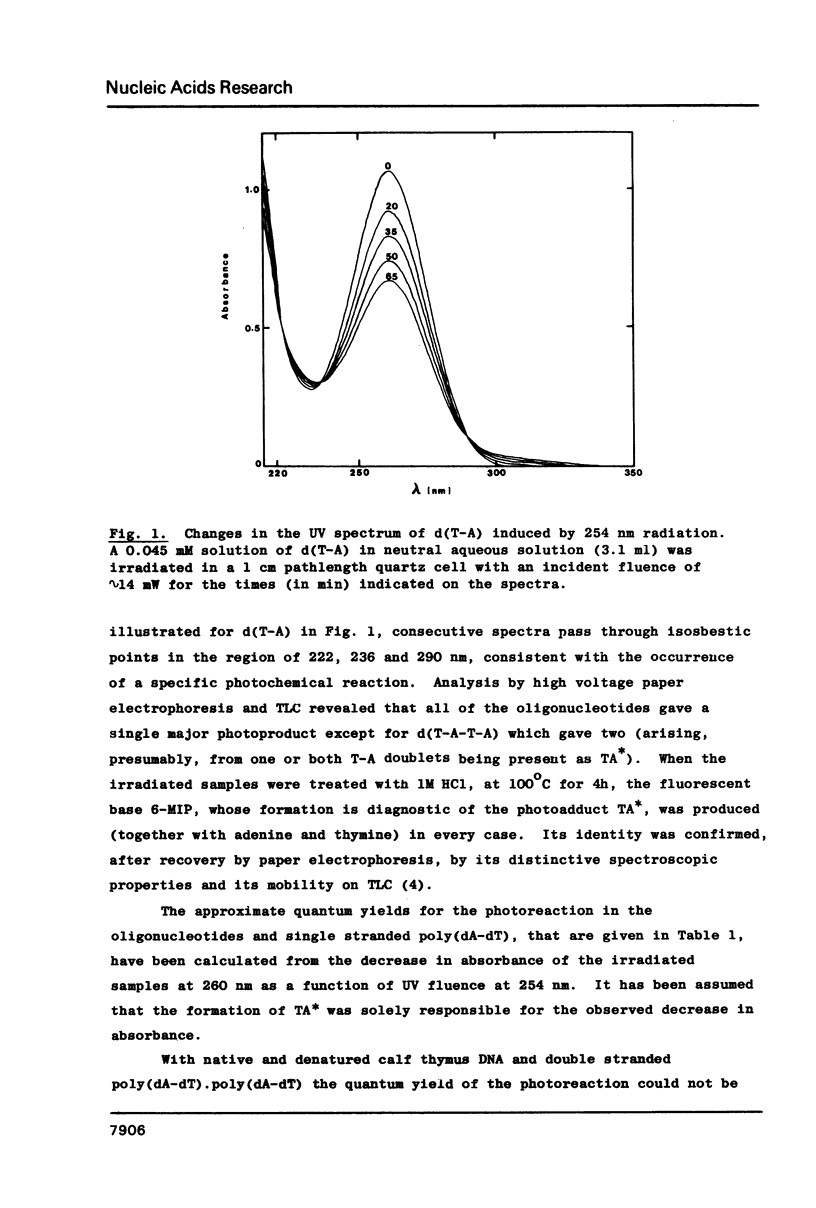
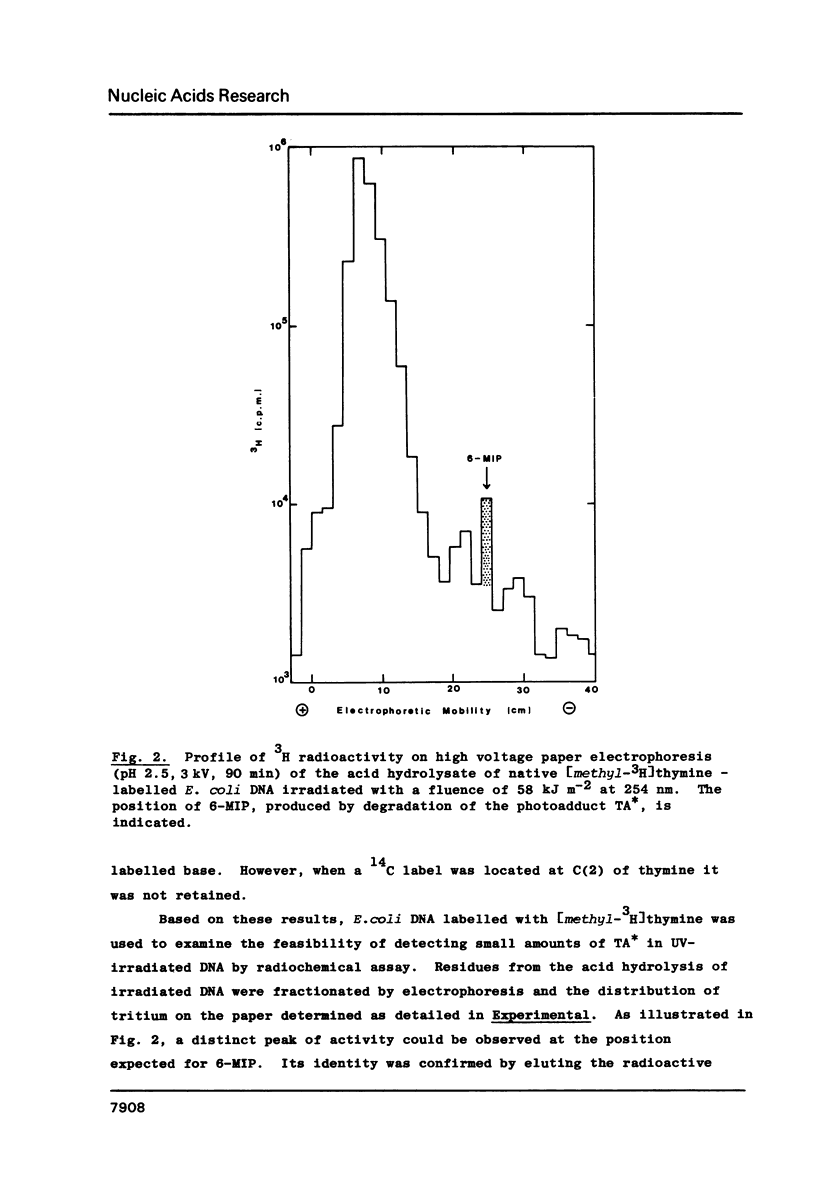
Photoaddition between adjacent adenine and thymine bases occurs, with a quantum yield of approximately 5 X 10(-4) mol einstein-1, when d(T-A), dT-A, d(pT-A), d(T-A-T), d(T-A-T-A) and poly(dA-dT) are irradiated, at 254 nm, in aqueous solution. The photoadduct thus formed is specifically degraded by acid to the fluorescent heterocyclic base 6-methylimidazo[4,5-b]pyridin-5-one (6-MIP) with retention of C(8) of adenine and the methyl group of thymine. This reaction, coupled with either spectrofluorimetric or radiochemical assay of 6-MIP isolated by high voltage paper electrophoresis, has been used to demonstrate formation of the adenine-thymine photoadduct on UV irradiation of poly(dA-dT).poly(dA-dT) and both native and denatured DNA from calf thymus and E. coli. Estimated quantum yields for this new type of photoreaction in DNA show that it is substantially quenched by base pairing. Possible biological implications of the photoreaction are discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assa-Munt N., Kearns D. R. Poly(dA-dT) has a right-handed B conformation in solution: a two-dimensional NMR study. Biochemistry. 1984 Feb 28;23(5):791–796. doi: 10.1021/bi00300a001. [DOI] [PubMed] [Google Scholar]
- Becker M. M., Wang J. C. Use of light for footprinting DNA in vivo. Nature. 1984 Jun 21;309(5970):682–687. doi: 10.1038/309682a0. [DOI] [PubMed] [Google Scholar]
- Bose S. N., Davies R. J., Sethi S. K., McCloskey J. A. Formation of an adenine-thymine photoadduct in the deoxydinucleoside monophosphate d(TpA) and in DNA. Science. 1983 May 13;220(4598):723–725. doi: 10.1126/science.6836308. [DOI] [PubMed] [Google Scholar]
- Bose S. N., Kumar S., Davies R. J., Sethi S. K., McCloskey J. A. The photochemistry of d(T-A) in aqueous solution and in ice. Nucleic Acids Res. 1984 Oct 25;12(20):7929–7947. doi: 10.1093/nar/12.20.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Warshaw M. M., Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9(9):1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Lucas J. L., Dannenberg A. A damage-specific DNA binding protein. Large scale purification from human placenta and characterization. J Biol Chem. 1982 Jun 10;257(11):6394–6401. [PubMed] [Google Scholar]
- Garcés F., Dávila C. A. Alterations in DNA irradiated with ultraviolet radiation-I. The formation process of cyclobutylpyrimidine dimers: cross sections, action spectra and quantum yields. Photochem Photobiol. 1982 Jan;35(1):9–16. doi: 10.1111/j.1751-1097.1982.tb03804.x. [DOI] [PubMed] [Google Scholar]
- Gordon L. K., Haseltine W. A. Quantitation of cyclobutane pyrimidine dimer formation in double- and single-stranded DNA fragments of defined sequence. Radiat Res. 1982 Jan;89(1):99–112. [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. Helix-random coil transitions in synthetic DNAs of alternating sequence. J Mol Biol. 1962 Aug;5:172–184. doi: 10.1016/s0022-2836(62)80082-5. [DOI] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- MOROWITZ H. J. Absorption effects in volume irradiation of microorganisms. Science. 1950 Mar 3;111(2879):229–229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Coulondre C., Farabaugh P. J. Correlation of nonsense sites in the lacI gene with specific codons in the nucleotide sequence. Nature. 1978 Aug 24;274(5673):770–775. doi: 10.1038/274770a0. [DOI] [PubMed] [Google Scholar]
- Pörschke D. Analysis of a specific photoreaction in oligo- and polydeoxyadenylic acids. J Am Chem Soc. 1973 Dec 12;95(25):8440–8446. doi: 10.1021/ja00806a040. [DOI] [PubMed] [Google Scholar]
- Rahn R. O. Search for an adenine photoproduct in DNA. Nucleic Acids Res. 1976 Apr;3(4):879–890. doi: 10.1093/nar/3.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]



