Abstract
Few studies have examined geographic variation in hypertension disparities, but studies of other health outcomes indicate that racial residential segregation may help to explain these variations. The authors used data from 8,071 black and white participants in the National Health and Nutrition Examination Survey (1999–2006) who were aged 25 years or older to investigate whether black-white hypertension disparities varied by level of metropolitan-level racial residential segregation and whether this was explained by race differences in neighborhood poverty. Racial segregation was measured by using the black isolation index. After adjustment for demographics and individual-level socioeconomic position, blacks had 2.74 times higher odds of hypertension than whites (95% confidence interval (CI): 2.32, 3.25). However, race differences were significantly smaller in low- than in high-segregation areas (Pinteraction = 0.006). Race differences in neighborhood poverty did not explain this heterogeneity, but poverty further modified race disparities: Race differences were largest in segregated, low-poverty areas (odds ratio = 4.14, 95% CI: 3.18, 5.38) and smallest in nonsegregated, high-poverty areas (odds ratio = 1.24, 95% CI: 0.77, 2.01). These findings suggest that racial disparities in hypertension are not invariant and are modified by contextual levels of racial segregation and neighborhood poverty, highlighting the role of environmental factors in the genesis of disparities.
Keywords: health status disparities, hypertension, prejudice, social environment
Hypertension prevalence is significantly higher among blacks than any other racial or ethnic group in the United States (1), and these disparities often persist after adjustment for individual-level socioeconomic position and traditional risk factors (2–4). Identification of environments in which black-white hypertension disparities are smaller or nonexistent may help to elucidate the role of contextual factors in perpetuating the unequal burden of hypertension and to provide important clues regarding the causes of these disparities.
A growing number of studies link place to hypertension disparities, but the mechanisms are not yet clear (5–8). No study, to our knowledge, has examined the impact of racial residential segregation on black-white disparities in hypertension, but there is evidence that disparities in other health outcomes may vary by levels of racial segregation (9–13). Researchers hypothesize that segregation leads to health disparities by leaving blacks more likely to live in concentrated poverty than whites (14, 15). Living in concentrated poverty is, in turn, associated with a wide range of deleterious exposures that could lead to hypertension disparities, including decreased neighborhood safety, limited access to healthy foods and recreational resources, and lower levels of educational attainment (5, 6, 16).
In this study, we used data on adult participants of the 1999–2006 National Health and Nutrition Examination Survey (NHANES) who resided in US metropolitan statistical areas (MSAs) to evaluate the contribution of racial residential segregation to black-white hypertension disparities. We hypothesized that higher levels of racial residential segregation would be associated with a larger race difference in hypertension prevalence. In addition, we hypothesized that larger race differences in neighborhood poverty would explain the stronger association of race with hypertension in more segregated areas.
MATERIALS AND METHODS
Study population
The data used in these analyses came from the 1999–2006 non-Hispanic black and white NHANES participants aged 25 years or older who resided in MSAs. Approximately 10% of all US MSAs were represented in NHANES. Of the 10,611 eligible black and white study participants, 1,349 were excluded for missing blood pressure. In addition, 721 participants were excluded for missing education or income data and another 470 for missing data on body mass index, cigarette smoking, diet, or exercise. This left 8,071 participants for the analyses. Blacks and women were slightly more likely to be excluded because of missing data.
NHANES is a multistage stratified probability sample of US households designed to examine health and nutrition in children and adults (17). National Center for Health Statistics Research Ethics Review Board approval was obtained for NHANES, and informed consent was obtained from all participants.
Measures
Hypertension.
Hypertension prevalence was defined as having a mean systolic blood pressure greater than or equal to 140 mm Hg, a mean diastolic blood pressure greater than or equal to 90 mm Hg, or a self-reported history of hypertension and report of being on medication for it (18). Resting seated blood pressure was measured up to 4 times in a single visit by a certified operator using a mercury sphygmomanometer. Approximately 95% of all participants in this study had at least 2 blood pressure measurements taken. Consistent with prior research, the average of the last 2 measurements was used for participants who had 3–4 measurements taken; the second measurement was taken for those who had only 2 measurements; and the only measurement was used for participants who had just 1 recorded measurement (4). Sensitivity analyses showed that results were similar when the study population was limited to those who had at least 3 blood pressure measurements.
Racial residential segregation.
Massey and Denton (19) conceptualized 5 geographic dimensions of racial/ethnic residential segregation: evenness, exposure, clustering, centralization, and concentration. All are empirically correlated, but each is thought to represent distinct aspects of residential segregation. In this study, the black isolation index, a measure of the exposure dimension, was used according to 2000 Census data. The black isolation index is a commonly used measure that estimates the extent to which blacks live in neighborhoods where they are exposed only to other blacks (14).
The isolation index ranges from near 0 to 1, where a score near 0 indicates that blacks are completely integrated with whites, and 1 means that blacks are completely isolated from whites. It is represented mathematically as follows:
where xi is the number of blacks in tract i, ti is the total population in tract i, and X is the number of blacks in the metropolitan area. This proportion is then summed across all census tracts in the MSA.
MSAs are geographic entities consisting of large urban areas and surrounding counties that have social or economic ties with the urban core. They were chosen as the geographic context for which to measure segregation as opposed to cities or counties because, by design, they represent regional housing and labor markets, which help to shape residential segregation and its potential impact on differential disadvantage and adverse health outcomes (20).
Covariates.
Census tracts were used as proxies for neighborhoods in these analyses. Neighborhood poverty was measured as the percentage of the population living below the 1999 US Census Bureau-defined poverty threshold and modeled continuously (21).
Individual-level education was measured as the highest level completed and categorized as less than high school, high school, some college, and college or more. Mean annual family income was broken into the following quartiles: ≤$14,999; $15,000–$34,999; $35,000–$64,999; and ≥$65,000. Gender was analyzed dichotomously as male versus female, and age was analyzed continuously. Cigarette smoking, based on self-report, was categorized as current, former, and never. Obesity (body mass index, ≥30 kg/m2) was included dichotomously on the basis of measured height and weight. Results were similar when body mass index was included as a continuous variable. Physical activity was dichotomized as yes versus no on the basis of whether or not participants reported engaging in any amount of moderate or vigorous activity lasting 10 minutes or longer over the last 30 days. Diet history was obtained by using a 24-hour dietary recall that was administered by a trained interviewer. Diet was assessed on the basis of adherence to the Dietary Approaches to Stop Hypertension trial diet by using data on levels of consumption of the following nutrients: saturated fat, total fat, protein, cholesterol, fiber, magnesium, calcium, potassium, and sodium (22, 23).
Analyses
Means with standard errors and frequencies were calculated for all continuous and categorical characteristics by race, taking into account the study design and unequal selection probabilities of the study participants. Continuous variables were compared by using analysis of variance, and categorical variables were compared by using the χ2 statistic. Descriptive analyses also examined the distribution of blacks and whites across cross-classified categories (based on quartiles) of black isolation score and neighborhood poverty.
Multilevel logistic regression was used to estimate black-white disparities in hypertension before and after adjustment for MSA-level residential segregation and neighborhood (census tract)-level poverty, as well as multiplicative interactions between race and both variables. Three-level random intercept models (with random intercepts for tracts and counties) were fitted by using the 8,071 study participants nested within 1,827 census tracts and 99 counties. A random intercept was not included to account for clustering at the MSA level, because NHANES participants were sampled at the county level, and the majority of MSAs in the study population were represented by only one (64.9%) or two (27%) counties. Allowing the coefficient for race to vary randomly across counties did not significantly improve the fit of the model. There was a median of 12 tracts per county and 8 participants per tract. These geographic identifiers are restricted-use variables that were accessed through the National Center for Health Statistics Research Data Center.
The first model (model 1) was run adjusted for age, gender, and race. Model 2 also adjusted for income and education. Model 3 added the black isolation score, and model 4 added a multiplicative interaction between race and black isolation to allow the race difference in hypertension to vary by level of segregation. Model 5 added a measure of neighborhood poverty to examine whether race differences in neighborhood poverty explained any heterogeneity in the association of race with hypertension by levels of black isolation score. Subsequent analyses (shown in figures) also added a race-neighborhood poverty interaction (to account for possible differences in the poverty-hypertension association in blacks and whites), as well as risk factors for hypertension. For a more meaningful interpretation of the results, estimates for segregation and neighborhood poverty correspond to a difference equivalent to a 1-standard deviation increase (standard deviations = 0.21% and 10.7%, respectively). Neighborhood poverty was also mean centered (mean = 12.1%).
Individual-level sampling weights were incorporated into the multilevel models to account for the study design and unequal selection probabilities. On the basis of previous research, these weights were scaled so that the new weights summed to the level-2 (census tract) cluster sample size (24). Level-2 and level-3 weights (accounting for selection probabilities of the census tracts and counties, respectively) were unavailable and were thus set to 1 in these analyses. All multilevel analyses were conducted by using the GLLAMM (generalized linear latent and mixed models) program (25) in STATA, version 11, software (StataCorp LP, College Station, Texas). Given the high prevalence of hypertension, the odds ratios presented do not approximate the prevalence ratios (26), although they are still meaningful estimates of the associations.
RESULTS
Hypertension prevalence was 40.0% among blacks compared with 30.8% among whites (Table 1). Blacks were more likely to have low individual and neighborhood socioeconomic position: Just under 30% of all blacks had less than a high school education compared with fewer than 11% of whites; 23.6% of blacks reported annual family incomes below $14,999 versus 9.5% of whites; and mean neighborhood poverty for blacks was 19.8% compared with 8.4% for whites. The mean body mass index was significantly higher for blacks than whites; blacks were also more likely to be current smokers and to have poor diets and less likely to exercise.
Table 1.
Demographic and Other Characteristics by Race, National Health and Nutrition Examination Survey, 1999–2006
| Black (n = 2,382) |
White (n = 5,689) |
P Value | |||
| Mean (SE) | % | Mean (SE) | % | ||
| Hypertensive | 40.0 | 30.8 | <0.0001 | ||
| Age, years | 45.7 (0.3) | 49.1 (0.3) | <0.0001 | ||
| Male gender | 45.0 | 48.9 | 0.002 | ||
| Education less than high school | 29.9 | 10.7 | <0.0001 | ||
| Income less than $14,999 | 23.6 | 9.5 | <0.0001 | ||
| Obese | 42.3 | 30.8 | <0.0001 | ||
| No intentional exercise | 80.2 | 73.8 | 0.0002 | ||
| Current cigarette smoker | 26.8 | 22.5 | <0.0001 | ||
| Former cigarette smoker | 16.2 | 29 | |||
| Poor diet | 93.8 | 88.4 | <0.0001 | ||
| Neighborhood poverty, % | 19.8 (0.7) | 8.4 (0.3) | <0.0001 | ||
| Black isolation, score | 0.50 (0.02) | 0.36 (0.02) | <0.0001 | ||
Abbreviation: SE, standard error.
Table 2 depicts the distribution of black and white study participants by level of segregation and neighborhood poverty. The smallest percentage of black participants were living in low-segregation, low-poverty areas (0.9% of all blacks), and the largest percentage were living in high-segregation, high-poverty areas (28.7%). Whites were more evenly distributed across the segregation-poverty subgroups, though smaller percentages of whites were living in higher than in lower poverty areas, regardless of level of segregation. Continuous measures of the black isolation score and neighborhood poverty were only weakly correlated (Pearson’s r = 0.17).
Table 2.
Percentage of All Study Participants by Level of Racial Residential Segregation and Neighborhood Poverty for Blacks and Whites, National Health and Nutrition Examination Surveys, 1999–2006
| Quartile of Black Isolation Score by Race | Quartile of Mean Neighborhood Poverty |
|||
| NP 1 (low) | NP 2 | NP 3 | NP 4 (high) | |
| Blacks (n = 2,382) | ||||
| BI 1 (low) | 0.9 | 1.6 | 2.2 | 3.4 |
| BI 2 | 2.3 | 3.5 | 5.5 | 13.2 |
| BI 3 | 3.1 | 4.0 | 5.4 | 10.6 |
| BI 4 (high) | 2.6 | 4.9 | 8.0 | 28.7 |
| Whites (n = 5,689) | ||||
| BI 1 (low) | 8.7 | 9.4 | 9.4 | 3.6 |
| BI 2 | 9.4 | 8.9 | 6.9 | 3.3 |
| BI 3 | 5.5 | 7.8 | 6.5 | 2.4 |
| BI 4 (high) | 7.8 | 3.9 | 3.6 | 2.8 |
Abbreviations: BI, black isolation score; NP, neighborhood poverty.
Table 3 shows that, after adjustment for age and gender, blacks had 2.92 (95% confidence interval (CI): 2.47, 3.45) times higher odds of hypertension compared with whites (model 1). This was attenuated some after adjustment for education and income (odds ratio (OR) = 2.74, 95% CI: 2.32, 3.25) and then increased slightly after adjustment for the isolation index (OR = 2.81, 95% CI: 2.37, 3.34). However, the magnitude of the black-white differences varied significantly by level of segregation (Pinteraction = 0.006). The estimates shown are those predicted by the model for the 10th percentile (defined as “low”) and the 90th percentile (defined as “high”) of segregation (0.06 and 0.65 isolation scores, respectively). Blacks living in low segregation areas had 1.67 (95% CI: 1.08, 2.57) times higher odds of hypertension than whites, compared with a 3.57 (95% CI: 2.88, 4.42) times higher odds for blacks versus whites residing in high segregation areas. This statistically significant interaction also indicated that the relation between segregation and hypertension was different for blacks than for whites. For blacks, each standard deviation increase in segregation was associated with a 1.18 (95% CI: 1.00, 1.39) times higher odds of hypertension, while a weaker, inverse association was seen for whites (OR = 0.90, 95% CI: 0.79, 1.02).
Table 3.
Odds Ratios of Hypertension Associated With Race and Other Covariates in Sequential Models, National Health and Nutrition Examination Survey, 1999–2006a
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 1.10 | 1.10, 1.11 | 1.10 | 1.09, 1.11 | 1.10 | 1.09, 1.11 | 1.10 | 1.09, 1.11 | 1.10 | 1.09, 1.11 |
| Gender | ||||||||||
| Male | 0.91 | 0.79, 1.06 | 0.89 | 0.77, 1.04 | 0.89 | 0.77, 1.04 | 0.89 | 0.77, 1.04 | 0.89 | 0.77, 1.03 |
| Female | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Race | ||||||||||
| Black | 2.92 | 2.47, 3.45 | 2.74 | 2.32, 3.25 | 2.81 | 2.37, 3.34 | ||||
| White | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||||
| Race difference at low (10th percentile) black isolation score | 1.67* | 1.08, 2.57 | 1.64** | 1.07, 2.52 | ||||||
| Race difference at high (90th percentile) black isolation score | 3.57* | 2.88, 4.42 | 3.37** | 2.66, 4.26 | ||||||
| Education | ||||||||||
| Less than high school | 1.37 | 1.06, 1.77 | 1.37 | 1.06, 1.77 | 1.36 | 1.05, 1.76 | 1.34 | 1.03, 1.74 | ||
| High school | 1.63 | 1.34, 1.98 | 1.63 | 1.34, 1.97 | 1.63 | 1.34, 1.97 | 1.62 | 1.33, 1.97 | ||
| Some college | 1.29 | 1.06, 1.57 | 1.29 | 1.0, 1.57 | 1.28 | 1.05, 1.56 | 1.28 | 1.05, 1.56 | ||
| Bachelor’s degree or more | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| Income | ||||||||||
| <$14,999 | 1.19 | 0.94, 1.51 | 1.20 | 0.94, 1.52 | 1.19 | 0.94, 1.51 | 1.16 | 0.92, 1.47 | ||
| $15,000–$34,999 | 0.99 | 0.78, 1.25 | 0.99 | 0.78, 1.26 | 1.00 | 0.78, 1.27 | 0.98 | 0.76, 1.25 | ||
| $35,000–$64,999 | 0.87 | 0.70, 1.10 | 0.88 | 0.70, 1.11 | 0.88 | 0.70, 1.10 | 0.87 | 0.69, 1.09 | ||
| ≥$65,000 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| Black isolation score | 0.96 | 0.86, 1.07 | ||||||||
| Black isolation score among blacks | 1.18* | 1.00, 1.39 | 1.17** | 0.99, 1.38 | ||||||
| Black isolation score among whites | 0.90* | 0.79, 1.02 | 0.90** | 0.79, 1.02 | ||||||
| Neighborhood poverty | 1.07 | 0.97, 1.18 | ||||||||
Abbreviations: CI, confidence interval; OR, odds ratio.
*Pinteraction = 0.006; **Pinteraction = 0.009.
Estimates are derived from a 3-level random intercept model as described in the text. Model 1 is adjusted for age, sex, and race. Model 2 is adjusted for model 1, education, and income. Model 3 is adjusted for model 2 and the black isolation score. Model 4 is adjusted for model 3 and race-black isolation interaction. Model 5 is adjusted for model 4 and neighborhood poverty.
Neighborhood poverty was not significantly associated with hypertension, and adjustment for neighborhood poverty did not attenuate the race-segregation interaction (Table 3, model 5). However, there was a statistically significant interaction between race and neighborhood poverty (P = 0.009) (not shown in table). The race-black isolation interaction remained statistically significant (P = 0.007) when the race-neighborhood poverty interaction was included in the model (not shown in table).
Figure 1 shows odds ratios of hypertension for blacks versus whites based on estimates predicted by models for low (Figure 1A) and high (Figure 1B) segregation at the 10th percentile, the mean, and the 90th percentile of poverty (low, medium, and high corresponding to 3%, 12.1%, and 28% poverty, respectively). Overall, the odds ratios of hypertension in blacks versus whites were lower in areas of low segregation than in areas of high segregation. In addition, within both low and high segregation areas, the odds ratios for blacks versus whites were greater in low poverty than in high poverty areas. For example, the odds ratios of hypertension for blacks versus whites living in very segregated MSAs ranged from 4.14 (95% CI: 3.18, 5.38) in low poverty neighborhoods to 2.61 (95% CI: 1.90, 3.57) in high poverty neighborhoods. Corresponding odds ratios for the low segregation MSAs were 1.97 (95% CI: 1.26, 3.08) in low poverty neighborhoods and 1.24 (95% CI: 0.77, 2.01) in high poverty neighborhoods.
Figure 1.
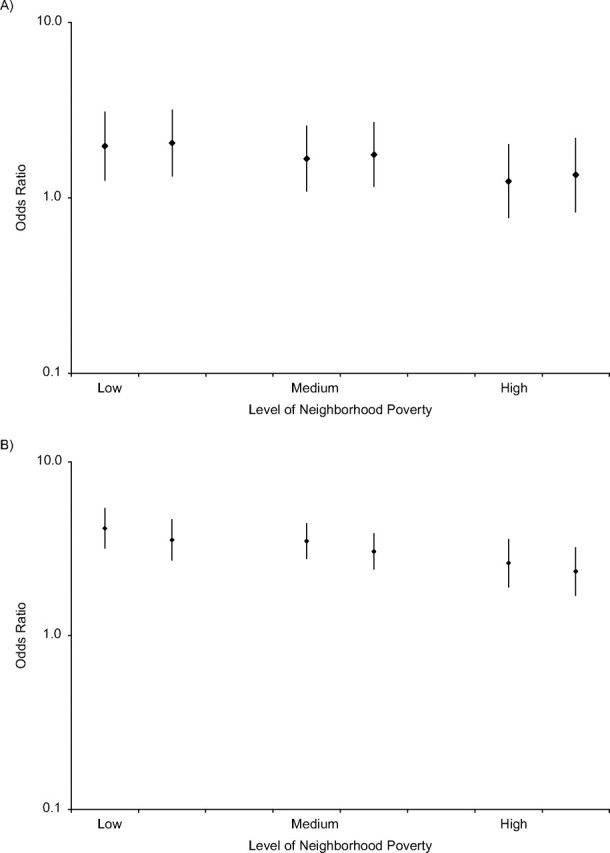
Odds ratios of hypertension prevalence in blacks versus whites by level of neighborhood poverty at low (part A; 10th percentile) and high (part B; 90th percentile) levels of segregation, National Health and Nutrition Examination Survey, 1996–2006. Each part has 3 sets of 2 odds ratios, with the left estimate of each set showing the odds ratio before adjustment for risk factors (i.e., body mass index and health behaviors) and the right estimate showing the odds ratio after adjustment for these risk factors. Odds ratios were derived from a 3-level random intercept model as described in the text. Models were adjusted for age, gender, race, education, income, black isolation score, race-black isolation score interaction, neighborhood poverty, and race-neighborhood poverty interaction. Low neighborhood poverty = 10th percentile; medium = mean; and high = 90th percentile. Bars, 95% confidence interval.
Adjustment for body mass index and behavioral risk factors attenuated race differences somewhat for those living in high segregation areas but had little impact on race differences in low segregation areas (Figure 1, right estimates). For example, at mean neighborhood poverty, the odds ratio for blacks versus whites living in highly segregated areas was 3.50 (95% CI: 2.78, 4.40) compared with 3.05 (95% CI: 2.41, 3.86) after adjustment for risk factors. In contrast, the odds ratio for blacks versus whites living in less segregated areas was not reduced after adjustment for risk factors (OR = 1.67 (95% CI: 1.09, 2.56) and OR = 1.76 (95% CI: 1.16, 2.68) before and after risk factor adjustment, respectively). Interactions between race and segregation and between race and poverty were still statistically significant after risk factor adjustment (Pinteraction = 0.04 and 0.02, respectively).
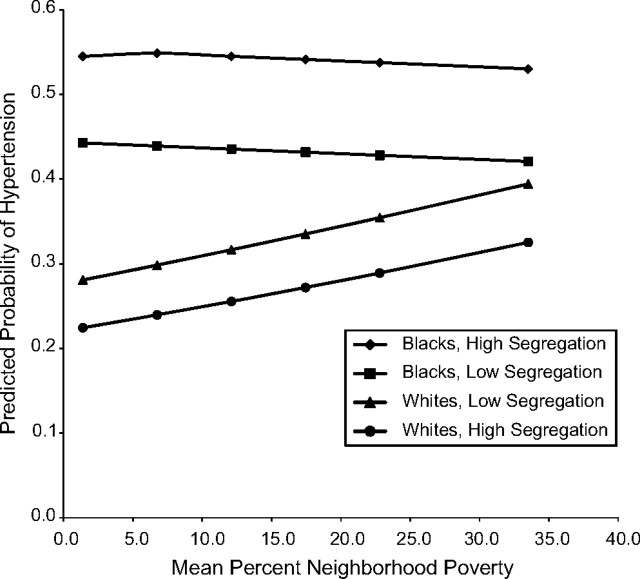
An examination of the predicted probabilities of hypertension by level of neighborhood poverty and segregation for blacks and whites (Figure 2) shows that, although hypertension prevalence among whites was slightly higher in less segregated areas than in more segregated areas, among blacks, living in less segregated areas was associated with lower hypertension prevalence. Figure 2 also indicates that the diminished race difference in hypertension observed in high compared with low poverty neighborhoods was due to the fact that greater neighborhood poverty was associated with greater hypertension prevalence in whites but not in blacks (OR of hypertension per standard deviation increase in neighborhood poverty = 1.18, 95% CI: 1.03, 1.36 in whites and OR = 0.97, 95% CI: 0.88, 1.07 in blacks; not shown on figure).
Figure 2.
Predicted probability of hypertension for blacks and whites by level of neighborhood poverty at low (10th percentile) and high (90th percentile) levels of segregation, National Health and Nutrition Examination Survey, 1999–2006. Estimates were derived from a 3-level random intercept model as described in the text. Models were adjusted for age, gender, race, education, income, black isolation score, race-black isolation score interaction, neighborhood poverty, and race-neighborhood poverty interaction.
DISCUSSION
This is among the first studies to investigate how racial residential segregation is related to black-white disparities in hypertension. The association between race and hypertension varied significantly by level of segregation; it was smallest among those living in more integrated areas and largest for those living in more segregated areas. Although segregation is often hypothesized to impact racial health disparities through aspects of the neighborhood environment, this has generally remained untested empirically. Comparison of results before and after adjustment for neighborhood poverty suggested that black-white differences in neighborhood poverty did not explain the larger race differences in more segregated areas. However, there was significant heterogeneity in the association between race and hypertension by neighborhood poverty in addition to segregation. When considered together, race differences in hypertension were greatest in segregated, low-poverty areas and weakest in nonsegregated, high-poverty areas.
Few studies have investigated associations between segregation and cardiovascular risk, and findings vary depending on the level at which segregation was measured. In a nationally representative US study of metropolitan-level segregation and body mass index, Chang (27) found that both body mass index and the odds of being overweight increased significantly with increasing metropolitan-level segregation for blacks, but no relation was observed for whites. These results are consistent with our finding that greater segregation at the metropolitan level was associated with higher odds of hypertension in blacks, but no association (or even the opposite association) was observed in whites. In contrast, a study of low-income, uninsured white, black, and Hispanic women living in 5 US states found no association between zip code-level segregation and body mass index in any group and a protective association with 10-year predicted heart disease risk among black and Hispanic women (28) after adjustment for several measures of the neighborhood environment. Similarly, a study in New York City found a protective association between neighborhood-level segregation and cardiovascular disease mortality rates (29). Differences in the geographic areas for which segregation has been measured, in the measure of segregation used, and in the individual-level variables for which estimates are adjusted may explain different results across studies.
A limited number of studies have assessed variation in the relation between race and hypertension by both level of segregation and neighborhood poverty. Consistent with our results, a Baltimore study found that race differences in hypertension were smaller in a low-income, integrated community than in a national sample (7). One explanation for these findings is that blacks and whites living in integrated but poor areas may be more comparable in their exposure to individual- and area-level stressors and in their access to health-enhancing resources than blacks and whites living in less poor or more segregated areas.
The racial disparities in hypertension seen in this study were somewhat attenuated by adjustment for body mass index and behavioral factors, but only among those living in more segregated areas. Racial residential segregation leads to the inequitable distribution of social and economic resources. One way to cope with this chronic disadvantage is to engage in behaviors that may reduce feelings of anxiety or stress at the expense of physical health (30–36). In addition, the ability to engage in healthy behaviors may be hindered by environmental factors associated with neighborhood segregation (37–39). Thus, it is possible that the contribution of these risk factors to black-white disparities in hypertension depends on the context in which individuals live. However, our ability to investigate the contributions of different individual-level factors to race differences was limited by the data available. In addition, the observational nature of our analyses prevents us from categorically ruling out the possibility that some of the patterns that we observed are attributable to the differential sorting of persons into different kinds of neighborhoods.
This study is not without limitations. Although NHANES is a nationally representative survey, the small percentage of MSAs represented may limit the generalizability of our findings regarding associations of segregation with hypertension. In addition, although both blacks and whites were represented across most of the continuum of MSA segregation and poverty (Table 2 shows there were observations in all segregation-poverty subgroups), the distributions were clearly different and, hence, our statistical analysis necessarily relies on some extrapolations. In the absence of clear evidence of threshold effects, we modeled both poverty and segregation as linear variables. Estimates presented for the 10th and 90th percentiles of segregation and poverty are predictions based on the associations observed across the full range of both distributions and not only on the data available at exactly the 10th and 90th percentiles. These estimates capture the general pattern observed across the continuum observed in the data but necessarily rely on extrapolations. Unfortunately, data sparseness made it impossible to fully investigate poverty and segregation as categorical variables, especially for the extremes of the distributions. Additional studies that take advantage of overlapping distributions are therefore necessary to confirm our findings.
We were unable to explain why race disparities in hypertension varied by level of segregation or neighborhood poverty. Adjustment for body mass index and behavioral risk factors somewhat attenuated the race-segregation and (to a lesser extent) the race-neighborhood poverty interactions, suggesting that these factors could play a mediating role, but the interactions remained statistically significant after risk factor adjustment. Measurement error in the behavioral factors or residual confounding due to either unmeasured psychosocial factors (that may be more strongly patterned by race in some geographic contexts than in others) or unmeasured neighborhood-level factors known to be associated with both hypertension and neighborhood poverty and segregation could play a role (6, 40–45). There may also be residual socioeconomic differences between blacks and whites, and the magnitude of these differences may vary by level of segregation and neighborhood poverty. Racial differences in wealth, for example, may be a better representation of disparities in economic status and resources than income and may be influenced by racial residential segregation (13, 46, 47). In addition, racial residential segregation may make blacks less able to convert improvements in individual-level socioeconomic position into better residential quality (46, 48). This residual confounding could contribute to the apparent effect modification that we observed. Another interpretation is that these factors themselves explain why the effect modification is observed (in a sense “mediate” rather than confound the effect modification).
Our results show that race differences in hypertension are not invariant across contexts and, in fact, are strongly modified by level of segregation and neighborhood poverty. These findings suggest that further research into environmental factors, as well as how environments may interact with genetic predispositions in both blacks and whites, is needed to better understand the causes of hypertension disparities and to develop more effective hypertension prevention interventions.
Acknowledgments
Author affiliations: Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Kiarri N. Kershaw); Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Kiarri N. Kershaw, Ana V. Diez Roux, Lynda D. Lisabeth); Department of Sociology, Population Studies Center, University of Michigan, Ann Arbor, Michigan (Sarah A. Burgard); Division of Epidemiology, School of Public Health, University of California, Berkeley, Berkeley, California (Mahasin S. Mujahid); and Department of Health Behavior and Health Education, School of Public Health, University of Michigan, Ann Arbor, Michigan (Amy J. Schulz).
This work was supported by a University of Michigan Rackham Graduate Student Research Grant and Michigan Center for Integrative Approaches to Health Disparities grant P60 MD002249. This work was also supported by grant T32-HL-069771-07. This research used data from the RAND Center for Population Health and Health Disparities, which is funded by grant 1-P50-ES012383 from the National Institute of Environmental Health Sciences; for further information on the Center for Population Health and Health Disparities, go to http://www.rand.org/health/centers/pophealth/index.html. The research in this paper was conducted while K. K. was a Special Sworn Status researcher of the US Census Bureau at the Michigan Census Research Data Center (which is supported by National Science Foundation awards SES-0004322 and ITR-0427889) and the Chicago Census Research Data Center.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the research data centers, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- MSA
metropolitan statistical area
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
References
- 1.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2004;17(10):963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Hertz RP, Unger AN, Cornell JA, et al. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165(18):2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 4.Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 5.Morenoff JD, House JS, Hansen BB, et al. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med. 2007;65(9):1853–1866. doi: 10.1016/j.socscimed.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujahid MS, Diez Roux AV, Morenoff JD, et al. Neighborhood characteristics and hypertension. Epidemiology. 2008;19(4):590–598. doi: 10.1097/EDE.0b013e3181772cb2. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe RJ, Jr, Brandon DT, LaVeist TA. Social context as an explanation for race disparities in hypertension: findings from the Exploring Health Disparities in Integrated Communities (EHDIC) Study. Soc Sci Med. 2008;67(10):1604–1611. doi: 10.1016/j.socscimed.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershaw KN, Diez Roux AV, Carnethon M, et al. Geographic variation in hypertension prevalence among blacks and whites: the Multi-Ethnic Study of Atherosclerosis. Am J Hypertens. 2010;23(1):46–53. doi: 10.1038/ajh.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polednak AP. Black-white differences in infant mortality in 38 standard metropolitan statistical areas. Am J Public Health. 1991;81(11):1480–1482. doi: 10.2105/ajph.81.11.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polednak AP. Poverty, residential segregation, and black/white mortality ratios in urban areas. J Health Care Poor Underserved. 1993;4(4):363–373. doi: 10.1353/hpu.2010.0094. [DOI] [PubMed] [Google Scholar]
- 11.Haas JS, Earle CC, Orav JE, et al. Racial segregation and disparities in cancer stage for seniors. J Gen Intern Med. 2008;23(5):699–705. doi: 10.1007/s11606-008-0545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laveist TA. Segregation, poverty, and empowerment: health consequences for African Americans. Milbank Q. 1993;71(1):41–64. [PubMed] [Google Scholar]
- 13.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acevedo-Garcia D, Lochner KA, Osypuk TL, et al. Future directions in residential segregation and health research: a multilevel approach. Am J Public Health. 2003;93(2):215–221. doi: 10.2105/ajph.93.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massey DS, Fischer MJ. How segregation concentrates poverty. Ethn Racial Stud. 2000;23(4):670–691. [Google Scholar]
- 16.Schulz AJ, Kannan S, Dvonch JT, et al. Social and physical environments and disparities in risk for cardiovascular disease: the healthy environments partnership conceptual model. Environ Health Perspect. 2005;113(12):1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHANES 1999–2000, 2001–2002, 2003–2004, and 2005–2006 documentation. Atlanta, GA: Centers for Disease Control and Prevention; 2011. ( http://www.cdc.gov/nchs/nhanes.htm) [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 19.Massey DS, Denton NA. The dimensions of residential segregation. Soc Forces. 1988;67(2):281–315. [Google Scholar]
- 20.Kramer MR, Hogue CR. Is segregation bad for your health? Epidemiol Rev. 2009;31(1):178–194. doi: 10.1093/epirev/mxp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Current Population Survey, 1999. Washington, DC: US Census Bureau; 2010. ( http://www.census.gov/apsd/techdoc/cps/cps-main.html) [Google Scholar]
- 22.Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168(3):308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 23.Sacks FM, Obarzanek E, Windhauser MM, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5(2):108–118. doi: 10.1016/1047-2797(94)00055-x. [DOI] [PubMed] [Google Scholar]
- 24.Carle AC. Fitting multilevel models in complex survey data with design weights: recommendations (electronic article) BMC Med Res Methodol. 2009;9:49. doi: 10.1186/1471-2288-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabe-Hesketh S, Skrondal A, Pickles A. Maximum likelihood estimation of limited and discrete dependent variable models with nested random effects. J Econometrics. 2005;128(2):301–323. [Google Scholar]
- 26.Rothman KJ, Greenland S, editors. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 27.Chang VW. Racial residential segregation and weight status among US adults. Soc Sci Med. 2006;63(5):1289–1303. doi: 10.1016/j.socscimed.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 28.Mobley LR, Root ED, Finkelstein EA, et al. Environment, obesity, and cardiovascular disease risk in low-income women. Am J Prev Med. 2006;30(4):327–332. doi: 10.1016/j.amepre.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Madhavan S, Bosworth W, et al. Residential segregation and mortality in New York City. Soc Sci Med. 1998;47(4):469–476. doi: 10.1016/s0277-9536(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 30.Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med. 1988;319(20):1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 31.Dallman MF, Akana SF, Laugero KD, et al. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79(1):3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 32.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food.”. Proc Natl Acad Sci U S A. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiLorenzo TM, Bargman EP, Stucky-Ropp R, et al. Long-term effects of aerobic exercise on psychological outcomes. Prev Med. 1999;28(1):75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- 34.Kirschbaum C, Wüst S, Strasburger CJ. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50(6):435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- 35.Steptoe A, Edwards S, Moses J, et al. The effects of exercise training on mood and perceived coping ability in anxious adults from the general population. J Psychosom Res. 1989;33(5):537–547. doi: 10.1016/0022-3999(89)90061-5. [DOI] [PubMed] [Google Scholar]
- 36.Jackson JS, Knight KM. Race and self-regulatory health behaviors: the role of the stress response and the HPA axis in physical and mental health disparities. In: Schaie KW, Cartensen L, editors. Social Structures, Aging, and Self-Regulation in the Elderly. New York, NY: Springer; 2006. pp. 189–207. [Google Scholar]
- 37.Burdette HL, Wadden TA, Whitaker RC. Neighborhood safety, collective efficacy, and obesity in women with young children. Obesity (Silver Spring) 2006;14(3):518–525. doi: 10.1038/oby.2006.67. [DOI] [PubMed] [Google Scholar]
- 38.Estabrooks PA, Lee RE, Gyurcsik NC. Resources for physical activity participation: does availability and accessibility differ by neighborhood socioeconomic status? Ann Behav Med. 2003;25(2):100–104. doi: 10.1207/S15324796ABM2502_05. [DOI] [PubMed] [Google Scholar]
- 39.Powell LM, Slater S, Chaloupka FJ, et al. Availability of physical activity-related facilities and neighborhood demographic and socioeconomic characteristics: a national study. Am J Public Health. 2006;96(9):1676–1680. doi: 10.2105/AJPH.2005.065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Harmer P, Cardinal BJ, et al. Built environment and changes in blood pressure in middle aged and older adults. Prev Med. 2009;48(3):237–241. doi: 10.1016/j.ypmed.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. Am J Public Health. 2006;96(2):325–331. doi: 10.2105/AJPH.2004.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore LV, Diez Roux AV, Evenson KR, et al. Availability of recreational resources in minority and low socioeconomic status areas. Am J Prev Med. 2008;34(1):16–22. doi: 10.1016/j.amepre.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore LV, Diez Roux AV, Nettleton JA, et al. Fast-food consumption, diet quality, and neighborhood exposure to fast food: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;170(1):29–36. doi: 10.1093/aje/kwp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenk SN, Schulz AJ, Israel BA, et al. Neighborhood racial composition, neighborhood poverty, and the spatial accessibility of supermarkets in metropolitan Detroit. Am J Public Health. 2005;95(4):660–667. doi: 10.2105/AJPH.2004.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams DR, Neighbors H. Racism, discrimination and hypertension: evidence and needed research. Ethn Dis. 2001;11(4):800–816. [PubMed] [Google Scholar]
- 46.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8(6):621–628. [PubMed] [Google Scholar]
- 47.Oliver ML, Shapiro TM. Black Wealth/White Wealth: A New Perspective on Racial Inequality. New York, NY: Routledge; 2006. [Google Scholar]
- 48.Villemez WJ. Race, class, and neighborhood: differences in the residential return on individual resources. Soc Forces. 1980;59(2):414–430. [Google Scholar]