Abstract
The authors prospectively examined bowel movement frequency at baseline in relation to future Parkinson’s disease risk in the Health Professionals Follow-up Study (HPFS) during 2000–2006 (33,901 men) and the Nurses’ Health Study (NHS) during 1982–2006 (93,767 women). During the follow-up (6 years for the HPFS and 24 years for the NHS), the authors identified 156 incident male Parkinson’s disease cases (HPFS) and 402 female cases (NHS). In the HPFS, compared with men with daily bowel movements, men with a bowel movement every 3 days or less had a multivariate-adjusted relative risk of 4.98 (95% confidence interval (CI): 2.59, 9.57) for developing Parkinson’s disease in the next 6 years. In the NHS, the corresponding relative risk was 2.15 (95% CI: 0.76, 6.10), and the risk of Parkinson’s disease was not elevated beyond 6 years of follow-up (relative risks = 1.25 for years 7–12, 0.54 for years 13–18, and 0.88 for years 19–24). When these 2 cohorts were combined, the pooled relative risks for Parkinson’s disease in the next 6 years were 0.75, 1 (referent), 2.62, and 3.93 (95% CI: 2.26, 6.84) (Ptrend < 0.0001) across 4 bowel movement categories. In conclusion, infrequent bowel movements may antedate the onset of cardinal motor symptoms of Parkinson’s disease and may contribute to the identification of populations with higher than average Parkinson’s disease risk.
Keywords: constipation, Parkinson disease, prospective studies
Patients with Parkinson’s disease suffer from a variety of nonmotor symptoms, such as olfactory dysfunction, constipation, sleep disorders, sexual dysfunction, and weight loss, in addition to motor dysfunctions (1–5). Some of these symptoms may develop prior to the clinical onset of Parkinson’s disease, and research on these nonmotor symptoms during disease development may improve our understanding of the pathoetiology of this disease and lead to earlier disease diagnosis and better clinical management. Constipation affects approximately 60%–80% of Parkinson’s disease patients and may develop prior to the cardinal motor signs of the disease (6). In Parkinson’s disease, constipation could be the result of lesions in the enteric nervous system (e.g., gastric myenteric plexus) or the dorsal motor nucleus of the vagus nerve, which are among the earliest affected regions in Parkinson’s disease pathogenesis prior to the involvement of substantia nigra (1, 6–13). Consistent with this hypothesis, in the men-only Honolulu-Asia Aging Study (Parkinson’s disease cases = 96), infrequent bowel movements were associated with higher future Parkinson’s disease risk (14). A higher Parkinson’s disease risk among individuals with a history of constipation, as assessed through review of medical records, was also observed in a nested case-control study, which included both men (cases = 121) and women (cases = 75) (15). However, the temporal relation between bowel movements and future risk of Parkinson’s disease was not systematically examined in these studies. Further, although the prevalence of constipation in women is approximately 2 times higher than that in men and the determinants of constipation may differ by gender (16), women were underrepresented in the previous studies. We therefore prospectively examined the temporal relation between bowel movement frequency and Parkinson’s disease risk among 33,901 men in the Health Professionals Follow-up Study (HPFS) and 93,767 women in the Nurses’ Health Study.
MATERIALS AND METHODS
Subjects
The Nurses’ Health Study (NHS) comprises 121,700 female registered nurses aged 30–55 years at the time of enrollment in 1976. The HPFS was established in 1986, when 51,529 male US health professionals aged 40–75 years completed a mailed questionnaire about their medical history and lifestyle. These 2 cohorts were then followed with biennial surveys to update information on exposures and the occurrence of major chronic diseases. As the information on bowel movement frequency was not collected until 1982 in the NHS and 2000 in the HPFS, we therefore used 1982 as baseline for the NHS and 2000 as baseline for the HPFS for the current study. Further, we excluded participants with a diagnosis of Parkinson’s disease at baseline or missing information on bowel movement, leaving us 33,901 men and 93,767 women in the current analyses.
The study was approved by the human research committees at the Harvard School of Public Health and the Brigham and Women’s Hospital with receipt of each questionnaire accepted as participant’s consent.
Assessment of bowel movement frequency, use of laxatives, and covariates
In the 1982 survey of the NHS and the 2000 survey of the HPFS, study participants were asked to report their frequency of bowel movement at the time of the survey; 7 categories are allowed: more than once daily; daily; every other day; every 3–4 days; every 5–6 days; and once a week or less. Further, participants were also asked how often they took laxatives (such as softeners, bulking agents, and suppositories) in the following categories: daily, at least once per week, 1–4 times per month, <1 time per month, and never.
Information on potential confounders, including age, weight, height, smoking status, use of medicines, and history of chronic diseases, was collected via biennial questionnaires through the follow-up. Dietary intakes were assessed with a semiquantitative food frequency questionnaire validated for use in this population (17, 18).
Ascertainment of Parkinson’s disease
The identification of Parkinson’s disease cases during the cohorts’ follow-up has been described elsewhere (19, 20). Briefly, physician-diagnosed Parkinson’s disease was first reported in the biennial questionnaires. After obtaining permission from potential Parkinson’s disease patients, we asked their treating neurologists (or internist if there was no neurologist) to complete a questionnaire to confirm or refute Parkinson’s disease diagnosis or to send us a copy of the medical records. A case was confirmed if a diagnosis of Parkinson’s disease was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of Parkinson’s disease made by a neurologist or evidence of at least 2 of the 3 cardinal signs (rest tremor, rigidity, bradykinesia) in the absence of features suggesting other diagnoses. The review of medical records was conducted by a movement disorder specialist (M. A. S.), blind to the exposure status. Overall, the diagnosis was confirmed by the treating neurologist in >90% of the cases. We also requested the death certificates of the deceased study participants and identified Parkinson’s disease diagnoses that were not reported in the regular follow-up (<2%). In this analysis, only confirmed Parkinson’s disease cases were included as in our previous studies (2, 5, 19–21).
Statistical analysis
We computed the person-time of follow-up for each participant from the return date of the baseline questionnaire (2000 for the HPFS and 1982 for the NHS) to the date of the occurrence of the first symptoms of Parkinson’s disease, the date of death, or the end of follow up in 2006, whichever came first. Bowel movement frequency was categorized into 4 categories: more than once daily, daily, every 2 days, and every 3 days or less. Relative risks and 95% confidence intervals were calculated by dividing the incidence rate in each category by the rate in the referent category (i.e., daily), separately in each cohort. Multivariate-adjusted relative risks were derived from Cox proportional hazard models controlling for age (in months), smoking status (never smoker, past smoker, or current smoker: cigarettes/day, 1–14 or ≥15), body mass index (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), use of nonsteroid antiinflammatory drugs (yes/no) and laxatives (none, <1 time/month, 1–4 times/month, or ≥1 time/week), and intakes of alcohol (none, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/day for women; none, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/day for men), caffeine (quintiles), and lactose (quintiles). The analyses were first conducted by cohorts (gender) and then pooled by a fixed-effects model, weighted by the inverse of their variances because there was no evidence of heterogeneity between these 2 cohorts (P > 0.1) (22).
We also examined potential interactions between bowel movement frequency and age (years), smoking status (never vs. ever), and caffeine intake (based on median intake) by adding multiplicative terms in the Cox models, adjusting for other potential confounders. In a secondary analysis, we excluded regular laxative users (≥1 time/week) because some participants may use laxatives for other purposes, such as weight control, rather than constipation. To test the robustness of our results, we conducted a sensitivity analysis by excluding the individuals with a Parkinson’s disease diagnosis made by a physician who was not a neurologist (n = 56 of 559 cases).
RESULTS
We identified 156 incident Parkinson’s disease cases during 6 years of follow-up (2000–2006) in the HPFS and 402 cases over 24 years of follow-up (1982–2006) in the NHS. Participants with fewer bowel movements were more likely to use laxatives regularly (≥1 time/week), had a lower body mass index, and consumed larger amounts of caffeine and less alcohol and lactose than those with a bowel movement daily or more often (Table 1), but no differences were observed with respect to the other covariates.
Table 1.
Baseline Characteristics According to Bowel Movement Frequency in the Health Professionals Follow-up Study (2000) and the Nurses’ Health Study (1982)a
| Bowel Movement Frequency |
||||||||||||||||
| Men |
Women |
|||||||||||||||
| >1/Day |
Daily |
Every 2 Days |
Every 3 Days or Less |
>1/Day |
Daily |
Every 2 Days |
Every 3 Days or Less |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Totals by bowel movement frequency and sex | 8,999 | 26.5 | 22,374 | 66.0 | 2,046 | 6.0 | 482 | 1.4 | 10,117 | 10.8 | 58,833 | 62.7 | 16,801 | 17.9 | 8,016 | 8.5 |
| Age, years | 65.9 | 67.1 | 69.3 | 71.6 | 49.5 | 48.8 | 47.3 | 47.3 | ||||||||
| Current smokers | 3.2 | 4.3 | 4.0 | 7.9 | 27.0 | 28.1 | 23.2 | 24.9 | ||||||||
| Past smokers | 52.5 | 53.1 | 52.4 | 51.3 | 29.3 | 28.7 | 30.7 | 30.3 | ||||||||
| Body mass index, kg/m2 | 26.3 | 26.1 | 25.8 | 25.8 | 26.5 | 24.6 | 24.2 | 24.1 | ||||||||
| Alcohol intake, g/day | 11.0 | 11.2 | 9.2 | 9.6 | 6.9 | 6.7 | 5.6 | 5.4 | ||||||||
| Caffeine intake, mg/day | 193 | 212 | 219 | 229 | 375 | 396 | 398 | 420 | ||||||||
| Lactose intake, g/day | 15.9 | 15.7 | 15.3 | 15.0 | 13.4 | 14.0 | 13.2 | 12.6 | ||||||||
| Use of laxatives weekly or more | 8.3 | 8.5 | 15.0 | 22.3 | 4.0 | 4.3 | 9.3 | 17.0 | ||||||||
| Use of nonaspirin nonsteroidal antiinflammatory drugs | 27.1 | 25.1 | 25.0 | 26.6 | 7.4 | 4.8 | 5.0 | 5.7 | ||||||||
Values were standardized to the age distribution of the overall cohort.
In the HPFS, we found a significant association between fewer bowel movements and higher risk of developing Parkinson’s disease in the next 6 years of follow-up (Table 2). The multivariate relative risks were 0.75 for those with >1 bowel movement/day, 3.08 for those with bowel movements every 2 days, and 4.98 (95% confidence interval (CI): 2.59, 9.57) (Ptrend < 0.0001) for those with bowel movements every 3 days or less in a comparison with men who had daily bowel movements. In the NHS, the results appeared to depend on the years of follow-up under investigation. When the analysis was limited to the first 6 years of follow-up, we identified an association that was similar to what we found in men. The multivariate-adjusted relative risks as compared with daily bowel movements were 0.74 for >1 per day, 1.42 for every 2 days, and 2.15 for every 3 days or less (95% CI: 0.76, 6.10) (Ptrend = 0.10). When we pooled the 2 cohorts together, the combined relative risks for Parkinson’s disease during the first 6 years of follow-up were 0.75 for >1 per day, 1 (referent) for daily, 2.62 for every 2 days, and 3.93 for every 3 days or less (95% CI: 2.26, 6.84) (Ptrend < 0.0001) (Figure 1). The association remained in both men and women after excluding participants who reported use of laxatives ≥1 time/week: The multivariate relative risk comparing a bowel movement every 3 days or less with daily was 4.35 (95% CI: 1.80, 10.5) (Ptrend < 0.0001) in men and 2.98 (95% CI: 1.09, 8.14) (Ptrend = 0.03) in women. Similar results were observed when we included only Parkinson’s disease cases confirmed by a neurologist: The multivariate-adjusted relative risk for a bowel movement every 3 days or less versus daily was 5.10 (95% CI: 2.63, 9.88) (Ptrend < 0.0001) in men and 3.07 (95% CI: 1.10, 8.5) (Ptrend = 0.08) in women. Further adjustment for consumption of fruits and vegetables, supplements of iron or calcium, physical activity, or use of drugs that may result in constipation (i.e., aspirin, diuretics, H-2 blockers, methyldopa, and valium or other minor tranquilizers) did not materially change the results observed (Ptrend = 0.10 in women and < 0.0001 in men).
Table 2.
Relative Risk of Developing Parkinson’s Disease According to Frequency of Bowel Movement in the Health Professionals Follow-up Study (2000–2006) and the Nurses’ Health Study (1982–2006)
| No. of Cases | >1/Day |
Daily |
Every 2 Days |
Every 3 Days or Less |
Ptrend | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| Men (HPFS) | 156 | |||||||||
| Multivariate RRa | 0.75 | 0.49, 1.17 | 1 | Referent | 3.08 | 1.97, 4.82 | 4.98 | 2.59, 9.57 | <0.0001 | |
| Excluding laxative regular users | 0.78 | 0.48, 1.25 | 1 | Referent | 3.31 | 1.96, 5.58 | 4.35 | 1.80, 10.5 | <0.0001 | |
| Women (NHS) | ||||||||||
| Parkinson’s disease onset during years 1–6 | 37 | |||||||||
| Multivariate RRa | 0.74 | 0.22, 2.52 | 1 | Referent | 1.42 | 0.59, 3.39 | 2.15 | 0.76, 6.10 | 0.10 | |
| Excluding laxative regular users | 0.85 | 0.25, 2.90 | 1 | Referent | 1.71 | 0.71, 4.10 | 2.98 | 1.09, 8.14 | 0.03 | |
| Parkinson’s disease onset during years 7–12 | 93 | |||||||||
| Multivariate RRa | 1.50 | 0.83, 2.72 | 1 | Referent | 0.79 | 0.42, 1.49 | 1.25 | 0.61, 2.59 | 0.50 | |
| Excluding laxative regular users | 1.19 | 0.62, 2.29 | 1 | Referent | 0.72 | 0.36, 1.41 | 1.33 | 0.63, 2.79 | 0.80 | |
| Parkinson’s disease onset during years 13–18 | 140 | |||||||||
| Multivariate RRa | 0.94 | 0.55, 1.61 | 1 | Referent | 0.88 | 0.55, 1.41 | 0.54 | 0.25, 1.19 | 0.23 | |
| Excluding laxative regular users | 0.89 | 0.50, 1.57 | 1 | Referent | 0.97 | 0.59, 1.58 | 0.56 | 0.23, 1.39 | 0.49 | |
| Parkinson’s disease onset during years 19–24 | 132 | |||||||||
| Multivariate RRa | 1.07 | 0.62, 1.85 | 1 | Referent | 1.09 | 0.69, 1.74 | 0.88 | 0.43, 1.79 | 0.83 | |
| Excluding laxative regular users | 1.15 | 0.67, 1.99 | 1 | Referent | 1.17 | 0.73, 1.87 | 1.03 | 0.51, 2.06 | 0.94 | |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; RR, relative risk.
Adjusted for age (in months), smoking (never smoker, past smoker, or current smoker: cigarettes/day, 1–14 or ≥15), body mass index (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), use of nonaspirin nonsteroidal antiinflammatory drugs (yes/no), laxatives (none, <1 time/month, 1–4 times/month, or ≥1 time/week), and intakes of alcohol (none, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/day for women; none, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/day for men), caffeine (quintiles), and lactose (quintiles).
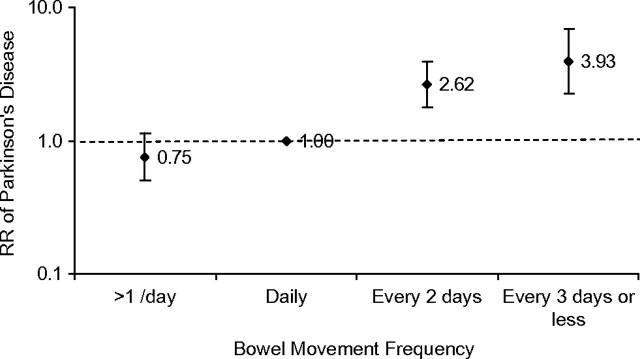
Figure 1.
Pooled relative risk (RR) of Parkinson’s disease onset during the first 6 years of follow-up in the Health Professionals Follow-up Study (2000–2006) and the Nurses’ Health Study (1982–1988). Adjusted for age (in months), smoking (never smoker, past smoker, or current smoker: cigarettes/day, 1–14 or ≥15), body mass index (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), use of nonaspirin nonsteroidal antiinflammatory drugs (yes/no), laxatives (none, <1 time/month, 1–4 times/month, or ≥1 time/week), and intakes of alcohol (none, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/day for women; none, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/day for men), caffeine (quintiles), and lactose (quintiles). Ptrend < 0.0001. Bars, confidence intervals.
In the NHS, however, we did not find a significant association between baseline bowel movement frequency and risk of Parkinson’s disease onset beyond the 6 years of follow-up (Table 2). The multivariate-adjusted relative risks comparing bowel movements every 3 days or less with daily were 1.25 (95% CI: 0.61, 2.59) for Parkinson’s disease onset during years 7–12, 0.54 (95% CI: 0.25, 1.19) for years 13–18, and 0.88 (95% CI: 0.43, 1.79) for years 19–24. Excluding regular users of laxatives did not materially change the results.
We did not find a significant interaction between age (years), smoking status (never vs. ever), use of hormone replacement therapy (never vs. ever, in women only), and caffeine intake (high vs. low, based on median value) and bowel movement frequency in relation to Parkinson’s disease risk (Pinteraction > 0.2 for all).
DISCUSSION
In this large prospective study, we found that infrequent bowel movements were associated with a higher future risk of Parkinson’s disease in the next 6 years in men and women. This association is independent of known risk factors of Parkinson’s disease, such as age, smoking, and caffeine intake. Further, the NHS data showed that less frequent bowel movements might not predict future Parkinson’s disease risk beyond 6 years of follow-up. Our study provides direct evidence to support the hypothesis that infrequent bowel movements precede Parkinson’s disease onset by many years. Constipation alone is insufficient to characterize Parkinson’s disease risk, but when integrated with other nonmotor symptoms and biomarkers it may contribute to identify individuals with high risk of developing Parkinson’s disease.
Our findings support the results from the all-male Honolulu-Asia Aging Study that an infrequent bowel movement was associated with higher future risk for Parkinson’s disease and further prospectively showed a similar association in women. In the Honolulu study (96 cases, men only), men with infrequent bowel movements had a higher risk of Parkinson’s disease (14) and a higher likelihood of pathologic findings of Lewy bodies and fewer nigral neurons in postmortem brains (23, 24). In a case-control study, constipation as assessed by medical records review was associated with a higher risk of developing Parkinson’s disease in both men (121 cases) and women (75 cases) (15).
In our study, we do not have data for men beyond 6 years of follow-up, and in women, we found little evidence that the infrequent bowel movements predated Parkinson’s disease risk for more than 6 years. This showed some discrepancy with the prospective Honolulu study and the later case-control report. The former has ∼15 years of follow-up, and infrequent bowel movement was associated with higher risk of Parkinson’s disease in both strata when stratified by the median years of follow-up (12 years), although the numbers in each stratum were small (14). The case-control study primarily had male cases (15). It is therefore not unreasonable to hypothesize that constipation is more predictive in men and may predate Parkinson’s disease clinical onset over a longer period than in women. This would be supported by the fact that the reasons for constipation may be more complicated in women, for example, due to pelvic floor dysfunction and pregnancy (16). This would be also consistent with the fact that the prevalence of constipation is generally higher in women than in men, but their Parkinson’s disease incidence is lower. However, we cannot exclude the possibility that the observed gender difference in the current study could be due to the difference in age at the time bowel movement frequency was assessed in the 2 cohorts, although we did not find significant interaction between age and bowel movement in relation to Parkinson’s disease risk.
Further, the results that constipation was more predictive of Parkinson’s disease risk in the immediate than in the distant future also conform to the hypothesis that it is an early marker of Parkinson’s disease. Alternately, one may speculate that constipation also increases the risk of Parkinson’s disease as constipation results in a longer stay of the feces in the bowel and thus more absorption of neurotoxicants. However, this hypothesis does not reconcile well with our finding that the infrequent bowel movement predicts increased Parkinson’s disease risk within a few years. Another potential interpretation is that individuals with constipation may visit physicians more frequently than the others and, consequently, they are more likely to be found to have Parkinson’s disease. In this case, the association between constipation and Parkinson’s disease risk would be expected to be stronger in women relative to men, as women are more likely to report having constipation (16).
Strengths of the current study include its prospective design, large sample sizes, inclusion of both men and women, and the assessment of temporal relation among women. A potential limitation is that questions on bowel movement frequency were asked only once, and bowel movement frequency may change over time. Because we did not have information on bowel movement frequency prior to the baseline, the length of infrequent bowel movement and Parkinson’s disease onset could be longer than the observed 6 years. Further, because of short follow-up in the HPFS, we are unable to examine in detail the temporal relation between bowel movement frequency and Parkinson’s disease in men. Another limitation is that we did not collect information on antidepressant use in the NHS until 1996. However, further adjustment for antidepressants in the HPFS did not materially change the results.
In conclusion, in this large prospective investigation, we found that infrequent bowel movements antedate the onset of cardinal motor symptoms of Parkinson’s disease. As information on bowel movement frequency and constipation is fairly easy to collect, it may be included in future nonmotor screening tools for early Parkinson’s disease identification.
Acknowledgments
Author affiliations: Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Xiang Gao, Alberto Ascherio); Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Honglei Chen); Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts (Michael A. Schwarzschild); and Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Alberto Ascherio).
The study was supported by National Institutes of Health grants R01 NS048517, R01 NS061858, and K24NS060991 and, in part, by grant Z01-ES-101986 from the intramural research program of the National Institute of Environmental Health Sciences.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- HPFS
Health Professionals Follow-up Study
- NHS
Nurses’ Health Study
References
- 1.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792(7):730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Chen H, Schwarzschild MA, et al. Erectile function and risk of Parkinson’s disease. Am J Epidemiol. 2007;166(12):1446–1450. doi: 10.1093/aje/kwm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63(2):167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Zhang SM, Hernán MA, et al. Weight loss in Parkinson’s disease. Ann Neurol. 2003;53(5):676–679. doi: 10.1002/ana.10577. [DOI] [PubMed] [Google Scholar]
- 6.Jost WH. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol Sci. 289(1-2):69–73. doi: 10.1016/j.jns.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, de Vos RA, Bohl J, et al. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Phillips RJ, Walter GC, Wilder SL, et al. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153(3):733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(2):79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Del Tredici K. Invited article: nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70(20):1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 13.Bloch A, Probst A, Bissig H, et al. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32(3):284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 14.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 15.Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73(21):1752–1758. doi: 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrea GL, Miaskowski C, Stotts NA, et al. A review of the literature on gender and age differences in the prevalence and characteristics of constipation in North America. J Pain Symptom Manage. 2009;37(4):737–745. doi: 10.1016/j.jpainsymman.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 18.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 19.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86(5):1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Chen H, Schwarzschild MA, et al. Perceived imbalance and risk of Parkinson’s disease. Mov Disord. 2008;23(4):613–616. doi: 10.1002/mds.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord. 2007;22(11):1581–1586. doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 24.Petrovitch H, Abbott RD, Ross GW, et al. Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov Disord. 2009;24(3):371–376. doi: 10.1002/mds.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]