Abstract
The authors conducted a meta-analysis of the association between smoking before a first pregnancy, when undifferentiated breast tissue may be vulnerable to tobacco carcinogens, and the risk of breast cancer. A search of the published literature through August 2010 identified 23 papers reporting on associations between smoking before a first pregnancy and breast cancer. Odds ratios or hazard ratios and 95% confidence intervals, adjusted for known or suspected breast cancer risk factors, were abstracted from each study. Data were pooled using both fixed- and random-effects models. The fixed-effect summary risk ratio for breast cancer among the women who smoked before their first pregnancy versus women who had never smoked was 1.10 (95% confidence interval: 1.07, 1.14); the random-effects estimate was similar. The separate fixed-effect risk ratios for smoking only before the first pregnancy (5 studies) or only after the first pregnancy (16 studies) were both 1.07, providing no evidence that breast tissue is more susceptible to malignant transformation from smoking before the first pregnancy. While these small summary risk ratios may represent causal effects, residual confounding could readily produce estimates of this size in the absence of any causal effect.
Keywords: breast neoplasms, pregnancy, smoking
Although many investigators report no association between smoking and breast cancer, it is possible that smoking during susceptible periods may increase risk (1). According to studies on breast development and cancer susceptibility, the relatively undifferentiated breast epithelial cells present before a first pregnancy may be particularly vulnerable to the carcinogenic effects of cigarette smoke (2, 3). Animal models have shown that cancer initiation can occur when chemical carcinogens come into contact with undifferentiated, highly proliferating mammary epithelium and is less likely after a full-term pregnancy, during which the mammary gland undergoes differentiation (2). In humans, the mammary gland is composed of developing lobules at menarche, and a first pregnancy and lactation trigger breast growth and differentiation.
We conducted a meta-analysis to examine the association between smoking before a first pregnancy and the risk of breast cancer. A previous meta-analysis by Lawlor et al. (4) used data from 11 papers; we used 23 studies and somewhat different selection criteria. We estimated the summary risk ratios for breast cancer among women who smoked before their first pregnancies, regardless of whether or not they continued to smoke after the pregnancy, compared with those who had never smoked. We also calculated separate summary risk ratio estimates for breast cancer among women who had smoked only before their first pregnancies and those who had smoked only after their first pregnancies to determine whether there were differences for these exposure periods. If cancer initiation by tobacco smoke were more likely in undifferentiated mammary gland epithelium, then the risk ratio for women who smoked only before their first pregnancy should be larger than the risk ratio for women who smoked only after, compared with otherwise similar women who had never smoked.
MATERIALS AND METHODS
Relevant studies were identified by querying MEDLINE (US National Library of Medicine) for articles published from 1949 through August 2010. To be included in the meta-analysis, a study had to 1) present data on incident cases of clinically diagnosed breast cancer and 2) examine the association between active smoking before a woman's first pregnancy and breast cancer. An advanced search was conducted in PubMed using the following Medical Subject Headings: (“smoking” [major:noexplode] or “tobacco smoke pollution” [major:noexplode]) and (“breast neoplasms/etiology” [major:noexplode] or “breast neoplasms/epidemiology” [major:noexplode] or “breast neoplasms/genetics” [major:noexplode]) and Journal Article [Publication Type], not males not animals. Of the 148 manuscripts identified, 41 were excluded and 107 were examined for analyses of smoking before pregnancy and breast cancer (Figure 1).
Figure 1.
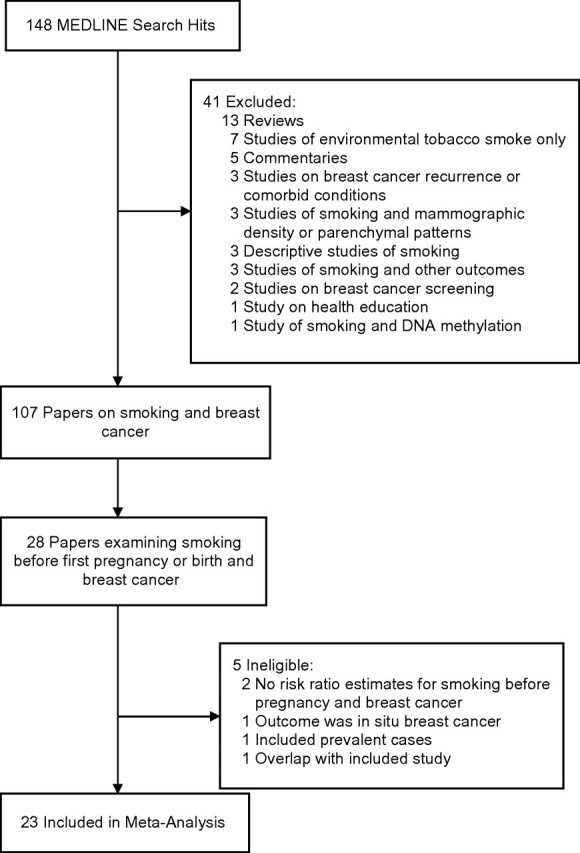
Search strategy and study selection process used in a meta-analysis of the association between smoking before the first pregnancy and risk of breast cancer, 1988–2009.
Of 28 papers that examined smoking before the first pregnancy, 5 were deemed ineligible. Two examined smoking before pregnancy and breast cancer but did not report estimated associations from those analyses (5, 6). One studied in situ breast cancer only, excluding cases with invasive cancer (7). One study included prevalent cases (8) and was excluded because estimates might have been biased if some eligible case subjects did not survive to take part in the study or if smoking is related to survival time (9). Finally, we excluded a case-control study (10) nested within the Nurses’ Health Study because the same subjects were also part of an included cohort study by Egan et al. (11). In addition, our eligibility criteria differed from those of the previous meta-analysis (4) as follows: We used 1 summary estimate from Band et al. (12) in our calculation of pooled estimates, whereas Lawlor et al. (4) used 2, and we did not include 2 studies of smoking during the first pregnancy that used linked birth and cancer registry data (13, 14) because nonsmokers in those studies included both never smokers and smokers who quit before the first pregnancy.
The 23 studies included in our meta-analysis were published between 1988 and 2009 and included 15 case-control studies (12, 15–28) and 8 cohort studies (4, 11, 29–34). Adjusted odds ratios or hazard ratios and 95% confidence intervals for the association between smoking before a first pregnancy and breast cancer were abstracted from each paper. Some investigators reported estimates stratified by years of smoking or pack-years of smoking before the first pregnancy (11, 15, 25, 26, 28–30, 33, 34), menopausal status (12, 27), N-acetyltransferase 2 acetylation genotype (20), or race/ethnicity (27). Others separated smokers into categories according to whether they smoked only before their first pregnancy or both before and after their first pregnancy (17–19, 21). When necessary, we created within-study estimates for the association between ever smoking before the first pregnancy and breast cancer risk by using inverse-variance weighting of the stratum-specific adjusted estimates reported in each study. When available, we also abstracted association estimates for women who had smoked only before their first pregnancy (11, 17–19, 21) and those who had smoked only after their first pregnancy (11, 12, 16–19, 21, 23–25, 27, 28, 30–32), and we used the same methods to calculate within-study estimates for stratified results.
We summarized risk ratios across the studies using inverse-variance weighting to calculate fixed-effect summary estimates. Random-effects estimates were also calculated using the method of DerSimonian and Laird (35). We used Cochran's Q statistic (36) to test the null hypothesis that risk ratios were homogeneous across studies (37). We set a conservative cutoff (10%) for significance because the Q statistic is poor at detecting heterogeneity under certain conditions, such as when the number of studies is small (38). We also calculated I2, a quantitative estimate of the percentage of total variation between studies that is due to heterogeneity rather than chance (39, 40). Funnel plots (41) and Begg's tests (42) were used to assess potential publication bias. Analyses were conducted using Stata software (43, 44).
RESULTS
Of the 23 studies used for this review, 13 were conducted in the United States (11, 17, 19–22, 25–27, 29, 30, 32, 34), 3 in Canada (12, 28, 33), 2 in Sweden and Norway combined (15, 31), and 1 each in Switzerland (16), Germany (18), the United Kingdom (4), Poland (23), and Sweden (24) (Table 1). Nearly all of the case-control studies identified breast cancer patients through population-based registries, and controls were selected using population registries (15, 16, 18, 23, 24, 28), random digit dialing (17, 19, 21), voter's lists (12), lists of licensed drivers (20, 26, 27), Health Care Financing Administration records of Medicare beneficiaries (17, 19–22, 26, 27), mortality registers (17, 19), and a neighborhood walk algorithm (25). The cohort studies drew subjects from population-based samples (4, 31, 32), women undergoing mammographic screening (33), and occupational groups, including nurses (11, 29), teachers (30), and radiologic technologists (34). Breast cancer cases were identified in the cohorts using self-reports validated by medical records (11, 29, 34), cancer registries (4, 30–33), and mortality databases (4, 33, 34). In all studies, information on smoking and possible confounders was collected using self-administered questionnaires (4, 11, 12, 24, 28–34) or interviews (15–23, 25–27). In most of the studies, investigators used breast cancer patients and controls who were alive at the time of the study; however, in 2 studies (17, 19), they also collected information on deceased subjects by interviewing their next of kin. Each study examined numerous covariates, including alcohol use, pregnancy history, and age at first pregnancy. For 15 studies, the published article specified that nulliparous women were excluded from analyses of smoking before pregnancy (11, 12, 15, 18, 19, 21–23, 25, 26, 28, 30–33).
Table 1.
Characteristics of Studies Included in a Meta-Analysis of the Association Between Smoking Before the First Pregnancy and Risk of Breast Cancer, by Study Design and Publication Year, 1988–2009
| First Author, Year (Reference No.) | Year(s) of Data Collection | Location of Study | Source of Information on Smoking | Source of Information on Breast Cancer | Age of Subjects, years | Potential Confounders Examined in the Analyses | Comments |
| Case-control studies | |||||||
| Adami, 1988 (15) | 1984–1985 | Sweden and Norway | In-person interview | Cancer registry | <45 | Age at menarche, age at first full-term pregnancy, parity, menopausal status, OC use, alcohol consumption, education, history of surgery for BBD, and family history of breast cancer | Cases and controls were matched on date of birth and country of residency |
| Morabia, 1996 (16) | 1992–1993 | Geneva, Switzerland | In-person interview | Cancer registry | <75 | Age, age at menarche, age at first livebirth, OC use, alcohol consumption, BMIa, education, and family history of breast cancer | Referent group was never-active, never-passive smokers |
| Lash, 1999 (17) | 1983–1986 | Massachusetts, United States | Telephone (86%) and in-person interviews | Cancer registry | All | Age, age at first birth, parity, menopausal status, HRT, BMI, history of BBD, history of breast cancer other than the index diagnosis, history of radiation therapy, and family history of breast cancer | Referent group was never-active, never-passive smokers |
| Band, 2002 (12) | 1988–1989 | British Columbia, Canada | Mailed questionnaire | Cancer registry | <75 | Age at menarche, no. of pregnancies, no. of livebirths, age at first pregnancy, age at first full-term pregnancy, breastfeeding, age at menopause, OC use, estrogen replacement therapy, cumulative alcohol score, weight and BMI at age 18 years and currently, change in BMI from age 18 years to the present, education, marital status, history of biopsy for BBD, ethnic origin, and family history of breast cancer | Cases and controls were matched on age |
| Kropp, 2002 (18) | 1992–1995 | Rhein-Neckar-Odenwald and Freiburg, Germany | Computer-assisted telephone interview | Hospital surveillance | ≤50 | Age, months of breastfeeding, menopausal status, average daily alcohol intake, BMI, education, and family history of breast cancer | Cases and controls were matched on age and study region |
| Referent group was never-active, never-passive smokers | |||||||
| Lash, 2002 (19) | 1987–1993 | Massachusetts, United States | Interviews | Cancer registry | All | Age at first birth, parity, alcohol consumption, BMI, history of BBD, history of radiation therapy, and family history of breast cancer | Cases and controls were matched on age and vital status |
| Referent group was never-active, never-passive smokers | |||||||
| Egan, 2003 (20) | 1997–1998 | Massachusetts and Wisconsin, United States | Telephone interviews | State tumor registries | 20–69 | Age, age at menarche, parity, age at first birth, menopausal status, age at menopause, alcohol consumption, BMI, education, state, history of BBD, and family history of breast cancer | |
| Gammon, 2004 (21) | 1996–1997 | Long Island Breast Cancer Study Project, New York, United States | In-person interview | Pathology reports confirmed by a physician | 24–98 | Age, age at menarche, age at first birth, parity, no. of livebirths, months of lactation, no. of miscarriages, history of fertility problems, OC use, HRT, alcohol intake, physical activity, fruit and vegetable intake, BMI at reference date and at age 20 years, education, race, ethnicity, religion, marital status, screening history, history of BBD, and family history of breast cancer | Referent group was never-active, never-passive smokers |
| Li, 2005 (22) | 1997–1999 | Washington, United States | In-person interview | Cancer registry | 65–79 | Age at first full-term pregnancy, parity, age at menopause, type of menopause, HRT (unopposed and combined estrogen/progestin), alcohol consumption, BMI, education, and family history of breast cancer | Cases and controls were matched on age |
| Lissowska, 2006 (23) | 2000–2003 | Warsaw and Lodz, Poland | Personal interview | Hospitals and cancer registry | 20–74 | Age at menarche, age at first full-term birth, no. of full-term births, age at menopause, OC use, HRT, alcohol consumption, BMI, education, prior benign breast biopsy, and family history of breast cancer | Cases and controls were matched on age and study site |
| Magnusson, 2007 (24) | 1993–1995 | Sweden | Mailed questionnaire | Cancer registry | 50–74 | Age at menarche, age at first birth, parity, age at menopause, HRT, alcohol consumption, BMI, socioeconomic position, history of BBD, and family history of breast cancer | Cases and controls were matched on age |
| Prescott, 2007 (25) | 1998–2003 | Los Angeles, California, United States | In-person interviews | Cancer registry | 20–49 | Age, age at menarche, no. of full-term pregnancies, alcohol consumption, education, race, and family history of breast or ovarian cancer | Cases and controls were matched on age and race |
| Rollison, 2008 (26) | 2000–2002 | Delaware, United States | Telephone interviews | Cancer registry | 40–79 | Age, age at menarche, age at first livebirth, menopausal status, OC use, other hormone use, alcohol consumption, BMI, education, and family history of breast cancer | Cases and controls were frequency-matched on age |
| Slattery, 2008 (27) | 1999–2004 | Arizona (7 counties), Colorado, New Mexico, and Utah, United States | Interviewer-administered computer questionnaire | Cancer registry | 25–79 | Age, center, age at menarche, age at first birth, parity, age at menopause, OC use, alcohol consumption, long-term physical activity, use of aspirin/nonsteroidal antiinflammatory drugs, and BMI | Cases and controls were matched on age |
| Young, 2009 (28) | 1996–1998 | Ontario Women's Health Study, Ontario, Canada | Mailed questionnaire | Cancer registry | 25–75 | Age at menarche, age at first livebirth, parity, menopausal status, OC use, HRT, alcohol consumption, BMI, household income, history of BBD, and family history of breast cancer | Cases and controls were matched on age |
| Referent group was never-active, never-passive smokers | |||||||
| Cohort studies | |||||||
| Egan, 2002 (11) | 1982–1996 | Nurses’ Health Study, United States | Mailed questionnaire | Self-report confirmed by medical record review | 36–61 | Age, age at menarche, age at first birth, parity, menopausal status, age at menopause, HRT, alcohol consumption, weight at age 18 years and adult weight change, adult height, total carotenoid intake, duration of postpartum smoking, history of BBD, and family history of breast cancer | |
| Al-Delaimy, 2004 (29) | 1989–1999 | Nurses’ Health Study II, United States | Mailed questionnaire | Self-report confirmed by hospital record and pathology reports | 25–42 | Age, age at menarche, age at first birth, parity, menopausal status, OC use, recent alcohol consumption, BMI, height, history of BBD, and family history of breast cancer | |
| Lawlor, 2004 (4) | 1999–2001 | British Women's Heart and Health Cohort Study, United Kingdom | Self-administered questionnaire | Cancer and mortality registries | 60–79 | Age, age at menarche, age at first birth, parity, age at menopause, hysterectomy and/or oophorectomy, OC use, HRT, alcohol consumption, BMI, and childhood and adult social class | Results for incident cases were used |
| Reynolds, 2004 (30) | 1995–2000 | California Teachers Study, California, United States | Mailed questionnaire | Cancer registry | All | Age, age at menarche, age at first full-term pregnancy, parity, menopausal status, HRT, alcohol consumption, physical activity, BMI, race, and family history of breast cancer | |
| Gram, 2005 (31) | 1991–1992 | Norwegian-Swedish Women's Lifestyle and Health Cohort Study, Sweden and Norway | Mailed questionnaire | Cancer registry | 30–50 | Age, age at menarche, age at first birth, parity, menopausal status, hormonal contraceptive use, alcohol consumption, BMI, and family history of breast cancer | Results for long-term smokers (≥20 years) were used |
| Olson, 2005 (32) | 1986 | Iowa Women's Health Study, Iowa, United States | Mailed questionnaire | Cancer registry | 55–69 | Age at menarche, age at menopause, OC use, HRT, alcohol consumption, physical activity, waist-to-hip ratio, height, BMI at age 18 years and currently, education, and family history of breast cancer | |
| Cui, 2006 (33) | 1980–1985 | Canadian National Breast Cancer Screening Study, Canada | Self-administered questionnaire | Cancer registry and mortality database | 40–59 | Age, randomization group, study center, age at menarche, parity, menopausal status, OC use, HRT, alcohol consumption, physical activity, BMI, education, breast self-examination, history of BBD, and family history of breast cancer | Cohort was created from a randomized controlled trial |
| Ha, 2007 (34) | 1983–1989 | US Radiologic Technologists Study, United States | Mailed questionnaire | Self-report (most validated) and National Death Index Plus | 22–92 | Age, birth cohort, year first worked as a radiologic technologist, age at menarche, age at first birth, parity, menopausal status, HRT, alcohol consumption, BMI, and family history of breast cancer |
Abbreviations: BBD, benign breast disease; BMI, body mass index; HRT, hormone replacement therapy; OC, oral contraceptive.
Weight (kg)/height (m)2.
Smoking before the first pregnancy
The 23 adjusted within-study odds ratios or hazard ratios ranged from 0.70 to 3.0 (Table 2, Figure 2). The fixed-effect summary risk ratio for breast cancer among the women who had smoked before their first pregnancy as compared with women who had never smoked was 1.10 (95% confidence interval (CI): 1.07, 1.14) across the 23 studies; the random-effects summary risk ratio was 1.11 (95% CI: 1.06, 1.16) (Table 3). Stratifying by study type, the 15 case-control studies had a fixed-effect summary risk ratio (risk ratio (RR) = 1.08, 95% CI: 1.03, 1.13) similar to that of the 8 cohort studies (RR = 1.12, 95% CI: 1.07, 1.17) (test of heterogeneity: P = 0.21). There was evidence of heterogeneity across the 23 studies (Cochran's Q test P = 0.06; I2 = 34.0%) but no evidence of publication bias based on funnel plots or Begg's test (P = 0.48). Removing the results of 2 studies that included deceased subjects (17, 19) reduced the heterogeneity (Cochran's Q test P = 0.26; I2 = 15%), and the summary risk ratio estimate for the remaining 21 studies was 1.11 (95% CI: 1.07, 1.14) using the fixed-effect method and 1.11 (95% CI: 1.07, 1.15) using the random-effects method. Fourteen studies (4, 11, 15, 20–26, 28, 30, 31, 34) used the outcome of first birth, first livebirth, or first full-term pregnancy (as opposed to a first pregnancy, which could end in abortion). The pooled fixed-effect risk ratio from these 14 studies was 1.10 (95% CI: 1.05, 1.14). For the other 9 studies that used first pregnancy as an outcome (12, 16–19, 27, 29, 32, 33), the fixed-effect risk ratio was 1.11 (95% CI: 1.06, 1.16).
Table 2.
Within-Study Subgroup and Summary Estimates for Risk of Breast Cancer Among Women Who Smoked Before Their First Pregnancy, Women Who Smoked Only Before Their First Pregnancy, and Women Who Smoked Only After Their First Pregnancy as Compared With Women Who Had Never Smoked, 1988–2009
| Smoking Exposure Variable and Study | Within-Study Subgroup Estimate | Within-Study Summary Estimate | ||
| OR or HR | 95% CI | RR | 95% CI | |
| Smoked before first pregnancy | ||||
| Adami, 1988 (15) | 0.8 | 0.6, 1.1 | ||
| <5 yearsa | 1.0 | 0.6, 1.6 | ||
| 5–9 years | 0.7 | 0.4, 1.1 | ||
| ≥10 years | 0.7 | 0.3, 1.4 | ||
| Morabia, 1996 (16) | 3.0 | 1.7, 7.0 | ||
| Lash, 1999b (17) | 1.5 | 0.8, 2.5 | ||
| Smoked only before first pregnancy | 5.6 | 1.5, 21 | ||
| Smoked before and after first pregnancy | 1.1 | 0.6, 2.0 | ||
| Band, 2002 (12) | 1.11 | 0.90, 1.35 | ||
| Premenopausal women | 1.47 | 1.02, 2.10 | ||
| Postmenopausal women | 0.97 | 0.76, 1.24 | ||
| Egan, 2002 (11) | 1.12 | 1.01, 1.23 | ||
| <5 yearsa | 1.10 | 0.96, 1.26 | ||
| ≥5 years | 1.13 | 0.99, 1.30 | ||
| Kropp, 2002 (18) | 1.16 | 0.82, 1.64 | ||
| Smoked only before first pregnancy | 0.92 | 0.52, 1.65 | ||
| Smoked before and after first pregnancy | 1.32 | 0.86, 2.03 | ||
| Lash, 2002b (19) | 0.70 | 0.52, 0.95 | ||
| Smoked only before first pregnancy | 0.73 | 0.42, 1.30 | ||
| Smoked before and after first pregnancy | 0.69 | 0.49, 0.96 | ||
| Egan, 2003 (20) | 1.22 | 0.87, 1.70 | ||
| Slow NAT2 acetylation genotype (>5 yearsa) | 1.38 | 0.87, 2.19 | ||
| Fast NAT2 acetylation genotype (>5 years) | 1.05 | 0.64, 1.72 | ||
| Al-Delaimy, 2004 (29) | 1.18 | 1.05, 1.32 | ||
| 1–4 yearsa | 1.02 | 0.72, 1.44 | ||
| 5–9 years | 1.12 | 0.91, 1.39 | ||
| 10–14 years | 1.19 | 0.97, 1.47 | ||
| 15–19 years | 1.42 | 1.10, 1.83 | ||
| ≥20 years | 1.10 | 0.80, 1.52 | ||
| Gammon, 2004 (21) | 0.94 | 0.75, 1.17 | ||
| Smoked only before first pregnancy | 0.72 | 0.49, 1.05 | ||
| Smoked before and after first pregnancy | 1.08 | 0.82, 1.43 | ||
| Lawlor, 2004 (4) | 1.08 | 0.39, 2.55 | ||
| Reynolds, 2004 (30) | 1.09 | 0.98, 1.21 | ||
| <5 yearsa | 0.99 | 0.80, 1.21 | ||
| ≥5 years | 1.13 | 1.00, 1.28 | ||
| Gram, 2005 (31) | 1.27 | 1.00, 1.62 | ||
| Li, 2005 (22) | 1.3 | 1.0, 1.6 | ||
| Olson, 2005 (32) | 1.21 | 1.07, 1.37 | ||
| Cui, 2006 (33) | 1.07 | 0.99, 1.15 | ||
| ≤5 yearsa | 1.01 | 0.91, 1.13 | ||
| >5 years | 1.13 | 1.01, 1.25 | ||
| Lissowska, 2006 (23) | 1.14 | 0.98, 1.32 | ||
| Ha, 2007 (34) | 1.14 | 0.80, 1.61 | ||
| <10 pack-years | 0.97 | 0.61, 1.54 | ||
| ≥10 pack-years | 1.39 | 0.82, 2.35 | ||
| Magnusson, 2007 (24) | 1.09 | 0.94, 1.26 | ||
| Current smokers | 1.2 | 0.9, 1.4 | ||
| Past smokers | 1.0 | 0.8, 1.2 | ||
| Prescott, 2007 (25) | 0.99 | 0.79, 1.22 | ||
| ≤10 yearsa | 0.95 | 0.48, 1.95 | ||
| >10 years | 1.03 | 0.75, 1.43 | ||
| Rollison, 2008 (26) | 1.24 | 0.92, 1.66 | ||
| <5 yearsa | 1.25 | 0.73, 2.13 | ||
| 5–9 years | 1.37 | 0.87, 2.16 | ||
| 10–14 years | 0.69 | 0.33, 1.45 | ||
| 15–39 years | 1.99 | 0.76, 5.18 | ||
| Slattery, 2008 (27) | 1.05 | 0.91, 1.22 | ||
| Premenopausal women | ||||
| Non-Hispanic white | 1.4 | 1.0, 1.9 | ||
| Hispanic/American Indian | 1.1 | 0.8, 1.7 | ||
| Postmenopausal women | ||||
| Non-Hispanic white | 1.0 | 0.8, 1.3 | ||
| Hispanic/American Indian | 0.9 | 0.7, 1.2 | ||
| Young, 2009 (28) | 1.07 | 0.98, 1.16 | ||
| ≤5 yearsa | 0.96 | 0.84, 1.09 | ||
| >5 years | 1.16 | 1.04, 1.31 | ||
| Smoked only before the first pregnancy | ||||
| Lash, 1999b (17) | 5.6 | 1.5, 21.0 | ||
| Egan, 2002 (11) | 1.15 | 0.99, 1.34 | ||
| <5 yearsa | 1.18 | 0.95, 1.46 | ||
| ≥5 years | 1.12 | 0.90, 1.40 | ||
| Kropp, 2002 (18) | 0.92 | 0.52, 1.65 | ||
| Lash, 2002b (19) | 0.73 | 0.42, 1.30 | ||
| Gammon, 2004 (21) | 0.72 | 0.49, 1.05 | ||
| Smoked only after the first pregnancy | ||||
| Morabia, 1996 (16) | 3.5 | 1.7, 7.0 | ||
| Lash, 1999b (17) | 2.1 | 1.1, 4.0 | ||
| Band, 2002 (12) | 0.68 | 0.47, 0.99 | ||
| Premenopausal women | 0.83 | 0.37, 1.85 | ||
| Postmenopausal women | 0.64 | 0.42, 0.98 | ||
| Egan, 2002 (11) | 1.01 | 0.78, 1.32 | ||
| Kropp, 2002 (18) | 1.64 | 0.90, 2.97 | ||
| Lash, 2002b (19) | 0.66 | 0.42, 1.00 | ||
| Gammon, 2004 (21) | 1.20 | 0.76, 1.89 | ||
| Reynolds, 2004 (30) | 0.89 | 0.65, 1.21 | ||
| Gram, 2005 (31) | 0.98 | 0.70, 1.62 | ||
| Li, 2005 (22) | 1.1 | 0.8, 1.5 | ||
| Olson, 2005 (32) | 1.03 | 0.88, 1.21 | ||
| Lissowska, 2006 (23) | 1.06 | 0.87, 1.29 | ||
| Magnusson, 2007 (24) | 1.13 | 0.88, 1.46 | ||
| Current smokers | 1.0 | 0.7, 1.4 | ||
| Past smokers | 1.3 | 0.9, 1.9 | ||
| Prescott, 2007 (25) | 0.97 | 0.48, 1.95 | ||
| Slattery, 2008 (27) | 1.08 | 0.86, 1.37 | ||
| Premenopausal women | ||||
| Non-Hispanic white | 1.2 | 0.6, 2.4 | ||
| Hispanic/American Indian | 1.0 | 0.5, 2.1 | ||
| Postmenopausal women | ||||
| Non-Hispanic white | 1.0 | 0.7, 1.4 | ||
| Hispanic/American Indian | 1.2 | 0.8, 1.8 | ||
| Young, 2009 (28) | 1.24 | 1.02, 1.52 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; NAT2, N-acetyltransferase 2; OR, odds ratio; RR, risk ratio.
Categories refer to the number of years of smoking before the first pregnancy.
Study included deceased subjects.
Figure 2.
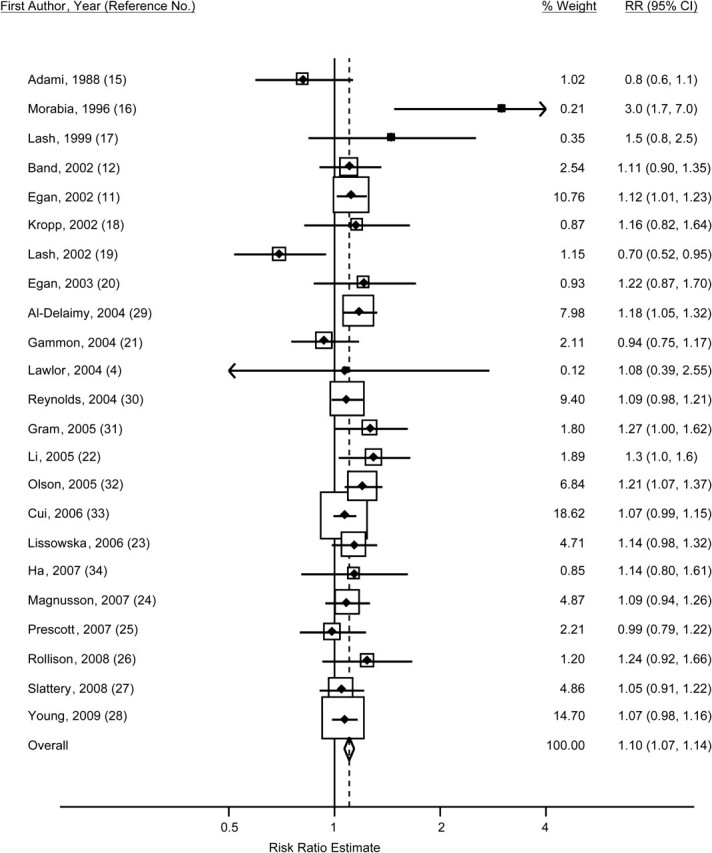
Study-specific odds and hazard ratios and fixed-effect summary risk ratio (RR) (diamond) for breast cancer among women who smoked before their first pregnancy as compared with women who had never smoked, 1988–2009. The size of each box indicates the relative weight of each study in the meta-analysis; the horizontal bars show the 95% confidence intervals (CIs).
Table 3.
Summary Risk Ratios for Breast Cancer Among Women Who Smoked Before Their First Pregnancy, Women Who Smoked Only Before Their First Pregnancy, and Women Who Smoked Only After Their First Pregnancy as Compared With Women Who Had Never Smoked, 1988–2009
| Smoking Exposure Variable and No. of Studies (Reference Nos.) | Fixed Effects |
Random Effects |
Cochran's QaP Value | I2b | Begg's TestcP Value | |||
| RR | 95% CI | RR | 95% CI | % | 95% CI | |||
| Smoked before the first pregnancy (4, 11, 12, 15–34) | ||||||||
| 23 | 1.10 | 1.07, 1.14 | 1.11 | 1.06, 1.16 | 0.06 | 34 | 0, 60 | 0.48 |
| 21d | 1.11 | 1.07, 1.14 | 1.11 | 1.07, 1.15 | 0.26 | 15 | 0, 45 | 0.43 |
| Smoked only before the first pregnancy (11, 17–19, 21) | ||||||||
| 5 | 1.07 | 0.93, 1.22 | 1.00 | 0.69, 1.44 | 0.01 | 69 | 22, 88 | 0.33 |
| 3d | 1.07 | 0.93, 1.23 | 0.95 | 0.69, 1.32 | 0.07 | 62 | 0, 89 | 0.60 |
| Smoked only after the first pregnancy (11, 12, 16–19, 21–25, 27, 28, 30–32) | ||||||||
| 16 | 1.07 | 0.99, 1.15 | 1.08 | 0.96, 1.21 | 0.007 | 53 | 17, 73 | 0.21 |
| 14d | 1.07 | 1.00, 1.16 | 1.08 | 0.97, 1.20 | 0.04 | 43 | 0, 70 | 0.30 |
Abbreviations: CI, confidence interval; RR, risk ratio.
Test of the null hypothesis that risk ratios were homogeneous across studies.
Estimate of the percentage of total variation between studies that was due to heterogeneity rather than chance.
Assessment of potential publication bias.
Two studies (17, 19) were eliminated to reduce heterogeneity.
Smoking only before the first pregnancy
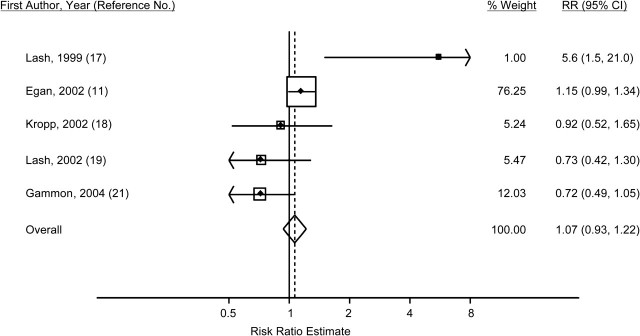
Five studies examined the risk ratio for breast cancer among women who had smoked only prior to their first pregnancy and not afterward, in comparison with women who had never smoked (11, 17–19, 21). The adjusted within-study odds ratios or hazard ratios ranged from 0.72 to 5.6 (Table 2, Figure 3). The summarized fixed-effect risk ratio across the 5 studies was 1.07 (95% CI: 0.93, 1.22); the random-effects estimate was 1.00 (95% CI: 0.69, 1.44) (Table 3). There was evidence of heterogeneity of results across the 5 studies (Cochran's Q test P = 0.01; I2 = 69%) but no evidence of publication bias (Begg's test P = 0.33). Removing the 2 studies that included deceased subjects (17, 19) did not eliminate the heterogeneity (Cochran's Q test P = 0.07; I2 = 62%). The fixed-effect summary risk ratio estimate for the remaining 3 studies was 1.07 (95% CI: 0.93, 1.23), and the random-effects estimate was 0.95 (95% CI: 0.69, 1.32).
Figure 3.
Study-specific odds and hazard ratios and fixed-effect summary risk ratio (RR) (diamond) for breast cancer among women who smoked only before their first pregnancy as compared with women who had never smoked, 1988–2009. The size of each box indicates the relative weight of each study in the meta-analysis; the horizontal bars show the 95% confidence intervals (CIs).
Smoking only after the first pregnancy
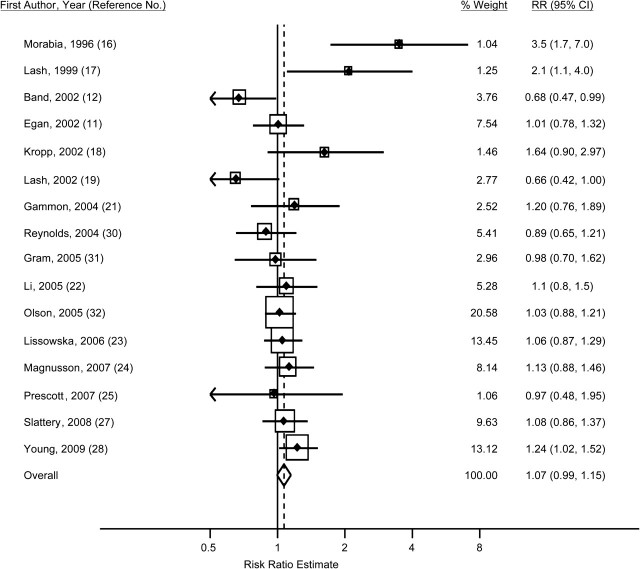
For 16 studies, authors reported the risk ratio for breast cancer among women who smoked only after their first pregnancy compared with women who had never smoked (11, 12, 16–19, 21–25, 27, 28, 30–32). The adjusted within-study odds ratio or hazard ratio ranged from 0.66 to 3.5 (Table 2, Figure 4). The fixed-effect summary risk ratio was 1.07 (95% CI: 0.99, 1.15), and the random-effects estimate was similar (RR = 1.08, 95% CI: 0.96, 1.21) (Table 3). There was evidence of heterogeneity across studies (Cochran's Q test P = 0.007; I2 = 53%) but little evidence of publication bias (Begg's test P = 0.21). Eliminating the 2 studies that included deceased subjects (17, 19) did not eliminate the heterogeneity (Cochran's Q test P = 0.04; I2 = 43%), and the summary risk ratio estimates for the remaining 14 studies were similar using the fixed-effect (RR = 1.07, 95% CI: 1.00, 1.16) and random-effects (RR = 1.08, 95% CI: 0.97, 1.20) methods.
Figure 4.
Study-specific odds and hazard ratios and fixed-effect summary risk ratio (RR) (diamond) for breast cancer among women who smoked only after their first pregnancy as compared with women who had never smoked, 1988–2009. The size of each box indicates the relative weight of each study in the meta-analysis; the horizontal bars show the 95% confidence intervals (CIs).
DISCUSSION
We found a weak association between smoking before a first pregnancy and breast cancer, with a 10% greater risk observed among women who smoked before their first pregnancy (regardless of whether or not they continued to smoke after the pregnancy) in comparison with women who had never smoked (summary RR = 1.10, 95% CI: 1.07, 1.14). Despite inclusion of 15 additional studies and exclusion of 3 studies, our results using 23 studies were consistent with a previous meta-analysis of 11 studies that calculated a pooled risk ratio estimate of 1.07 (95% CI: 0.94, 1.22) (4). The summary fixed-effect estimates for smoking only before or only after the first pregnancy were both 1.07, which does not support the idea that undeveloped mammary epithelium before a first pregnancy is more vulnerable to tobacco carcinogens than the more differentiated epithelium present after a first pregnancy.
A limitation of this meta-analysis was the small number of studies (n = 5) that specifically examined smoking only before the first pregnancy. Most of the analyses pertaining to smoking before a first pregnancy and breast cancer risk were not the main focus of the studies but instead were subgroup analyses reported in papers that focused primarily on current or lifetime smoking and breast cancer risk. Publication bias was a concern, because researchers may be more likely to report the results of subgroup analyses when they are statistically significant (45); however, we found little evidence of this.
Subjects who smoked before pregnancy may have also smoked at some time during the pregnancy, making it difficult to disentangle the possible effects of smoking prior to the first pregnancy and smoking during the first pregnancy. Three of the published studies used linked birth record and cancer registry data to specifically examine smoking during the first pregnancy and subsequent risk of breast cancer. One of these studies (13) found an increased risk ratio of 4.8 (95% CI: 1.6, 14.6) for breast cancer among women who smoked during the first pregnancy compared with those who did not. However, 2 larger studies using similar methods but with better control for age and other confounders (14, 46) did not find an increased risk ratio (RR = 0.9 (95% CI: 0.7, 1.3) and RR = 1.0 (95% CI: 0.8, 1.1), respectively), suggesting that smoking during the first pregnancy is not associated with an increased risk of breast cancer. If that is the case, then our inability to account for the fact that some women who smoked prior to their first pregnancy also smoked during their first pregnancy may not have been a source of bias for this meta-analysis. However, we acknowledge that for many of the studies whose data we summarized, we could not separate out any possible association between later breast cancer and the exposures of smoking prior to a first pregnancy and smoking during a first pregnancy.
Removing the results of 2 studies that collected information from the next of kin of deceased subjects (17, 19) reduced the heterogeneity between studies but did not substantially change the summary risk ratio estimates. In both of these studies, the percentage of information obtained from surrogate respondents was greater for controls (45%) than for cases (33%). The surrogate respondents’ recall of smoking habits before the subject's first pregnancy may have been less accurate than information collected from subjects themselves. This may have resulted in differential misclassification of exposure, which could have biased the study estimates (47).
Despite extensive study (48), there is little evidence that smoking increases the risk of breast cancer after taking confounders into account. An International Agency for Research on Cancer Working Group concluded that the cumulative evidence weighed against there being a causal association between smoking and breast cancer (49). The weak association (RR = 1.10) estimated in this meta-analysis does little to contradict that view.
Acknowledgments
Author affiliations: Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington (Lisa A. DeRoo, Beth A. Mueller); and Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Lisa A. DeRoo, Peter Cummings, Beth A. Mueller).
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- RR
risk ratio
References
- 1.Phillips DH, Garte S. Smoking and breast cancer: is there really a link? Cancer Epidemiol Biomarkers Prev. 2008;17(1):1–2. doi: 10.1158/1055-9965.EPI-07-2856. [DOI] [PubMed] [Google Scholar]
- 2.Russo J, Hu YF, Silva ID, et al. Cancer risk related to mammary gland structure and development. Microsc Res Tech. 2001;52(2):204–223. doi: 10.1002/1097-0029(20010115)52:2<204::AID-JEMT1006>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Russo J, Russo IH. Toward a physiological approach to breast cancer prevention. Cancer Epidemiol Biomarkers Prev. 1994;3(4):353–364. [PubMed] [Google Scholar]
- 4.Lawlor DA, Ebrahim S, Smith GD. Smoking before the birth of a first child is not associated with increased risk of breast cancer: findings from the British Women's Heart and Health Cohort Study and a meta-analysis. Br J Cancer. 2004;91(3):512–518. doi: 10.1038/sj.bjc.6601916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishibe N, Hankinson SE, Colditz GA, et al. Cigarette smoking, cytochrome P450 1A1 polymorphisms, and breast cancer risk in the Nurses’ Health Study. Cancer Res. 1998;58(4):667–671. [PubMed] [Google Scholar]
- 6.Breast Cancer Family Registry; Kathleen Cuningham Consortium for Research into Familial Breast Cancer (Australasia); Ontario Cancer Genetics Network (Canada) Smoking and risk of breast cancer in carriers of mutations in BRCA1 or BRCA2 aged less than 50 years. Breast Cancer Res Treat. 2008;109(1):67–75. doi: 10.1007/s10549-007-9621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trentham-Dietz A, Nichols HB, Egan KM, et al. Cigarette smoking and risk of breast carcinoma in situ. Epidemiology. 2007;18(5):629–638. doi: 10.1097/EDE.0b013e318127183a. [DOI] [PubMed] [Google Scholar]
- 8.Ghadirian P, Lubinski J, Lynch H, et al. Smoking and the risk of breast cancer among carriers of BRCA mutations. Int J Cancer. 2004;110(3):413–416. doi: 10.1002/ijc.20106. [DOI] [PubMed] [Google Scholar]
- 9.Rothman KJ, Greenland S, Lash TL. In: Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. Case-control studies; pp. 111–127. [Google Scholar]
- 10.Hunter DJ, Hankinson SE, Hough H, et al. A prospective study of NAT2 acetylation genotype, cigarette smoking, and risk of breast cancer. Carcinogenesis. 1997;18(11):2127–2132. doi: 10.1093/carcin/18.11.2127. [DOI] [PubMed] [Google Scholar]
- 11.Egan KM, Stampfer MJ, Hunter D, et al. Active and passive smoking in breast cancer: prospective results from the Nurses’ Health Study. Epidemiology. 2002;13(2):138–145. doi: 10.1097/00001648-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Band PR, Le ND, Fang R, et al. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360(9339):1044–1049. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- 13.Innes KE, Byers TE. Smoking during pregnancy and breast cancer risk in very young women (United States) Cancer Causes Control. 2001;12(2):179–185. doi: 10.1023/a:1008961512841. [DOI] [PubMed] [Google Scholar]
- 14.Fink AK, Lash TL. A null association between smoking during pregnancy and breast cancer using Massachusetts registry data (United States) Cancer Causes Control. 2003;14(5):497–503. doi: 10.1023/a:1024922824237. [DOI] [PubMed] [Google Scholar]
- 15.Adami HO, Lund E, Bergström R, et al. Cigarette smoking, alcohol consumption and risk of breast cancer in young women. Br J Cancer. 1988;58(6):832–837. doi: 10.1038/bjc.1988.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morabia A, Bernstein M, Héritier S, et al. Relation of breast cancer with passive and active exposure to tobacco smoke. Am J Epidemiol. 1996;143(9):918–928. doi: 10.1093/oxfordjournals.aje.a008835. [DOI] [PubMed] [Google Scholar]
- 17.Lash TL, Aschengrau A. Active and passive cigarette smoking and the occurrence of breast cancer. Am J Epidemiol. 1999;149(1):5–12. doi: 10.1093/oxfordjournals.aje.a009727. [DOI] [PubMed] [Google Scholar]
- 18.Kropp S, Chang-Claude J. Active and passive smoking and risk of breast cancer by age 50 years among German women. Am J Epidemiol. 2002;156(7):616–626. doi: 10.1093/aje/kwf093. [DOI] [PubMed] [Google Scholar]
- 19.Lash TL, Aschengrau A. A null association between active or passive cigarette smoking and breast cancer risk. Breast Cancer Res Treat. 2002;75(2):181–184. doi: 10.1023/a:1019625102365. [DOI] [PubMed] [Google Scholar]
- 20.Egan KM, Newcomb PA, Titus-Ernstoff L, et al. Association of NAT2 and smoking in relation to breast cancer incidence in a population-based case-control study (United States) Cancer Causes Control. 2003;14(1):43–51. doi: 10.1023/a:1022517506689. [DOI] [PubMed] [Google Scholar]
- 21.Gammon MD, Eng SM, Teitelbaum SL, et al. Environmental tobacco smoke and breast cancer incidence. Environ Res. 2004;96(2):176–185. doi: 10.1016/j.envres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Li CI, Malone KE, Daling JR. The relationship between various measures of cigarette smoking and risk of breast cancer among older women 65–79 years of age (United States) Cancer Causes Control. 2005;16(8):975–985. doi: 10.1007/s10552-005-2906-6. [DOI] [PubMed] [Google Scholar]
- 23.Lissowska J, Brinton LA, Zatonski W, et al. Tobacco smoking, NAT2 acetylation genotype and breast cancer risk. Int J Cancer. 2006;119(8):1961–1969. doi: 10.1002/ijc.22044. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson C, Wedren S, Rosenberg LU. Cigarette smoking and breast cancer risk: a population-based study in Sweden. Br J Cancer. 2007;97(9):1287–1290. doi: 10.1038/sj.bjc.6604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott J, Ma H, Bernstein L, et al. Cigarette smoking is not associated with breast cancer risk in young women. Cancer Epidemiol Biomarkers Prev. 2007;16(3):620–622. doi: 10.1158/1055-9965.EPI-06-0873. [DOI] [PubMed] [Google Scholar]
- 26.Rollison DE, Brownson RC, Hathcock HL, et al. Case-control study of tobacco smoke exposure and breast cancer risk in Delaware. BMC Cancer. 2008;8:157. doi: 10.1186/1471-2407-8-157. (doi: 10.1186/1471-2407-8-157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slattery ML, Curtin K, Giuliano AR, et al. Active and passive smoking, IL6, ESR1, and breast cancer risk. Breast Cancer Res Treat. 2008;109(1):101–111. doi: 10.1007/s10549-007-9629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young E, Leatherdale S, Sloan M, et al. Age of smoking initiation and risk of breast cancer in a sample of Ontario women. Tob Induc Dis. 2009;5(1):4. doi: 10.1186/1617-9625-5-4. (doi: 10.1186/1617-9625-5-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Delaimy WK, Cho E, Chen WY, et al. A prospective study of smoking and risk of breast cancer in young adult women. Cancer Epidemiol Biomarkers Prev. 2004;13(3):398–404. [PubMed] [Google Scholar]
- 30.Reynolds P, Hurley S, Goldberg DE, et al. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J Natl Cancer Inst. 2004;96(1):29–37. doi: 10.1093/jnci/djh002. [DOI] [PubMed] [Google Scholar]
- 31.Gram IT, Braaten T, Terry PD, et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev. 2005;14(1):61–66. [PubMed] [Google Scholar]
- 32.Olson JE, Vachon CM, Vierkant RA, et al. Prepregnancy exposure to cigarette smoking and subsequent risk of postmenopausal breast cancer. Mayo Clin Proc. 2005;80(11):1423–1428. doi: 10.4065/80.11.1423. [DOI] [PubMed] [Google Scholar]
- 33.Cui Y, Miller AB, Rohan TE. Cigarette smoking and breast cancer risk: update of a prospective cohort study. Breast Cancer Res Treat. 2006;100(3):293–299. doi: 10.1007/s10549-006-9255-3. [DOI] [PubMed] [Google Scholar]
- 34.Ha M, Mabuchi K, Sigurdson AJ, et al. Smoking cigarettes before first childbirth and risk of breast cancer. Am J Epidemiol. 2007;166(1):55–61. doi: 10.1093/aje/kwm045. [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 37.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. London, United Kingdom: BMJ Publishing Group; 2001. pp. 285–312. [Google Scholar]
- 38.Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;14:154–176. doi: 10.1093/oxfordjournals.epirev.a036084. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 43.StataCorp LP. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 44.Sterne JA, Bradburn MJ, Egger M. Meta-analysis in Stata. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. London, United Kingdom: BMJ Publishing Group; 2001. pp. 347–372. [Google Scholar]
- 45.Hahn S, Williamson PR, Hutton JL, et al. Assessing the potential for bias in meta-analysis due to selective reporting of subgroup analyses within studies. Stat Med. 2000;19(24):3325–3336. doi: 10.1002/1097-0258(20001230)19:24<3325::aid-sim827>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 46.DeRoo LA, Cummings P, Daling JR, et al. Smoking during first pregnancy and breast cancer: a case-control study using Washington State registry data. Ann Epidemiol. 2011;21(1):53–55. doi: 10.1016/j.annepidem.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothman KJ, Greenland S, Lash TL. In: Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. Validity in epidemiologic studies; pp. 128–147. [Google Scholar]
- 48.Hamajima N, Hirose K, Tajima K, et al. Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Collaborative Group on Hormonal Factors in Breast Cancer. Br J Cancer. 2002;87(11):1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 83) Lyon, France: International Agency for Research on Cancer; 2004. pp. 551–582. [PMC free article] [PubMed] [Google Scholar]