Abstract
Background: Type I and II endometrial cancer are biologically and clinically distinct, with type II cancers having a high frequency of p53 mutations and an association with chromosomal instability. This raises the hypothesis that one-carbon nutrients (folate, methionine, and the enzymic cofactors vitamins B2, B6, and B12), which mediate chromosomal stability and DNA methylation, may be protective for type II but not type I endometrial cancer.
Methods: Using a prospective cohort of 23 356 postmenopausal women followed 20 years, we estimated the relative risks (RRs) of type I (N = 471) and II (N = 71) endometrial cancers according to intake of one-carbon nutrients, adjusting for confounders.
Results: No associations were observed between dietary or supplemental intake of any one-carbon nutrient and risk of type I cancer. For type II cancer, positive associations were due to supplemental, rather than dietary, intake of these nutrients: supplemental folate (RR = 1.80 for >228.6 versus 0 μg/day; P trend = 0.027) and vitamins B2 (RR = 1.94 for >1.70 versus 0 mg/day; P trend = 0.011), B6 (RR = 2.08 for >2.00 versus 0 mg/day; P trend = 0.012), and B12 (RR = 2.10 for >3.43 versus 0 μg/day; P trend = 0.0060).
Conclusion: Contrary to our hypothesis, use of supplements containing folate and vitamins B2, B6, and B12 was associated with an increased risk of type II endometrial cancer.
Keywords: one-carbon nutrients, diet, endometrial cancer, prospective study
introduction
In the United States, endometrial cancer is the most common malignancy of the female reproductive tract [1]. Epidemiologic research has identified several risk factors for the development of this malignancy, such as obesity, unopposed estrogen exposure, and diabetes [2–5]. However, currently known risk factors do not completely explain endometrial cancer risk. Recently, attention has focused on dietary intake in order to identify food and nutrients that might be associated with an increased or decreased risk of developing endometrial cancer [6–11].
Alterations in one-carbon metabolism have been hypothesized to be carcinogenic, in part mediated by chromosomal instability and altered DNA methylation. Negri et al. [12] suggested an inverse association between folate intake and the risk of this malignancy, which was replicated in a case–control study by Xu et al. [13]. Conversely, others have reported no association between folate intake and endometrial cancer risk [14, 15].
One possible reason for the discrepancies observed in the literature is the failure to stratify endometrial cancer on the basis of the two main histological categories: type I endometrioid and type II non-endometrioid [16]. This pathological classification reflects important differences in terms of clinical characteristics, molecular abnormalities and prognosis. From a molecular point of view, type II endometrial cancer is often associated with p53 mutations, which commonly lead to DNA derangement, chromosomal instability and a more aggressive clinical behavior [17]. Women affected by type II cancers have a higher incidence of advanced-stage disease at diagnosis, a marked tendency to recurrence, and consequently a worse prognosis [18]. Further, uterine carcinosarcoma and type II endometrial cancers share similar molecular characteristics [19] and have similar clinical outcomes [20]. Conversely, PTEN (phosphatase and tensin homolog) mutations are an early event in the vast majority of type I cancers, along with other commonly mutated genes in type I including Bcl-2, KRAS, and β-catenin. Alterations of p53 have been reported in a small proportion of type I tumors and, when they occur, they are usually a late event [17].
Based on this literature, we hypothesized that low intake of nutrients in the one-carbon metabolism pathway, such as folate and methionine, as well as the related enzymic cofactors (vitamins B2, B6, and B12), might be specifically associated with an increased risk of type II endometrial cancer, since this subtype is most strongly associated with altered DNA methylation and possible chromosomal instability.
methods
study population and dietary assessment
This study was reviewed and approved by the human subjects review board at the University of Minnesota. A detailed description of the Iowa Women's Health Study (IWHS) has been previously published [21]. Briefly, 41 836 older women, age 55–69 years, were enrolled in 1986. Participants completed a self-administered survey that included a semiquantitative food-frequency questionnaire (FFQ), which was adapted from a 126-item instrument developed by Willett et al. [22]. For each food, a commonly used portion size or unit was specified, and respondents were asked how often on average over the last year they had consumed that amount of each food item. Respondents were also asked if they used multivitamins (including frequency of use) as well as selected supplements including any regular use of folic acid, B-complex vitamins, and vitamin B6. For the latter supplements, dose and duration of use were not collected.
The daily intake of total energy and selected nutrients was calculated by multiplying the frequency of consumption of each unit of food by the nutrient content of the specified portions, using the Harvard Food Composition Database, which is based on U.S. Department of Agriculture data [23]. Three types of nutrient variables were considered: intake from diet only, intake from supplements only, and combined dietary and supplemental intake.
The FFQ was found to be reliable and valid in this population [24]. The reproducibility of this FFQ instrument given 6 months apart as measured by Pearson's correlation coefficient (r) was quite high for folate (r = 0.76) and vitamins B2 (r = 0.83), B6 (r = 0.88), and B12 (r = 0.73). With respect to validity, energy-adjusted intakes assessed by the FFQ estimate and five 24-h recalls were moderately correlated for folate (r = 0.43) and more strongly correlated for vitamins B2 (r = 0.93), B6 (r = 0.69), and B12 (r = 0.76).
cohort follow-up and case ascertainment
Vital status of cohort members was ascertained via follow-up surveys (1987, 1989, 1992, 1997 and 2004) and yearly linkage with Iowa death certificates and the National Death Index through 2005. Migration out of Iowa has been low for this cohort, and we have estimated that <1% of the cohort has been lost to follow-up [25].
Incident endometrial cancer cases were identified through annual linkage with the Iowa Cancer Registry, a Surveillance, Epidemiology and End Results (SEER) program member. The Iowa Cancer Registry collects cancer data including primary site and morphology. All pathology data were derived from pathology reports of the diagnosing pathologist, and there was no central review. Topographic and morphologic data were coded according to the International Classification of Diseases for Oncology (ICD-O), 2nd [26] or 3rd [27] edition. Using these codes, we classified endometrial cancer into type I or type II (Table 1), based on prior literature [16–20] and systematic review of the ICD-O codes by the authors (SU and AM).
Table 1.
Definition of type I and II endometrial cancer
| Morphology code | Morphology | Type I endometrial cancer |
Type II endometrial cancer |
||
| n | % | n | % | ||
| 8000 | Neoplasm, malignant | 1 | 0.2 | ||
| 8010 | Carcinoma, NOS | 3 | 0.6 | ||
| 8041 | Small-cell carcinoma, NOS | 1 | 0.2 | ||
| 8050 | Papillary carcinoma, NOS | 1 | 1.4 | ||
| 8140 | Adenocarcinoma, NOS | 343 | 72.8 | ||
| 8210 | Adenocarcinoma in adenomatous polyp | 1 | 0.2 | ||
| 8260 | Papillary adenocarcinoma | 13 | 18.3 | ||
| 8262 | Villous adenocarcinoma | 1 | 0.2 | ||
| 8263 | Adenocarcinoma in tubulovillous adenoma | 2 | 0.4 | ||
| 8310 | Clear cell adenocarcinoma, NOS | 7 | 9.9 | ||
| 8323 | Mixed cell adenocarcinoma | 1 | 1.4 | ||
| 8380 | Endometrioid adenocarcinoma, NOS | 91 | 19.3 | ||
| 8441 | Serous cystadenocarcinoma, NOS | 5 | 7.0 | ||
| 8460 | Papillary serous cystadenocarcinoma | 27 | 38.0 | ||
| 8480 | Mucinous adenocarcinoma | 2 | 0.4 | ||
| 8560 | Adenosquamous carcinoma | 13 | 2.8 | ||
| 8570 | Adenocarcinoma with squamous metaplasia | 13 | 2.8 | ||
| 8950 | Mullerian mixed tumor | 10 | 14.1 | ||
| 8951 | Mesodermal mixed tumor | 1 | 1.4 | ||
| 8980 | Carcinosarcoma, NOS | 6 | 8.5 | ||
| Total | 471 | 100 | 71 | 100 | |
NOS, not otherwise specified.
data analysis
Women with history of cancer before baseline, except nonmelanoma skin cancer (n = 3830), hysterectomy before baseline (n = 14 350), extreme dietary intake (<600 or >5000 kcal/day), or incomplete FFQs (≥30 blank food items) (n = 3096) or who were not postmenopausal at baseline (n = 569) were excluded from the present analysis, yielding a final sample size of 23 356 study participants. Each woman accumulated person-years of follow-up from baseline to the date of endometrial cancer diagnosis, move from Iowa, death, or administrative censoring on 31 December 2005.
Data were descriptively summarized using means and standard deviations for continuous variables and frequencies and percents for categorical variables. We assessed pairwise associations of the one-carbon nutrient variables with each other using Spearman correlation coefficients. Relative risks (RRs) and 95% confidence intervals (CIs) examining the association between exposure variables and risk of endometrial cancer were estimated using Cox proportional hazards regression models, modeling age as the time variable [28]. Separate analyses were carried out for type I and type II endometrial cancer. For each, the outcome variable of interest was endometrial cancer of that specific type, and women with endometrial cancer not of that type were censored at the time of diagnosis. Nutrient variables were categorized into approximate quartiles based on their distribution of consumption of all women in the analytic cohort at study baseline. Tests for trend were carried out by ordering the intake quartiles from lowest to highest and including the resulting variable as a 1 degree-of-freedom linear term in the Cox regression models.
We formally determined whether risk ratios for the exposure variables differed by type of endometrial cancer using a competing risk form of Cox proportional hazards regression [29]. This approach allowed us to specifically model and test the ordered interaction between a given risk factor (modeled as a covariate) and endometrial cancer type (included as a stratum variable). Following the analyses of categorized intake variables, we re-assessed associations using the actual continuously distributed nutrient values via penalized smoothing splines or P-splines [30]. Briefly, this is a nonparametric modeling approach that is a generalization of polynomial splines. It allowed us to examine the unrestricted association of the nutrient variables with endometrial cancer, without regard to functional form. All models described above initially accounted for total energy and other potential confounding (Table 2). Total energy was modeled as a continuous covariate in the Cox model and was included to adjust for systematic over- and underreporting of food intake [31]. Subsequent analyses assessed the independent associations of the nutrient variables with risk of endometrial cancer by simultaneously including each in one multivariate Cox model, along with the potential confounding variables listed above. All statistical tests were two sided, and analyses were carried out using the SAS (SAS Institute Inc., Cary, NC) and S-Plus (Insightful, Seattle, WA) software systems.
Table 2.
Distribution of baseline (1986) factors by level of folate intake
| Variable | Total folate intake (diet and supplements) |
|||
| Quartile 1 (<250.2 μg/day), N = 5840 |
Quartile 2 (250.2–348.6 μg/day), N = 5838 |
Quartile 3 (348.7–560.9 μg/day), N = 5839 |
Quartile 4 (>560.9 μg/day), N = 5839 |
|
| Age (years), mean ± SD | 61.9 ± 4.2 | 62.2 ± 4.2 | 62.2 ± 4.1 | 62.3 ± 4.2 |
| Body mass index (kg/m2), mean ± SD | 27.0 ± 5.1 | 27.0 ± 5.2 | 26.9 ± 5.2 | 26.4 ± 4.9 |
| Waist-to-hip ratio, mean ± SD | 0.84 ± 0.08 | 0.84 ± 0.09 | 0.84 ± 0.08 | 0.83 ± 0.08 |
| Total energy (kcal/day), mean ± SD | 1394 ± 397 | 1796 ± 446 | 2094 ± 634 | 1955 ± 683 |
| Smoking (pack-years), mean ± SD | 11.6 ± 19.1 | 9.4 ± 18.0 | 8.5 ± 16.9 | 8.9 ± 17.3 |
| Adult-onset diabetes (%) | 4.7 | 6.2 | 6.0 | 5.8 |
| History of hypertension (%) | 35 | 35 | 34 | 34 |
| Age at menarche >12 years (%) | 59 | 59 | 58 | 59 |
| Age at menopause >50 years (%) | 59 | 62 | 64 | 65 |
| Ever-used hormone replacement therapy (%) | 24 | 25 | 26 | 31 |
| Smoking history (%) | ||||
| Current | 20 | 15 | 14 | 13 |
| Former | 19 | 19 | 18 | 21 |
| Never | 61 | 66 | 68 | 66 |
| Any alcohol use (%) | 42 | 47 | 48 | 47 |
SD, standard deviation.
results
During the 20-year follow-up period, we identified a total of 542 incident cases, including 471 cases of type I endometrial cancer and 71 cases of type II endometrial cancer (including carcinosarcoma). The mean age at diagnosis of type I endometrial cancer was 71.8 years (range 57.2–89.6), while the mean age at diagnosis of type II endometrial cancer was 72.8 years (range 60.2–89.3).
Table 2 describes the correlation of folate intake with key endometrial cancer risk factors. Higher intake was associated with greater intake of total energy, lower body mass index, greater use of hormone replacement therapy, less current smoking, and slightly greater use of alcohol, although absolute differences were very modest. There was little association of folate intake with age at study baseline, waist-to-hip ratio, prevalence of adult-onset diabetes, history of hypertension, age at menarche, and age at menopause.
Table 3 summarizes the results for one-carbon nutrients and risk of type I and II endometrial cancer. There was no association of total, dietary, or supplemental intake of folate or vitamins B2 and B12 with risk of type I endometrial cancer. There was also no association of total or supplemental intake of vitamins B6 with risk of type I endometrial cancer, while there were suggestive but not statistically significant inverse trends for dietary intake of vitamin B6 (RR = 0.79 for highest versus lowest quartile of intake, 95% CI 0.55–1.13, P trend = 0.25) and methionine (RR = 0.82 for highest versus lowest quartile of intake, 95% CI 0.55–1.20, P trend = 0.13). Multivitamin use was not associated with type I endometrial cancer.
Table 3.
Nutrients implicated in the one-carbon metabolism and the risk of type I and type II endometrial cancer
| Nutrient | Level of intake | Person-years | Type I |
Type II |
|||||
| Events | RRa (95% CI) | P | Events | RRa (95% CI) | P | ||||
| Folate (μg/day) | Total | 43.5–250.1 | 94 093 | 115 | 1.00 (reference) | 0.59 | 18 | 1.00 (reference) | 0.09 |
| 250.2–348.6 | 93 944 | 107 | 0.85 (0.64–1.13) | 15 | 0.93 (0.45–1.95) | ||||
| 348.7–560.9 | 93 772 | 128 | 1.08 (0.81–1.44) | 13 | 0.97 (0.44–2.12) | ||||
| >560.9 | 92 110 | 121 | 1.00 (0.76–1.32) | 25 | 1.71 (0.87–3.35) | ||||
| Food only | 43.5–225.1 | 93 312 | 114 | 1.00 (reference) | 0.74 | 18 | 1.00 (reference) | 0.76 | |
| 225.2–293.8 | 93 668 | 118 | 1.00 (0.76–1.31) | 23 | 1.48 (0.75–2.9) | ||||
| 293.9–373.6 | 93 589 | 119 | 0.93 (0.70–1.25) | 14 | 1.05 (0.47–2.3) | ||||
| >373.6 | 93 350 | 120 | 1.09 (0.79–1.52) | 16 | 1.34 (0.55–3.23) | ||||
| Supplements only | 0 | 264 898 | 319 | 1.00 (reference) | 0.56 | 42 | 1.00 (reference) | 0.027 | |
| 0.1–228.6 | 22 056 | 44 | 1.61 (1.15–2.26) | 4 | 1.33 (0.47–3.72) | ||||
| >228.6 | 86 965 | 108 | 1.00 (0.80–1.25) | 25 | 1.80 (1.07–3.02) | ||||
| Vitamin B6 (mg/day) | Total | 0.21–1.71 | 93 923 | 121 | 1.00 (reference) | 0.97 | 14 | 1.00 (reference) | 0.012 |
| 1.72–2.39 | 95 272 | 102 | 0.72 (0.54–0.95) | 16 | 1.40 (0.64–3.04) | ||||
| 2.40–3.80 | 93 217 | 133 | 1.06 (0.80–1.40) | 16 | 1.71 (0.78–3.75) | ||||
| >3.80 | 91 507 | 115 | 0.88 (0.66–1.17) | 25 | 2.42 (1.17–4.99) | ||||
| Food only | 0.21–1.49 | 94 596 | 128 | 1.00 (reference) | 0.25 | 20 | 1.00 (reference) | 0.60 | |
| 1.50–1.92 | 93 141 | 107 | 0.74 (0.56–0.97) | 18 | 1.11 (0.55–2.24) | ||||
| 1.93–2.41 | 93 346 | 122 | 0.80 (0.59–1.07) | 19 | 1.27 (0.59–2.75) | ||||
| >2.41 | 92 836 | 114 | 0.79 (0.55–1.13) | 14 | 1.23 (0.47–3.24) | ||||
| Supplements only | 0 | 243 670 | 290 | 1.00 (reference) | 0.40 | 37 | 1.00 (reference) | 0.012 | |
| 0.01–2.00 | 71 382 | 106 | 1.23 (0.97–1.55) | 15 | 1.53 (0.83–2.81) | ||||
| >2.00 | 58 866 | 75 | 1.01 (0.77–1.31) | 19 | 2.08 (1.16–3.73) | ||||
| Vitamin B12 (μg/day) | Total | 0.03–5.78 | 94 026 | 115 | 1.00 (reference) | 0.72 | 15 | 1.00 (reference) | 0.21 |
| 5.79–11.10 | 94 527 | 116 | 0.97 (0.74–1.28) | 17 | 1.36 (0.65–2.85) | ||||
| 11.11–16.66 | 93 132 | 115 | 0.98 (0.75–1.30) | 21 | 1.56 (0.77–3.17) | ||||
| >16.66 | 92 235 | 125 | 1.05 (0.79–1.39) | 18 | 1.58 (0.75–3.33) | ||||
| Food only | 0.03–4.76 | 93 066 | 114 | 1.00 (reference) | 0.84 | 22 | 1.00 (reference) | 0.33 | |
| 4.77–7.70 | 94 720 | 111 | 0.92 (0.70–1.23) | 11 | 0.61 (0.28–1.36) | ||||
| 7.71–14.42 | 92 832 | 128 | 1.09 (0.83–1.44) | 19 | 1.15 (0.58–2.25) | ||||
| >14.42 | 93 302 | 118 | 0.97 (0.71–1.31) | 19 | 1.19 (0.57–2.48) | ||||
| Supplements only | 0 | 247 271 | 293 | 1.00 (reference) | 0.25 | 37 | 1.00 (reference) | 0.0060 | |
| 0.01–3.43 | 35 940 | 57 | 1.28 (0.95–1.74) | 6 | 1.27 (0.53–3.04) | ||||
| >3.43 | 90 708 | 121 | 1.10 (0.88–1.36) | 28 | 2.10 (1.26–3.50) | ||||
| Vitamin B2 (mg/day) | Total | 0.23–1.61 | 94 423 | 112 | 1.00 (reference) | 0.50 | 13 | 1.00 (reference) | 0.026 |
| 1.62–2.34 | 93 864 | 113 | 1.03 (0.78–1.36) | 18 | 1.84 (0.85–3.96) | ||||
| 2.35–3.57 | 93 977 | 125 | 1.14 (0.85–1.51) | 18 | 2.12 (0.97–4.63) | ||||
| >3.57 | 91 655 | 121 | 1.08 (0.81–1.44) | 22 | 2.41 (1.13–5.13) | ||||
| Food only | 0.23–1.40 | 95 486 | 118 | 1.00 (reference) | 0.98 | 17 | 1.00 (reference) | 0.25 | |
| 1.41–1.84 | 91 324 | 116 | 0.99 (0.75–1.3) | 19 | 1.42 (0.69–2.95) | ||||
| 1.85–2.39 | 93 880 | 114 | 0.96 (0.71–1.29) | 22 | 2.04 (0.95–4.37) | ||||
| >2.39 | 93 228 | 123 | 1.02 (0.71–1.45) | 13 | 1.47 (0.56–3.88) | ||||
| Supplements only | 0 | 246 663 | 293 | 1.00 (reference) | 0.18 | 37 | 1.00 (reference) | 0.011 | |
| 0.01–1.70 | 47 026 | 65 | 1.09 (0.82–1.45) | 11 | 1.73 (0.88–3.41) | ||||
| >1.70 | 80 230 | 113 | 1.16 (0.93–1.46) | 23 | 1.94 (1.12–3.34) | ||||
| Methionine (g/day) | Food only | 0.18–1.36 | 93 314 | 115 | 1.00 (reference) | 0.13 | 22 | 1.00 (reference) | 0.36 |
| 1.37–1.76 | 92 627 | 131 | 1.03 (0.79–1.36) | 17 | 1.10 (0.55–2.23) | ||||
| 1.77–2.22 | 94 121 | 108 | 0.78 (0.57–1.06) | 12 | 0.91 (0.39–2.13) | ||||
| >2.22 | 93 857 | 117 | 0.82 (0.55–1.20) | 20 | 1.89 (0.70–5.09) | ||||
| Multivitamins | Any use | No | 250 324 | 300 | 1.00 (reference) | 0.30 | 38 | 1.00 (reference) | 0.022 |
| Yes | 118 545 | 164 | 1.11 (0.91–1.36) | 31 | 1.78 (1.09–2.92) | ||||
RRs and 95% CIs from Cox proportional hazards regression analysis, adjusted for age, total energy, body mass index, waist-to-hip ratio, diabetes, hypertension, age at menarche, age at menopause, use of hormone replacement therapy, smoking status, pack-years of smoking, and alcohol use. P value is a test for trend, assessing the dose–response effect of the variable of interest with risk of endometrial cancer.
RR, relative risk; CI, confidence interval. Significant P values (<0.05) are reported in bold.
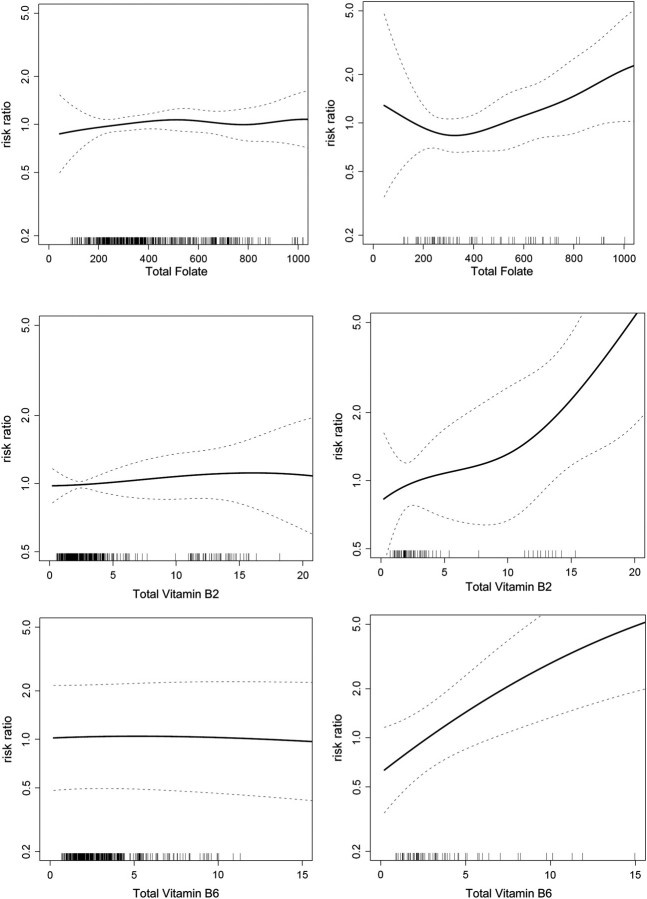
In contrast to type I endometrial cancer, greater intake (highest versus lowest quartile) of total folate (RR = 1.71, 95% CI 0.87–3.35; P trend = 0.09), vitamin B2 (RR = 2.41, 95% CI 1.13–5.13; P trend = 0.026), and vitamin B6 (RR = 2.42, 95% CI 1.17–4.99; P trend = 0.012) were positively associated with risk of type II endometrial cancer. Methionine (only available from dietary intake) was not associated with risk of type II endometrial cancer (P trend = 0.36), although the top quartile of intake showed an excess risk (RR = 1.89, 95% CI 0.70–5.09) that was not statistically significant. Figure 1 shows the multivariate-adjusted associations of total folate and vitamins B2 and B6 with type I versus type II endometrial cancer using smoothing splines. These splines show the lack of association with type I endometrial cancer, a J-shaped association for folate with type II endometrial cancer, and mainly linear and positive associations for vitamins B2 and B6 with type II endometrial cancer. In formal testing for any difference in the association of the one-carbon nutrients between type I and type II endometrial cancer, we found strong evidence for differences for total vitamin B6 intake (P = 0.021), suggestive evidence for total vitamin B2 (P = 0.072), and limited evidence for total folate (P = 0.17).
Figure 1.
Multivariate-adjusted association of total folate with risk of type I (upper left) versus type II (upper right), vitamin B2 with risk of type I (middle left) versus type II (middle right), and total vitamin B6 with risk of type I (lower left) versus type II (lower right) endometrial cancer risk. Relative risks modeled using P-splines (solid lines) and 95% confidence intervals (dashed lines).
Further inspection of these associations for type II endometrial cancer showed that they were mainly driven by supplemental versus dietary intake. Compared with no supplement use, there were positive associations with higher supplemental intake of folate (RR = 1.80 for >228.6 μg/day, 95% CI 1.07–3.02; P trend = 0.027) and vitamins B2 (RR = 1.94 for >1.70 mg/day, 95% CI 1.12–3.34; P trend = 0.011), B6 (RR = 2.08 for >2.00 mg/day, 95% CI 1.16–3.73; P trend = 0.012), as well as vitamin B12 (RR = 2.10 for >3.43 μg/day, 95% CI 1.26–3.50; P trend = 0.0060). Multivitamin use was associated with an increased risk of type II endometrial cancer (RR = 1.78, 95% CI 1.09–2.92; P trend = 0.022). The difference in risk ratios for type I versus type II endometrial cancer was statistically significant for intakes of supplemental vitamins B12 (P = 0.036) and B6 (P = 0.046), while there was suggestive evidence for intakes of supplemental folate (P = 0.068) and supplemental vitamin B2 (P = 0.067).
When the results for folate were stratified by era of diagnosis (1986–1996, 1997–2005; pre- and post-folate fortification in the United States), the associations reported in Table 3 were consistent with the overall results (data not shown).
Intake of total one-carbon nutrients was modestly to strongly correlated (range of r's: 0.37–0.89), and intake of these nutrients as supplements was very strongly correlated (range of r's: 0.82–0.97). We were able to fit a multivariate model for risk of type II endometrial cancer that simultaneously included all the one-carbon nutrients (as total intake) and the other endometrial cancer risk factors, and the association with vitamin B6 intake showed the clearest positive association (RRs = 1.00, 1.35, 1.56, 2.47), while the association with methionine intake attenuated (RRs = 1.00, 0.94, 0.73, 1.52), vitamin B2 intake became unstable (RRs = 1.00, 2.13, 1.90, 1.31), and intakes of folate (RRs = 1.00, 0.68, 0.56, 0.90) and vitamin B12 (RRs = 1.00, 0.89, 0.98, 0.82) were no longer associated with risk. The high correlations among intake of supplements precluded us from fitting a similar model.
discussion
Contrary to our initial hypothesis, we found that higher total intake of folate and vitamins B2 and B6 was associated with an increased risk of type II endometrial cancer, and this was mainly due to positive associations with the use of supplemental intake of these nutrients. Supplemental vitamin B12 intake was also associated with type II endometrial cancer risk. These results were not confounded by a wide variety of endometrial cancer risk factors, including body mass index, estrogen use, smoking, and alcohol use. In contrast, we found that one-carbon nutrients from the diet or supplements were not associated with type I endometrial cancer.
An accumulating body of evidence suggests that folate and B-vitamins may play a role in the etiology of several malignancies, including cancers of the colon, rectum, breast, pancreas, lung, and prostate [32–38]. A majority of the available reports indicate an inverse association between folate status and the risk of these malignancies [32–35]. Several experimental studies have substantiated these epidemiologic observations: the anticancer properties of folate appear to be due at least in part to its role in the synthesis of nitrogenous bases and in the mechanism of DNA replication and repair. Moreover, folate is crucial for the one-carbon metabolism, and an adequate folate status is essential for the production of S-adenosylmethionine, the donor of methyl groups involved in the methylation of DNA [39]. A normal pattern of DNA methylation is at the basis of gene silencing or expression and has been described to be substantially associated with a reduced risk of cancer [40].
However, the role of folate and perhaps other one-carbon nutrients in carcinogenesis may be more complex than it was initially believed [41]. Animal experiments suggest that modest supplementation of folic acid can be protective against the development of cancer, whereas higher intakes may facilitate tumor growth [42]. This dichotomous effect could be explained by the observation that the synthesis of nucleotides is essential not only for DNA repair processes but also for extensive DNA derangement [43]. Moreover, supplements contain folic acid that is more bioavailable than the form of folate in food [44] and thus they could be more potent in promoting cell replication. In this context, there have been provocative findings from epidemiologic studies that have observed a direct association between folic acid supplementation and the risk of cancer [36, 37]. An emerging view suggests that a very low folate status may be related to a higher risk of cancer; normal intake of folate is related to a modest protective effect, and higher levels of this nutrient may promote cancer [45].
Our finding of an association that is specific for folic acid supplementation and type II but not type I endometrial cancer could be due to the different characteristics of these two tumor subtypes. In support of this hypothesis, folate receptors have been shown to be preferentially overexpressed in cells of high-grade non-endometrioid cancers [43]. Our study is one of the few investigating the association between folate intake and endometrial cancer and the only to evaluate type I versus type II endometrial cancer. Our results for type II endometrial cancer are consistent with animal studies, which found that folate has a complex, nonlinear and, perhaps, dual relationship with carcinogenesis [42].
Another interesting finding of the present study is that multivitamin supplements in general, and B-vitamin supplements in particular, appear to increase the risk of type II endometrial cancer. The possible explanation for this observation is that B-vitamins have a synergistic effect in the one-carbon metabolism and in the synthesis of nucleotides, and they are implicated along with folic acid in the above-mentioned processes. Finally, our data suggest that an assessment of potential negative health consequences of vitamin supplement use needs to be more fully considered.
Our study has several key strengths, including the prospective cohort study design from a defined population, comprehensive dietary assessment that was found to be valid and reliable [24], identification of cancer cases using a SEER cancer registry, and minimal loss to follow-up. We were also able to adjust for a variety of potential confounding factors. A limitation of the present study is that we do not have data about the basal folate status of the women or regular updates of their diet during follow-up. We do note that women who participated in both the 1986 baseline and the 2004 follow-up showed an increase in the prevalence of the use of folate supplements from 1.2% to 6.9% [46]. Furthermore, the introduction of the fortification of grain products in the mid-1990s could potentially bias our observations, particularly if these agents act late in the carcinogenic process. However, even when analyzing only the cases of type II endometrial cancer stratified on diagnosis through 1996 versus after 1996, the associations with folic acid supplementation and endometrial cancer risk were present for both eras. The cases in this study were not reviewed by a central pathologist but rather relied on coding from original pathology reports by SEER abstractors. Given that this is an initial and unexpected observation, our findings require replication. Finally, the cohort consists of an older population of women from the upper Midwest United States, and the results may not generalize to other populations.
In conclusion, use of supplements containing folate, vitamins B2, B6, and B12 was associated with increased risk of type II but not type I endometrial cancer. While mandatory folate fortification of enriched grain products at the population level has lead to a decline in the incidence of neural tube defects [47], this and other recent studies raise concerns about potential negative health effects of excess levels of folic acid [41] and thus need to be fully evaluated.
funding
National Cancer Institute (R01 CA39742).
disclosure
The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Parslov M, Lidegaard O, Klintorp S, et al. Risk factors among young women with endometrial cancer: a Danish case-control study. Am J Obstet Gynecol. 2000;182:23–29. doi: 10.1016/s0002-9378(00)70486-8. [DOI] [PubMed] [Google Scholar]
- 4.Potischman N, Hoover RN, Brinton LA, et al. Case-control study of endogenous steroid hormones and endometrial cancer. J Natl Cancer Inst. 1996;88:1127–1135. doi: 10.1093/jnci/88.16.1127. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KE, Anderson E, Mink PJ, et al. Diabetes and endometrial cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev. 2001;10:611–616. [PubMed] [Google Scholar]
- 6.Wang L, Lee IM, Zhang SM, et al. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr. 2009;89:905–912. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCann SE, Yeh M, Rodabaugh K, Moysich KB. Higher regular coffee and tea consumption is associated with reduced endometrial cancer risk. Int J Cancer. 2009;124:1650–1653. doi: 10.1002/ijc.24125. [DOI] [PubMed] [Google Scholar]
- 8.Bravi F, Scotti L, Bosetti C, et al. Food groups and endometrial cancer risk: a case-control study from Italy. Am J Obstet Gynecol. 2009;200:293.e1–293.e7. doi: 10.1016/j.ajog.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Kasum CM, Nicodemus K, Harnack LJ, et al. Whole grain intake and incident endometrial cancer: the Iowa Women's Health Study. Nutr Cancer. 2001;39:180–186. doi: 10.1207/S15327914nc392_4. [DOI] [PubMed] [Google Scholar]
- 10.Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534–1543. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh M, Moysich KB, Jayaprakash V, et al. Higher intakes of vegetables and vegetable-related nutrients are associated with lower endometrial cancer risks. J Nutr. 2009;139:317–322. doi: 10.3945/jn.108.099960. [DOI] [PubMed] [Google Scholar]
- 12.Negri E, La Vecchia C, Franceschi S, et al. Intake of selected micronutrients and the risk of endometrial carcinoma. Cancer. 1996;77:917–923. doi: 10.1002/(sici)1097-0142(19960301)77:5<917::aid-cncr17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Xu WH, Shrubsole MJ, Xiang YB, et al. Dietary folate intake, MTHFR genetic polymorphisms, and the risk of endometrial cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2007;16:281–287. doi: 10.1158/1055-9965.EPI-06-0798. [DOI] [PubMed] [Google Scholar]
- 14.Potischman N, Swanson CA, Brinton LA, et al. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control. 1993;4:239–250. doi: 10.1007/BF00051319. [DOI] [PubMed] [Google Scholar]
- 15.Jain MG, Rohan TE, Howe GR, Miller AB. A cohort study of nutritional factors and endometrial cancer. Eur J Epidemiol. 2000;16:899–905. doi: 10.1023/a:1011012621990. [DOI] [PubMed] [Google Scholar]
- 16.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 17.Doll A, Abal M, Rigau M, et al. Novel molecular profiles of endometrial cancer—new light through old windows. J Steroid Biochem Mol Biol. 2008;108:221–229. doi: 10.1016/j.jsbmb.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Mendivil A, Schuler KM, Gehrig PA. Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control. 2009;16:46–52. doi: 10.1177/107327480901600107. [DOI] [PubMed] [Google Scholar]
- 19.Kounelis S, Jones MW, Papadaki H, et al. Carcinosarcomas (malignant mixed mullerian tumors) of the female genital tract: comparative molecular analysis of epithelial and mesenchymal components. Hum Pathol. 1998;29:82–87. doi: 10.1016/s0046-8177(98)90394-x. [DOI] [PubMed] [Google Scholar]
- 20.Kernochan LE, Garcia RL. Carcinosarcomas (malignant mixed Mullerian tumor) of the uterus: advances in elucidation of biologic and clinical characteristics. J Natl Compr Canc Netw. 2009;7:550–556. doi: 10.6004/jnccn.2009.0037. ; quiz 557. [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Kaye SA, Prineas RJ, et al. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol. 1990;131:794–803. doi: 10.1093/oxfordjournals.aje.a115570. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Agriculture. Composition of Foods—Raw, Processed and Prepared, 1963–1992. Agricultural Handbook No. 8 series. Washington, DC: Department of Agriculture, Government Printing Office; 1993. [Google Scholar]
- 24.Munger RG, Folsom AR, Kushi LH, et al. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136:192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 26.Percy C, Van Holten V, Muir C. International Classification of Diseases for Oncology. 2nd edition. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 27.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3rd edition. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 28.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 29.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 30.Eilers PH, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11:89–121. [Google Scholar]
- 31.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 32.Harnack L, Jacobs DR, Jr., Nicodemus K, et al. Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer. 2002;43:152–158. doi: 10.1207/S15327914NC432_5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Hunter DJ, Hankinson SE, et al. A prospective study of folate intake and the risk of breast cancer. JAMA. 1999;281:1632–1637. doi: 10.1001/jama.281.17.1632. [DOI] [PubMed] [Google Scholar]
- 34.Larsson SC, Hakansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst. 2006;98:407–413. doi: 10.1093/jnci/djj094. [DOI] [PubMed] [Google Scholar]
- 35.Voorrips LE, Goldbohm RA, Brants HA, et al. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:357–365. [PubMed] [Google Scholar]
- 36.Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101:432–435. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2006;83:895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SM, Cook NR, Albert CM, et al. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA. 2008;300:2012–2021. doi: 10.1001/jama.2008.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 40.Laird PW. Cancer epigenetics. Hum Mol Genet. 2005;14(suppl 1):R65–R76. doi: 10.1093/hmg/ddi113. [DOI] [PubMed] [Google Scholar]
- 41.Osterhues A, Holzgreve W, Michels KB. Shall we put the world on folate? Lancet. 2009;374:959–961. doi: 10.1016/S0140-6736(09)61646-9. [DOI] [PubMed] [Google Scholar]
- 42.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44:10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- 43.Brown Jones M, Neuper C, Clayton A, et al. Rationale for folate receptor alpha targeted therapy in “high risk” endometrial carcinomas. Int J Cancer. 2008;123:1699–1703. doi: 10.1002/ijc.23686. [DOI] [PubMed] [Google Scholar]
- 44.Suitor CW, Bailey LB. Dietary folate equivalents: interpretation and application. J Am Diet Assoc. 2000;100:88–94. doi: 10.1016/S0002-8223(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr. 2007;86:271–273. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 46.Park K, Harnack L, Jacobs DR., Jr. Trends in dietary supplement use in a cohort of postmenopausal women from Iowa. Am J Epidemiol. 2009;169:887–892. doi: 10.1093/aje/kwn410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rader JI, Schneeman BO. Prevalence of neural tube defects, folate status, and folate fortification of enriched cereal-grain products in the United States. Pediatrics. 2006;117:1394–1399. doi: 10.1542/peds.2005-2745. [DOI] [PubMed] [Google Scholar]