Abstract
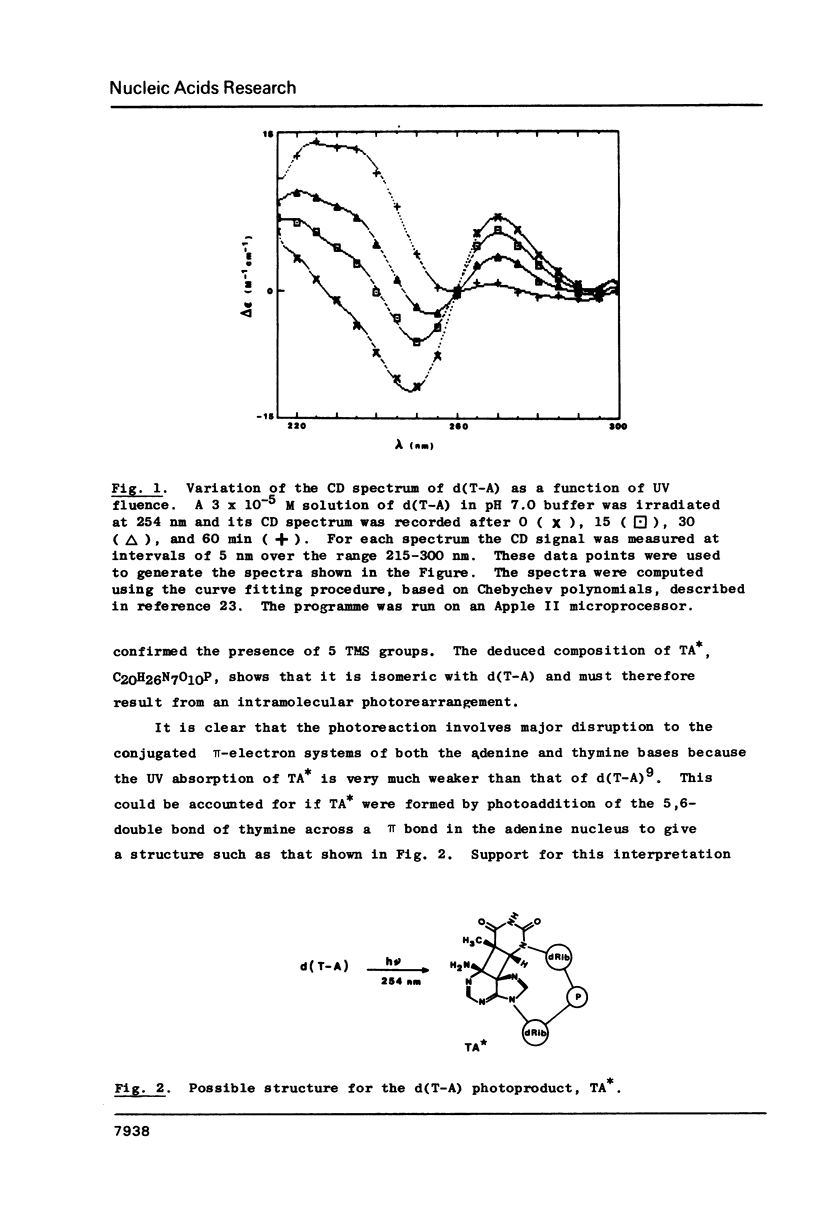
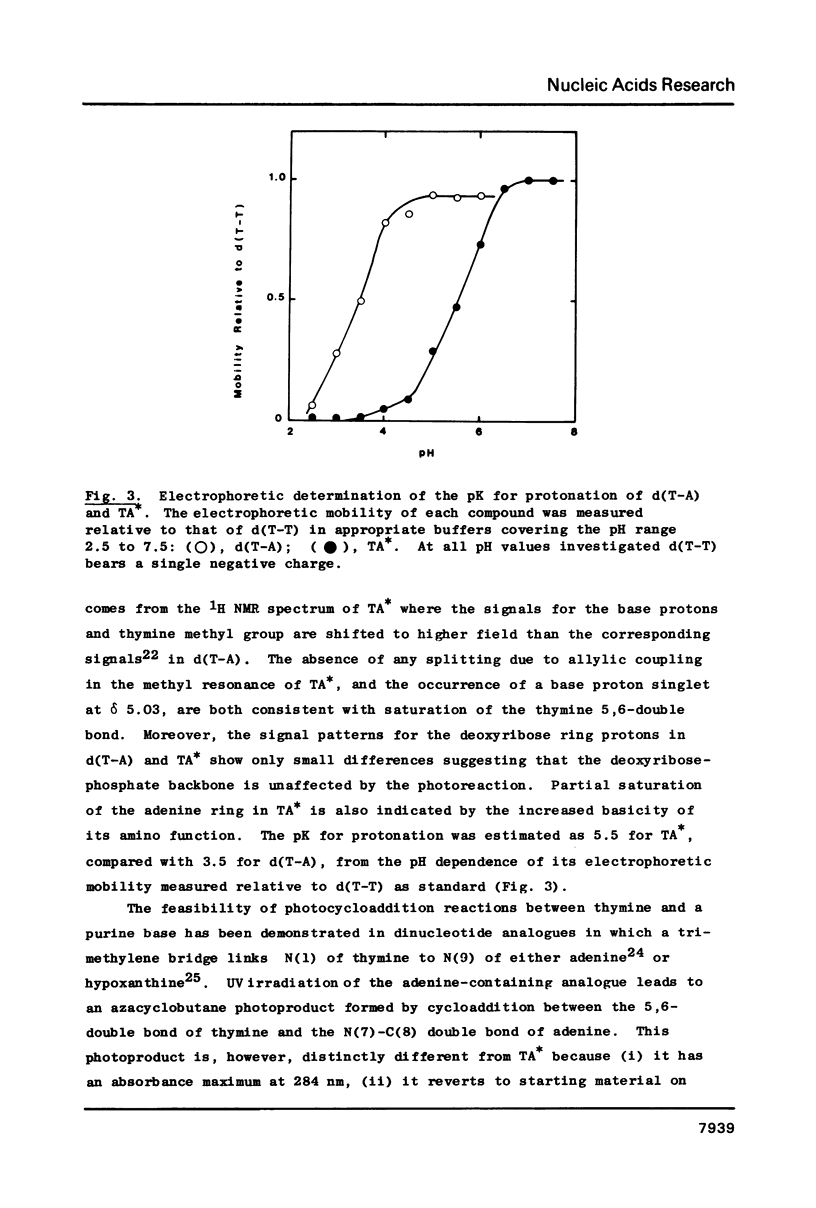
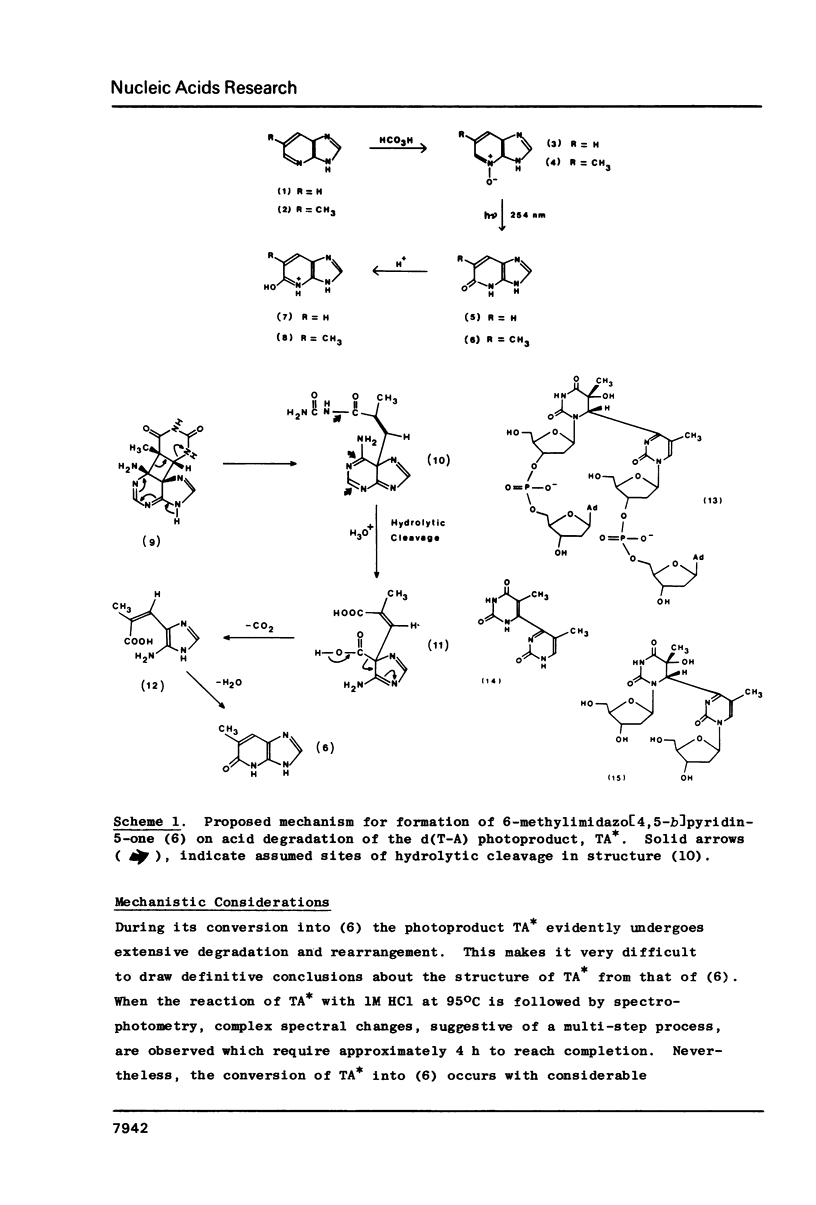
When d(T-A) is irradiated at 254 nm in aqueous solution an internal photoadduct is formed between its constituent adenine and thymine bases. The resultant photoproduct, designated TA*, arises from a singlet excited state precursor; a similar photoreaction is not observed with d(C-A) or d(T-G). In contradistinction, irradiation of d(T-A) in frozen aqueous solution yields a dimeric photoproduct in which two d(T-A) molecules are coupled together by a (6-4) photoadduct linkage between their respective thymine bases. Both photoproducts have been extensively characterised by a combination of electron impact and fast atom bombardment mass spectrometry, UV, CD, 1H NMR and fluorescence spectroscopy. Acid treatment of TA* gives 6-methylimidazo[4,5-b]pyridin-5-one whose identity was established by an independent chemical synthesis involving photorearrangement of 6-methyl-imidazo[4,5-b]pyridine N(4)-oxide. A tentative mechanism is presented to account for the acid degradation of TA*. The structure of the dimeric ice photoproduct follows from its cleavage, by snake venom phosphodiesterase, to 5'-dAMP and the (6-4) bimolecular photoadduct of thymidine; on acid hydrolysis it gives adenine and 6-(5'-methyl-2'-oxopyrimidin-4'-yl) thymine.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose S. N., Davies R. J., Sethi S. K., McCloskey J. A. Formation of an adenine-thymine photoadduct in the deoxydinucleoside monophosphate d(TpA) and in DNA. Science. 1983 May 13;220(4598):723–725. doi: 10.1126/science.6836308. [DOI] [PubMed] [Google Scholar]
- Bose S. N., Davies R. J. The photoreactivity of T-A sequences in oligodeoxyribonucleotides and DNA. Nucleic Acids Res. 1984 Oct 25;12(20):7903–7914. doi: 10.1093/nar/12.20.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D. M., Sarma R. H. Intimate details of the conformational characteristics of deoxyribodinucleoside monophosphates in aqueous solution. J Am Chem Soc. 1977 Oct 26;99(22):7333–7348. doi: 10.1021/ja00464a038. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Lo K. M., Haseltine W. A. Alkaline lability of fluorescent photoproducts produced in ultraviolet light-irradiated DNA. J Biol Chem. 1982 Nov 25;257(22):13535–13543. [PubMed] [Google Scholar]
- Horne D. S., Parker T. G. Polynomial representation of digital spectrophotometric data of amino acids and proteins. Biochim Biophys Acta. 1980 Sep 23;625(1):18–27. doi: 10.1016/0005-2795(80)90104-x. [DOI] [PubMed] [Google Scholar]
- Karle I. L. Crystal structure of a thymine-thymine adduct from irradiated thymine. Acta Crystallogr B. 1969 Oct 15;25(10):2119–2126. doi: 10.1107/s056774086900522x. [DOI] [PubMed] [Google Scholar]
- Lawson A. M., Stillwell R. N., Tacker M. M., Tsuboyama K., McCloskey J. A. Mass spectrometry of nucleic acid components. Trimethylsilyl derivatives of nucleotides. J Am Chem Soc. 1971 Feb 24;93(4):1014–1023. doi: 10.1021/ja00733a039. [DOI] [PubMed] [Google Scholar]
- Rahn R. O. Search for an adenine photoproduct in DNA. Nucleic Acids Res. 1976 Apr;3(4):879–890. doi: 10.1093/nar/3.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese A. J. Photochemistry of thymidine in ice. Biochemistry. 1970 Nov 24;9(24):4781–4787. doi: 10.1021/bi00826a023. [DOI] [PubMed] [Google Scholar]
- Varghese A. J., Wang S. Y. Thymine-thymine adduct as a photoproduct of thymine. Science. 1968 Apr 12;160(3824):186–187. doi: 10.1126/science.160.3824.186. [DOI] [PubMed] [Google Scholar]



