Abstract
Fulminant hepatic failure (FHF) is a serious clinical condition that is associated with high mortality. There is evidence that FHF is an inflammatory disease, which is supported clinically by elevated serum levels of cytokines. In an effort to develop hepatocytes with additional functions for use in our bioartificial liver (BAL) device, we focused on interleukin-1 (IL-1) blockade as a therapeutic modality. Primary porcine hepatocytes were isolated from the livers of miniature swine and then transfected with an adenoviral vector encoding human interleukin-1 receptor antagonist (AdIL-1Ra). The transfected hepatocytes secreted human IL-1Ra. These transfected hepatocytes were incorporated into a flat-plate BAL device to evaluate their efficacy in treating D-galactosamine (GalN)-induced FHF in a rat model. After extracorporeal perfusion with the BAL device containing the transfected hepatocytes, there were significant reductions in the plasma levels of hepatic enzymes (aspartate aminotransferase and alanine aminotransferase) and cytokines (IL-1 and IL-6), indicating a beneficial effect. Animal survival was significantly improved in the treated group compared to the control group. These experiments demonstrate that combining inflammatory cytokine blockade with a functional BAL device may be an effective therapeutic option in the treatment of FHF.
INTRODUCTION
FULMINANT HEPATIC FAILURE (FHF) is a serious clinical condition that is associated with a high mortality rate. Orthotopic liver transplantation is the treatment of choice for FHF and end-stage liver disease,1 although it has limitations primarily due to a lack of readily available donors. The development of an effective alternative therapy is necessary to address the limited availability of donor organs. One solution is a hybrid bioartificial liver (BAL) device containing functioning hepatocytes. It would ideally serve as a bridge to transplantation or liver regeneration while providing hepatic support to patients with FHF.
In patients with FHF, there is rapid and extensive necrosis and apoptosis of hepatocytes. There is evidence that FHF is an inflammatory disease, supported clinically by elevated serum levels of cytokines, including IL-1,2,3 TNF,2–4 IL-6,3–5 and IL-18.6 Approaches involving the blockade of inflammatory cytokines have been shown to be beneficial in the treatment of liver injury. IL-1 receptor antagonist (IL-1Ra) is a naturally occurring cytokine whose function is to block a biological response to IL-1.7 In a rat hepatic ischemia-reperfusion model, pre-treatment with IL-1 receptor antagonist (IL-1Ra) resulted in an improved animal survival rate compared to that of the untreated animals.8
To assess the effect of IL-1Ra in the treatment of galactosamine (GalN)-induced FHF in a rat model, our previous work transfected primary rat hepatocytes or immortalized human hepatocytes with an adenoviral vector (AdIL-1Ra) encoding the human IL-1Ra gene and incorporated these cells into a BAL device. After extra-corporeal perfusion, there were reductions in plasma hepatic enzyme and inflammatory cytokine levels and an improved animal survival rate (data unpublished). In the present study, we have continued to focus on IL-1 blockade to control inflammation in FHF. We transfected primary porcine hepatocytes with AdIL-1Ra, cultured these hepatocytes in our BAL device, and evaluated their efficacy in treating rats with GalN-induced FHF. The results demonstrate that IL-1Ra is beneficial in the treatment of FHF, and that IL-1Ra producing porcine hepatocytes could become an effective cell source for BAL devices.
MATERIALS AND METHODS
Primary porcine hepatocyte isolation and culture
Hepatocytes were isolated from the livers of adult female miniature swine (from the herd of Dr. David H. Sachs in Massachusetts General Hospital, Boston, MA), weighing 30-100 kg. Typically, a liver lobule weighing between 60–100 g was excised from heart-beating animals, and hepatocytes were isolated by a modified procedure from Dunn et al.9 Routinely, between 108 and 109 cells were isolated, with cell viabilities ranging between 70 and 90%, as judged by trypan blue exclusion. Culture medium was Dulbecco's modified Eagle's medium (DMEM, 4.5 g/L glucose, Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Life Technologies), 0.5 U/mL insulin (Novo Nordisk A/S, Bagsuaerd, Denmark), 7 ng/mL glucagon (Eli Lilly, Indianapolis, IN), 20 ng/mL epidermal growth factor (Becton Dickinson, Bedford, MA), 7.5 g/mL hydrocortisone, 200 U/mL penicillin (Life Technologies), and 200 g/mL streptomycin (Life Technologies), unless otherwise specified. For in vitro testing, hepatocytes were seeded in tissue culture dishes (35 mm) at a density of 1.0 106 per dish (1 mL). Cultures were incubated in a humidified gas mixture of 90% air/10% CO2 at either 37 or 39°C. All in vitro experiments were done in triplicate.
Culture conditions for porcine hepatocytes
Culture temperature
Porcine hepatocyte function was evaluated at culture temperatures of 37°C and 39°C using supplemented DMEM culture medium. The dishes were incubated for 1 h at 37°C with 0.25 mg/mL collagen type I solution (1 mL), which was prepared from Lewis rat tail tendons, as described elsewhere.10 After the incubation, all the solution was aspirated, followed by rinsing with 1 mL of the culture medium. Isolated hepatocytes were seeded in the culture dishes and incubated at 37°C for 24 h. Starting on the following day, cultures were incubated at either 37°C or 39°C. Culture medium was replaced daily. Medium samples collected on days 1, 5, and 10 post-seeding were used for urea and albumin measurements.
Culture medium
. Porcine hepatocyte function was evaluated at 37°C using two culture media: DMEM or Williams’ E (Sigma Chemical, St. Louis, MO), both of which were supplemented (at concentrations as described above) with fetal bovine serum, insulin, glucagon, epidermal growth factor, hydrocortisone, penicillin, and streptomycin. Isolated hepatocytes were seeded in collagen-coated culture dishes with 1 mL of culture medium containing DMEM. Starting on the following day, culture media were replaced daily with either DMEM or Williams’ E. Medium samples collected on days 1, 5, and 10 post-seeding were used for urea and albumin measurements.
Measurement of urea and porcine albumin
Urea concentrations were measured using a commercially available kit (Sigma Chemical). Pig albumin concentrations were determined by enzyme-linked immunosorbent assay utilizing a goat antibody to pig albumin conjugated with horse radish peroxidase (Bethyl Laboratories, Montgomery, TX) using the modified procedure from Dunn et al.10 A standard curve was derived using chromatographically purified pig albumin (Sigma Chemical).
Construction of adenoviral vector
Human IL-1Ra cDNA was derived from the chemically stimulated human monocytic cell line U937, as previously described.11–14 The replication-defective adenoviral vector containing CAG promoter LacZ and poly-A signal sequences (referred to as AdLacZ) was prepared as previously described.15 Another replication-defective adenoviral vector, referred to as AdIL-1Ra, contained an expression cassette encoding the human IL-1Ra gene under the control of CAG promoter, deleting the viral E1 and E3 regions. Construction of AdIL-1Ra was performed using a modification of the COS-TPC method.15 Adenoviral vectors were purified by cesium chloride ultracentrifugation and titered by plaque assay as a plaque-forming unit.
Adenoviral gene transfer of human IL-1Ra into porcine hepatocytes
Isolated primary porcine hepatocytes (1.0 × 106) were seeded in collagen-coated culture dishes with supplemented DMEM and incubated at 37°C. The following day, the culture medium was replaced with fresh supplemented DMEM, and the cells were transfected with AdIL-1Ra or AdLacZ at a multiplicity of infection (MOI) of 1, 5, 25, 125, or 625. After 1 h of incubation at 37°C, the culture medium and virus were removed and the dishes were rinsed twice with culture medium. Culture media were replaced daily. Medium samples collected on days 1, 2, 3, 4, and 10 after transfection were stored at 4°C for subsequent analysis of urea, albumin, and human IL-1Ra content.
BAL device
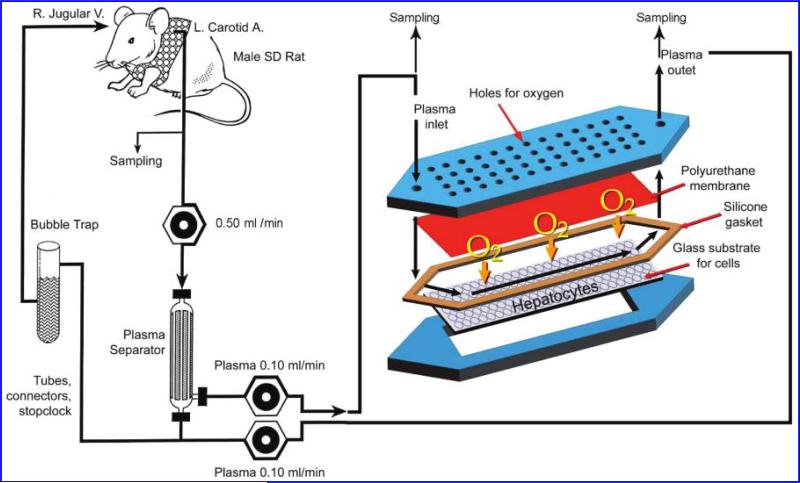
The flat-plate BAL design consisted of two plates fabricated of polycarbonate as described previously (Fig. 1).16 Using a custom-fabricated cover, the lower plate functioned as a tissue culture dish for static cultures prior to assembling the BAL for perfusion experiments. The flow channel heights in the assembled BALs averaged 550 μm.
FIG. 1.
Schematic of the extracorporeal perfusion system, including the flat-plate bioartificial liver device with an internal membrane oxygenator. (Color images are available online at www.liebertonline.com/ten).
Incorporation of hepatocytes into the BAL device
Four experimental groups of BAL devices were established for extracorporeal perfusion studies: no cell AdIL-1Ra(–) (n = 14); porcine AdIL-1Ra(–) (n = 11); porcine AdIL-1Ra(+) (n = 8); and no cell rIL-1Ra(+) (n = 9). In all the groups, the glass surface of the lower plate of the BAL device was pre-coated with 0.25 mg/mL rat tail collagen solution. In the no cell AdIL-1Ra(–) group, the BAL device contained no cells. In the porcine AdIL-1Ra(–) group, the BAL device contained primary porcine hepatocytes (30 × 106). On the day after hepatocyte seeding, the BAL device was assembled and a 10 h extracorporeal perfusion was initiated. In the porcine AdIL-1Ra(+) group, the BAL device contained primary porcine hepatocytes (30 × 106) seeded one day prior to transfection. The following day, the hepatocytes were incubated with AdIL-1Ra for 1 h at MOI 125. On day 2 after hepatocyte transfection, the BAL device was assembled and a 10 hour extracorporeal perfusion was initiated. In the no cell rIL-1Ra(+) group, the BAL device contained no cells and the recombinant protein of human IL-1Ra was continuously infused through a venous line, at a dose of 1 mg/kg/h, during the extracorporeal perfusion. This dose was based on our preliminary experiments in which we injected the recombinant IL-1Ra into rats and measured the plasma levels of human IL-1Ra.
Extracorporeal perfusion
Male Sprague-Dawley rats (Charles River Laboratories, Boston, MA), weighing 300–400 g, were used for this study. The animals were cared for in accordance with the guidelines set forth by the Committee on Laboratory Resources, National Institutes of Health, and Subcommittee on Research Animal Care and Laboratory Animal Resources of Massachusetts General Hospital. All animals were acclimated to the animal research laboratory for 5 days before initiating the experiment, and all had free access to food and water, both before and after the operation.
On day –1, rats were anesthetized with intraperitoneal injections of 110 mg/kg ketamine (Abbott Laboratories, Chicago, IL) and 0.4 mg/kg xylazine (Phoenix Pharmaceutical, St. Joseph, MO), and the carotid artery and jugular vein were cannulated (PE 50 polyethylene tubes, Becton Dickinson, Sparks, MD). On day 0, hepatitis was induced by an intraperitoneal administration of 1.4 g/kg GalN. One hour after the induction, a 10 h extracorpo-real perfusion was initiated through the perfusion circuit (Fig. 1).17 At the end of the 10 h perfusion (11 h after hepatitis induction), plasma samples were collected from the inlet and outlet of the BAL device to determine human IL-1Ra levels. Blood samples were collected from the artery line 24 h after hepatitis induction to determine plasma levels of hepatic enzymes and cytokines. This time point was chosen based on our prior study which showed that plasma hepatic enzyme levels and tissue cytokine levels were significantly elevated at 24 h.18 Animal survival was observed for 28 days.
The plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) 24 h after FHF induction were determined in a biochemistry laboratory (SRL, Tokyo, Japan). The levels of human IL-1Ra at the inlet and the outlet of the BAL device 24 h after FHF induction and plasma IL-1β and IL-6 levels,were determined using a commercially available ELISA kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical analysis
The results are expressed as means ± SD. With respect to the parametric data, the differences between the groups were evaluated using the student's t test for un-paired data based on the assumption that the data came from populations with equal SDs. If the difference between the two SDs was significant, the alternate Welch t test was used. Animal survival data at 28 days were evaluated using the Kaplan–Meier test and the general ized Wilcoxon's test. Differences were considered significant for p values less than 0.05.
RESULTS
Culture conditions for porcine hepatocytes
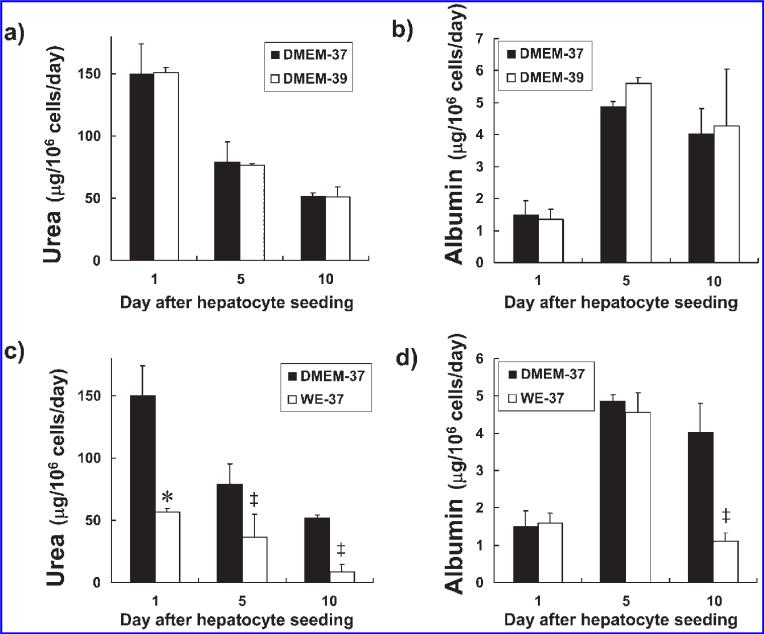
In an effort to determine the culture conditions that would produced the best performance of the porcine hepatocytes, we measured urea and albumin synthesis rates for the hepatocytes cultured in two temperature conditions (37°C or 39°C) and two media conditions (Williams’ E or DMEM). Since the body core temperature of a pig is 39°C, we compared porcine hepatocyte function at 37°C to that at 39°C when cultured on collagen-coated dishes in supplemented DMEM. The urea synthesis rate was 150 23 μg/106 hepatocytes/day on day 1 after hepatocyte seeding when incubated at 37°C (Fig. 2a). It then significantly decreased on days 5 (p < 0.05 vs. day 1) and 10 (p < 0.05 vs. day 1). There was no significant difference in the daily urea synthesis rates when cultured at 37°C compared to 39°C on days 1, 5, and 10 after hepatocyte seeding. On culture day 1, the albumin synthesis rate was 1.52 ± 0.40 μg/106 hepatocytes/day (Fig. 2b). It then significantly increased on days 5 (p < 0.005 vs. day 1) and 10 (p < 0.05 vs. day 1) compared to day 1. There was no significant difference in the daily albumin synthesis rates when cultured at 37°C compared to 39°C on days 1, 5, and 10 after hepatocyte seeding.
FIG. 2.
Urea and albumin synthesis rates at different incubation temperatures (37°C vs. 39°C) and for different culture media (DMEM vs. Williams’ E). Urea (a) and albumin (b) synthesis rates for porcine hepatocytes at incubation temperatures of 37°C (black bars) or 39°C (white bars). The culture dish surface was coated with 0.25 mg/mL collagen type I. The culture medium was supplemented DMEM. Urea (c) and albumin (d) synthesis rates in DMEM (black bars) or Williams’ E medium (WE, white bars). The culture dish surface was coated with 0.25 mg/mL rat tail derived collagen type I. Incubation temperature was 37°C. The results are expressed as means ± SD. *p < 0.05; ‡p < 0.01 vs. DMEM.
When cultured in Williams’ E medium at 37°C, the daily urea synthesis rates for the porcine hepatocytes on days 1, 5, and 10 after hepatocyte seeding were significantly lower than those when cultured in DMEM (p < 0.05 on day 1 and p < 0.01 on days 5 and 10 vs. DMEM on the same day) (Fig. 2c). Their albumin synthesis rate on culture day 10 in Williams’ E medium was significantly lower than that in DMEM on day 10 when incubated at 37°C (p < 0.01 on day 10 vs. DMEM on the same day) (Fig. 2d).
Human IL-1Ra production from gene transferred porcine hepatocytes
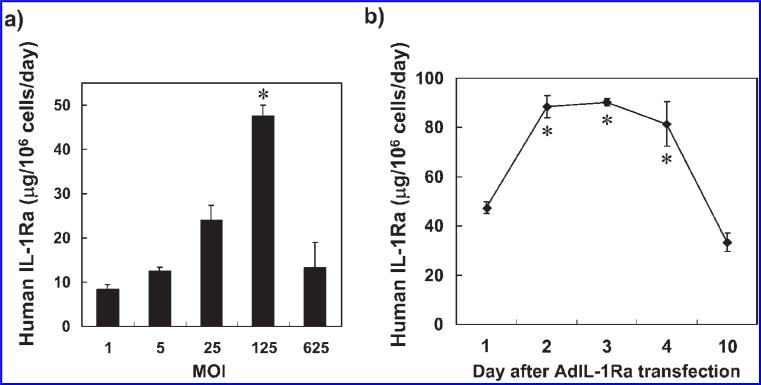
Based on the above results, the following tissue culture experiments transfecting porcine hepatocytes with human IL-1Ra were conducted in collagen-coated dishes with supplemented DMEM at an incubation temperature of 37°C. The level of human IL-1Ra secreted by the AdIL-1Ra transfected porcine hepatocytes was significantly higher at MOI 125 than at MOIs 1, 5, 25, and 625 (p < 0.001 in each comparison) (Fig. 3a). The levels of human IL-1Ra secreted at MOI 125 on days 2, 3, and 4 after transfection were significantly increased compared to the level on day 1 (p < 0.005) (Fig. 3b). The levels of human IL-1Ra secreted by porcine hepatocytes transfected with AdLacZ were lower than the detectable limit for all MOIs tested (data not shown).
FIG. 3.
Human IL-1Ra production from gene-transferred porcine hepatocytes. (a) The levels of human IL-1Ra produced from AdIL-1Ra transfected porcine hepatocytes on day 1 after transfection. *p < 0.001 vs. MOI 1, 5, 25, and 625. (b) Time course of human IL-1Ra production from porcine hepatocytes after MOI 125 transfection. The results are expressed as means ± SD. *p < 0.05 vs. day 1; IL-1Ra, interleukin-1 receptor antagonist; MOI, multiplicity of infection; AdIL-1Ra, adenoviral vector encoding human IL-1Ra gene.
Urea and albumin synthesis rates of human IL-1Ra transfected porcine hepatocytes
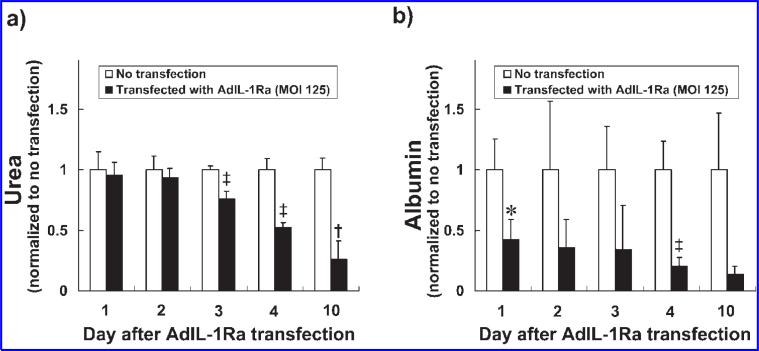
To evaluate the effect of IL-1Ra transfection on the synthetic function of porcine hepatocytes, the urea and albumin synthesis rates of the hepatocytes were measured over 10 days in culture. The urea synthesis rate (normalized to the synthesis rate for non-transfected hepatocytes) for the porcine hepatocytes transfected with AdIL-1Ra (MOI 125) was 0.96 ± 0.10 on day 1 after transfection and remained stable through day 2 (Fig. 4a). Starting on day 3, the urea synthesis rates of the transfected hepatocytes decreased in a time-dependent manner, with significant decreases occurring on days 3 (p < 0.005), 4 (p < 0.005), and 10 (p < 0.001) after transfection compared to the non-transfected hepatocytes. The albumin synthesis rate (normalized to the synthesis rate for non-transfected hepatocytes) for the porcine hepatocytes transfected with AdIL-1Ra (MOI 125) was significantly lower than that for the non-transfected hepatocytes on day 1 (p < 0.05) and day 4 (p < 0.005) after transfection (Fig. 4b).
FIG. 4.
Urea and albumin synthesis rates for the AdIL-1Ra transfected porcine hepatocytes. Urea (a) and albumin (b) synthesis rates for the AdIL-1Ra transfected porcine hepatocytes (MOI 125). The results are presented as normalized to non-transfected hepatocytes from the same culture day and are expressed as means ± SD. *p < 0.05, ‡p < 0.005, and †p < 0.001 vs. rate for non-transfection hepatocytes from the same culture day. IL-1Ra, interleukin-1 receptor antagonist; MOI, multiplicity of infection; AdIL-1Ra, adenoviral vector encoding human IL-1Ra gene.
Human IL-1Ra levels at the inlet and the outlet of the bioreactor device
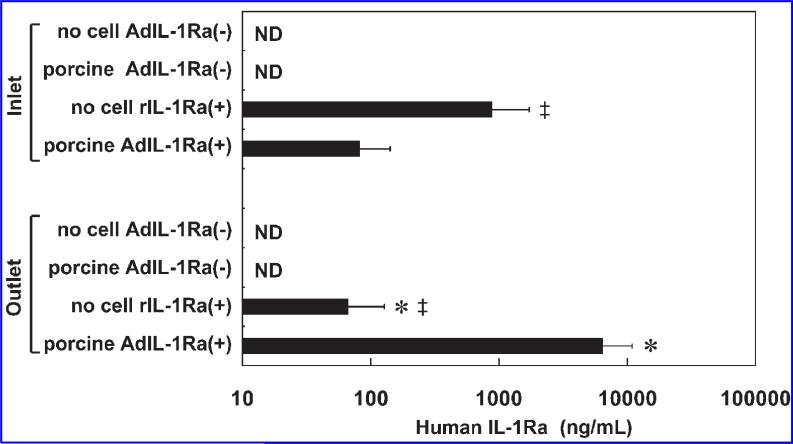
To determine the levels of circulating human IL-1Ra in the perfusion circuit, we measured human IL-1Ra at the inlet and the outlet of the BAL device. The outlet value represents the IL-1Ra level produced by the transfected hepatocytes in the BAL device, and the inlet value represents the plasma IL-1Ra level circulating through the animal. In the porcine AdIL-1Ra(+) group, the IL-1Ra concentration at the inlet (81 ± 17 pg/mL) was significantly lower than the outlet concentration (6460 ± 1660 pg/mL) (p < 0.05) (Fig. 5). In the no cell rIL-1Ra(+) group, the inlet concentration of IL-1Ra (871 ± 191 pg/mL) was significantly higher than the outlet concentration (65 ± 19 pg/mL) (p < 0.05). The inlet concentration in this group was significantly higher than the inlet concentration in the porcine AdIL-1Ra(+) group (p < 0.05), and the outlet concentration in this group was significantly lower than that in the porcine AdIL-1Ra(+) group (p < 0.05). The levels of human IL-1Ra at the inlet or the outlet were undetectable in the no cell AdIL-1Ra(–) group and in the porcine AdIL-1Ra(–) group.
FIG. 5.
Human IL-1Ra levels at the inlet and outlet of the BAL device at the end of 10 h of extracorporeal perfusion. The results are expressed as means ± SD. *p < 0.05 vs. the same group (no cell rIL-1Ra(+) or porcine AdIL-1Ra(+)) at the inlet; ‡p < 0.05 vs. porcine AdIL-1Ra(+) group in the same group (inlet or outlet); no cell AdIL-1Ra(–), no cells in the BAL device; porcine AdIL-1Ra(–), non-transfected porcine hepatocytes in the BAL device; no cell rIL-1Ra(+), BAL device without cells, but recombinant human IL-1Ra was continuously infused through a venous line; porcine AdIL-1Ra(+), porcine hepatocytes transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra, interleukin-1 receptor antagonist; BAL, bioartificial liver; ND, not detected.
Hepatic enzyme and cytokine levels 24 hours after hepatitis induction
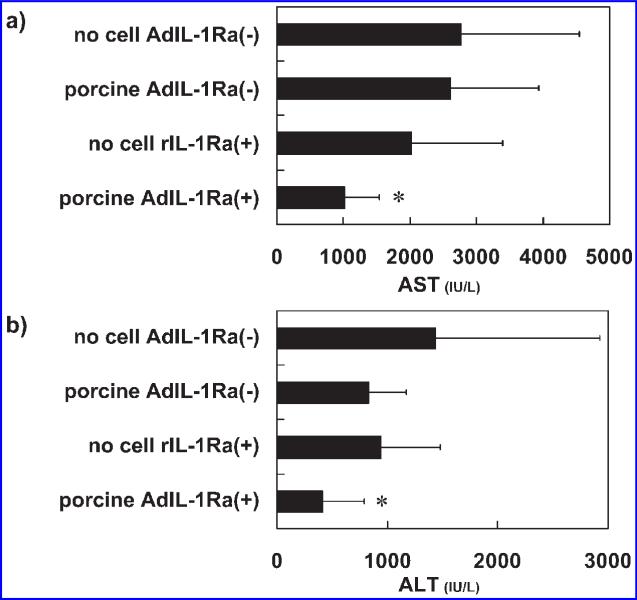
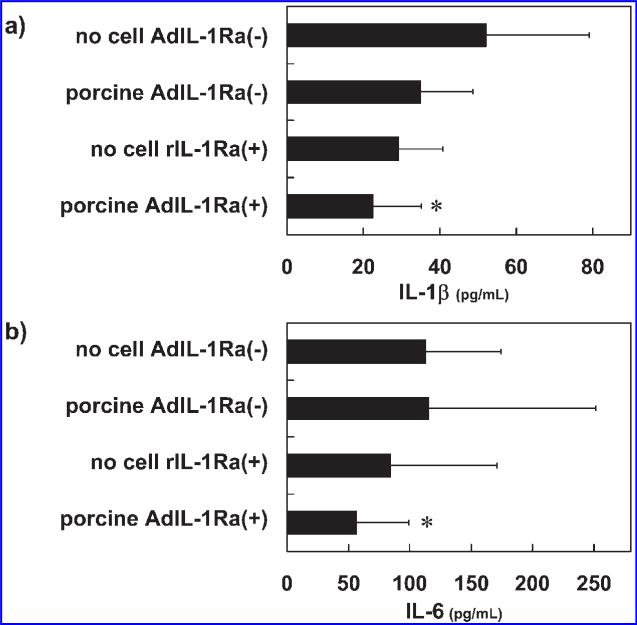
Twenty-four hours after the induction of hepatitis, plasma levels of AST and ALT were measured in the experimental animals. The plasma AST (Fig. 6a) and ALT (Fig. 6b) levels were as follows: 2774 ± 1770 and 1439 ± 1487 IU/L in no cell AdIL-1Ra(–) group, 2614 ± 1318 and 829 ± 342 IU/L in porcine AdIL-1Ra(–) group, 1018 ± 518 and 417 ± 372 IU/L in porcine AdIL-1Ra(+) group, and 2019 ± 1363 and 942 ± 597 IU/L in the no cell rIL-1Ra(+) group. There were statistically significant decreases in the plasma AST and ALT levels in the porcine AdIL-1Ra(+) group compared to those in the no cell AdIL-1Ra(–) group (p < 0.05). The plasma levels of IL-1β and IL-6 were also measured 24 h after hepatitis induction. In the porcine AdIL-1Ra(+) group, there were significant reductions in both the plasma IL-1β (Fig. 7a) and IL-6 (Fig. 7b) levels compared to the no cell AdIL-1Ra(–) group (p < 0.05).
FIG. 6.
Effect of BAL treatment on hepatic enzymes in fulminant hepatic failure rats. Aspartate aminotransferase (AST) (a) and alanine aminotransferase (ALT) (b) levels 24 h after hepatitis induction. The results are expressed as means ± SD. *p < 0.05 vs. no cell AdIL-1Ra(–) group. No cell AdIL-1Ra(–), no cells in the BAL device; porcine AdIL-1Ra(–), non-transfected porcine hepatocytes in the BAL device; no cell rIL-1Ra(+), BAL device without cells but recombinant human IL-1Ra was continuously infused through a venous line; porcine AdIL-1Ra(+), porcine hepatocytes transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra, interleukin-1 receptor antagonist; BAL, bioartificial liver.
FIG. 7.
Effect of BAL treatment on the plasma levels of IL-1β and IL-6 in fulminant hepatic failure rats. IL-1β (interleukin-1 beta) (a) and IL-6 (interleukin-6) (b) levels 24 h after hepatitis induction. The results are expressed as means ± SD. *p < 0.05 vs. no cell AdIL-1Ra(–) group. No cell AdIL-1Ra(–), no cells in the BAL device; porcine AdIL-1Ra(–), non-transfected porcine hepatocytes in the BAL device; no cell rIL-1Ra(+), BAL device without cells but recombinant human IL-1Ra was continuously infused through a venous line; porcine AdIL-1Ra(+), porcine hepatocytes transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra, interleukin-1 receptor antagonist; BAL, bioartificial liver.
Animal survival
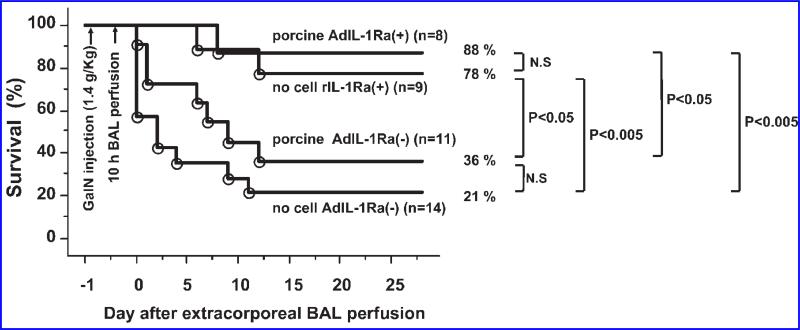
The therapeutic efficacy of treatment was evaluated by comparing animal survival in the four treatment groups for up to 28 days after the BAL perfusion. At 28 days, there were 3 of 14 (21%) animals alive in the no cell AdIL-1Ra(–) group and 4 of 11 (36%) animals alive in the porcine AdIL-1Ra(–) group (p = 0.14 vs. no cell AdIL-1Ra(–) group) (Fig. 8). In the no cell rIL-1Ra(+) group, 7 of 9 (78%) animals were alive at 28 days, which was a statistically significant increase in survival over the no cell AdIL-1Ra(–) group (p < 0.005) and the porcine AdIL-1Ra(–) group (p < 0.05). In the porcine AdIL-1Ra(+) group, 7 of 8 (88%) animals survived at 28 days, a survival increase that was statistically significant compared to the no cell AdIL-1Ra(–) group (p < 0.005) and the porcine AdIL-1Ra(–) group (p < 0.05).
FIG. 8.
Effect of BAL treatment on animal survival in fulminant hepatic failure rats. Survival curves of the no cell AdIL-1Ra(–), porcine AdIL-1Ra(–), no cell rIL-1Ra(+), and porcine AdIL-1Ra(+) groups. No cell AdIL-1Ra(–), no cells in the BAL device; porcine AdIL-1Ra(–), non-transfected porcine hepatocytes in the BAL device; no cell rIL-1Ra(+), BAL device without cells but recombinant human IL-1Ra was continuously infused through a venous line; porcine AdIL-1Ra(+), porcine hepatocytes transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra, interleukin-1 receptor antagonist; GalN, D-galactosamine; BAL, bioartificial liver.
DISCUSSION
Fulminant hepatic failure (FHF) represents a devastating clinical condition that is associated with high morbidity and mortality. Despite advanced medical management, treatment options are very limited. The only available proven therapy is liver transplantation. As a means of assessing potential new therapies in the treatment of FHF, an appropriate animal model is important. GalN is a hepatotoxin that has been used in many FHF animal models. Its mechanism of action is depletion of intracellular uridine, which blocks transcription. Reduced protein synthesis results in hepatocyte apoptosis and necrosis.19 It has also been suggested that GalN enhances intestinal endotoxins.20 In our prior work using a GalN-induced FHF rat model, we showed that IL-1β and TNF-α are involved in the pathogenesis of FHF.18 More recently, as a means of blocking inflammation mediated by IL-1, we showed that portal vein injection of AdIL-1Ra into rats with GalN-induced FHF produced significant reductions in the plasma levels of hepatic enzymes (AST and ALT), suggesting that IL-1Ra is beneficial for the treatment of FHF (unpublished data). Transfecting primary rat hepatocytes or immortalized human hepatocytes with AdIL-1Ra and incorporating these cells into a flat-plate BAL device also demonstrated improved survival in the GalN-induced FHF rats receiving extracorporeal perfusion with the device containing the transfected cells. In the present study, the overall goals were to determine the functionality of AdIL-1Ra transfected porcine hepatocytes and to evaluate the therapeutic efficacy of extra-corporeal perfusion with a BAL containing AdIL-1Ra transfected porcine hepatocytes in the GalN-induced FHF rat model. Porcine hepatocytes were successfully transfected with the human IL-1Ra gene using an adenoviral vector. Transfection at MOI 125 resulted in the greatest production of human IL-1Ra from the transfected hepatocytes. Incorporating the transfected porcine hepatocytes into the BAL device and testing in the GalN-induced FHF rat model resulted in significant reductions in the plasma levels of AST, ALT, IL-1β, and IL-6 and significant improvements in animal survival rates in the treated animals compared to the those in the control group. These results suggest that porcine hepatocytes producing human IL-1Ra are a promising cell source for BAL devices in the treatment of FHF.
Important considerations when choosing a source of hepatocytes for a BAL device are cell availability and cell immunogenicity. Human hepatocytes would be the ideal choice since they produce human proteins. However, the shortage of human donor livers limits this option. Porcine hepatocytes, anatomically and physiologically similar to human hepatocytes, and readily available, produce many important liver-specific functions, including detoxification.21–23 While swine are most often considered the source species for xenografts, there is the concern of transmission of pathogens from pigs to humans.24 Although a family of porcine endogenous retroviruses (PERV) has been identified in swine that are capable of infecting human cells in vitro,25,26 to date, there is no evidence of infection of human cells in vivo, and no disease has been described in humans resulting from this family of viruses.27–31
In our prior study, the urea and albumin synthesis rates of primary rat hepatocytes or immortalized human hepatocytes remained stable and were not significantly different when compared to the non-transfected cells throughout 4 days of culture after AdIL-1Ra transfection at MOI 125 (unpublished data). In the present study, the urea synthesis rate of porcine hepatocytes transfected at MOI 125 decreased starting on day 3 after transfection, and the albumin synthesis rate decreased starting on day 1 following transfection. Decreased urea synthesis rates (albumin synthesis rates were not measured) were also seen in the AdLacZ transfected hepatocytes (data not shown). Although transfecting the porcine hepatocytes at MOI of 125 resulted in secretion of the highest levels of human IL-1Ra on day 1 after transfection compared to other MOI tested, it is possible that transfecting at a lower MOI would result in more stable urea and albumin synthesis rates. This effect was reported in baculovirus transfected primary rat hepatocytes, where a dose-dependent decrease in albumin mRNA expression levels occurred from MOI 100 to 300.32 At MOI 10, no decreases in albumin expression were reported.
As a means of delivering human IL-1Ra to the rats with GalN-induced FHF, AdIL-1Ra transfected porcine hepatocytes were cultured in our flat-plate BAL device with an internal membrane oxygenator, and a 10 h extracorporeal perfusion was performed on the animals. We chose the 10 h extracorporeal perfusion time based on our prior study, which showed the therapeutic efficacy of our flat-plate BAL device containing non-genetically modified porcine hepatocytes in treating GalN-induced FHF in a rat model.17 In the present study, the levels of human IL-1Ra were measured at the inlet and outlet of the BAL device at the end of 10 h of perfusion. For the porcine AdIL-1Ra(+) group, the outlet IL-1Ra concentration was significantly higher than the inlet concentration. This difference between the inlet and outlet levels of human IL-1Ra could be due to the short half-life (~3 min) of IL-1Ra in plasma,33 dilutional effects, and/or consumption within the pig body. For the no cell rIL-1Ra(+) group, the inlet IL-1Ra concentration was significantly higher than the outlet concentration. This decreased concentration at the outlet would be expected since the residence time in the BAL device is 60 min (i.e., BAL device volume of 6 mL and flow rate of 0.1 mL/min) and the half-life of IL-1Ra in plasma is 3 min.33
In the rats treated with the BAL device containing the transfected porcine hepatocytes (porcine AdIL-1Ra(+) group), there were significant decreases in the plasma levels of hepatic enzymes (AST and ALT) compared to the control group, indicating a therapeutic benefit in the treatment of FHF. There were also significant decreases in the plasma levels of IL-1β and IL-6 in this treatment group. In human monocytes, IL-1Ra has been reported to block the production of IL-1: 50% inhibition at equimolar ratios and complete inhibition at a 10-fold molar excess of IL-1Ra to IL-1.34 In a rat ischemia-reper-fusion model, pretreatment with IL-1Ra gene delivery into the liver has been shown to attenuate serum levels of IL-6.14
One of the most important parameters for assessing the therapeutic efficacy of a BAL device is animal survival. In the present study, significant improvements in the animal survival rate were seen on day 28 in the porcine AdIL-1Ra(+) group and in the no cell rIL-1Ra(+) group compared to the no cell AdIL-1Ra(–) group or the porcine AdIL-1R(–) group. Although not reaching statistical significance, a greater percentage of animals in the porcine AdIL-1Ra(+) group were alive at 28 days compared to the no cell rIL-1Ra(+) group. In comparing these two treatment groups (i.e., porcine AdIL-1R(+) group and no cell rIL-1Ra(+) group), it is important to realize that they are not necessarily equivalent. The plasma levels of human IL-1Ra in animals in the no cell rIL-1Ra(+) group, as measured at the inlet of the BAL device, were significantly higher than the plasma levels of human IL-1Ra in animals in the porcine AdIL-1Ra(+) group, therefore, suggesting that there may be different ways of treating acute liver failure. In all cases there may be room for improvement; for example, no studies were conducted to optimize hepatocyte number, so conceivably, a larger cell mass could be even more therapeutic.
In conclusion, the results of this study demonstrate the therapeutic efficacy of incorporating human IL-1Ra producing porcine hepatocytes into a BAL device for the treatment of GalN-induced FHF in a rat model. There were significant reductions in the plasma levels of hepatic enzymes (AST and ALT) and inflammatory cytokines (IL-1 and IL-6), as well as a significant improvement in animal survival in the group receiving treatment with the BAL device containing the AdIL-1Ra transfected porcine hepatocytes. It is our belief that this approach of combining inflammatory cytokine blockade with a functional BAL device will be an effective therapeutic option in the treatment of FHF. Further preclinical trials will help to determine the therapeutic value of such an approach.
This article has been cited by:
1. François Berthiaume, Timothy J. Maguire, Martin L. Yarmush. 2011. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annual Review of Chemical and Biomolecular Engineering 2:1, 403-430. [CrossRef]
2. Kiminori Takano, Masahiro Shinoda, Minoru Tanabe, Taku Miyasho, Shingo Yamada, Shigeshi Ono, Yohei Masugi, Koichi Suda, Koichi Fukunaga, Tetsu Hayashida, Taizo Hibi, Hideaki Obara, Hiroya Takeuchi, Shigeyuki Kawachi, Kazufumi Kawasako, Minoru Okamoto, Hiroshi Yokota, Ikuro Maruyama, Yuko Kitagawa. 2010. Protective Effect of High-Mobility Group Box 1 Blockade on Acute Liver Failure in Rats. Shock 34:6, 573-579. [CrossRef]
3. Hiroshi Yagi , Biju Parekkadan , Kazuhiro Suganuma , Alejandro Soto-Gutierrez , Ronald G. Tompkins , Arno W. Tilles , Martin L. Yarmush . 2009. Long-Term Superior Performance of a Stem Cell/Hepatocyte Device for the Treatment of Acute Liver FailureLong-Term Superior Performance of a Stem Cell/Hepatocyte Device for the Treatment of Acute Liver Failure. Tissue Engineering Part A 15:11, 3377-3388. [Abstract] [Full Text] [PDF] [PDF Plus]
4. Eleni Mylona, Styliani Golfinopoulou, Michael Samarkos, Panagiotis Fanourgiakis, Vasilios Papadakos, Athanasios Skoutelis. 2008. Acute hepatitis in adult Still's disease during corticosteroid treatment successfully treated with anakinra. Clinical Rheumatology 27:5, 659-661. [CrossRef]
5. Tim Maguire, Alexander E. Davidovich, Eric J. Wallenstein, Eric Novik, Nripen Sharma, Henrik Pedersen, Ioannis P. Androulakis, Rene Schloss, Martin Yarmush. 2007. Control of hepatic differentiation via cellular aggregation in an alginate microenvironment. Biotechnology and Bioengineering 98:3, 631-644. [CrossRef]
ACKNOWLEDGMENTS
This work was partially supported by grants from Shriners Hospitals for Children, The Whitaker Foundation (Grant no. RG-01-0220), and National Institutes of Health (Contract grant no. DK43371). The authors would like to thank Dr. Saito (University of Tokyo) for kindly providing the adenoviral vector containing LacZ (Ad-LacZ) and Amgen for supplying the recombinant human IL-1Ra.
REFERENCES
- 1.Bismuth H, Samuel D, Castaing D, Adam R, Saliba F, Johann M, Azoulay D, Ducot B, Chiche L. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann. Surg. 1995;222:109. doi: 10.1097/00000658-199508000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72. doi: 10.1016/s0140-6736(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 3.Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin. Exp. Immunol. 1994;98:71. doi: 10.1111/j.1365-2249.1994.tb06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wigmore SJ, Walsh TS, Lee A, Ross JA. Proinflammatory cytokine release and mediation of the acute phase protein response in fulminant hepatic failure. Intensive Care Med. 1998;24:224. doi: 10.1007/s001340050554. [DOI] [PubMed] [Google Scholar]
- 5.Izumi S, Hughes RD, Langley PG, Pernambuco JR, Williams R. Extent of the acute phase response in fulminant hepatic failure. Gut. 1994;35:982. doi: 10.1136/gut.35.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yumoto E, Higashi T, Nouso K, Nakatsukasa H, Fujiwara K, Hanafusa T, Yumoto Y, Tanimoto T, Kurimoto M, Tanaka N, Tsuji T. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J. Gastroenterol. Hepatol. 2002;17:285. doi: 10.1046/j.1440-1746.2002.02690.x. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 2000;343:732. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 8.Shito M, Wakabayashi G, Ueda M, Shimazu M, Shirasugi N, Endo M, Mukai M, Kitajima M. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation. 1997;63:143. doi: 10.1097/00007890-199701150-00026. [DOI] [PubMed] [Google Scholar]
- 9.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 10.Dunn JC, Tompkins RG, Yarmush ML. Longterm in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol. Prog. 1991;7:237. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 11.Carter DB, Deibel MR, Jr., Dunn CJ, Tomich CS, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF, McEwan RN, Harris PKW, Yem AW, Waszak GA, Chosay JG, Sieu LC, Hardee MM, Zurcher-Neely HA, Readon IM, Heinrikson RL, Truesdell SE, Shelly JA, Eessalu TE, Taylor BM, Tracey DE. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg SP, Evans RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, Thompson RC. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 13.Sumitomo M, Tachibana M, Murai M, Hayakawa M, Nakamura H, Takayanagi A, Shimizu N. Overexpression of IL-1ra gene up-regulates interleukin-1beta converting enzyme (ICE) gene expression: possible mechanism underlying IL-1beta-resistance of cancer cells. Br. J. Cancer. 1999;81:277. doi: 10.1038/sj.bjc.6690688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada H, Wakabayashi G, Takayanagi A, Shimazu M, Matsumoto K, Obara H, Shimizu N, Kitajima M. Transfer of the interleukin-1 receptor antagonist gene into rat liver abrogates hepatic ischemia-reperfusion injury. Transplantation. 2002;74:1434. doi: 10.1097/00007890-200211270-00016. [DOI] [PubMed] [Google Scholar]
- 15.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl. Acad. Sci. USA. 1996;93:1320. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shito M, Kim NH, Baskaran H, Tilles AW, Tompkins RG, Yarmush ML, Toner M. In vitro and in vivo evaluation of albumin synthesis rate of porcine hepatocytes in a flat-plate bioreactor. Artif. Organs. 2001;25:571. doi: 10.1046/j.1525-1594.2001.025007571.x. [DOI] [PubMed] [Google Scholar]
- 17.Shito M, Tilles AW, Tompkins RG, Yarmush ML, Toner M. Efficacy of an extracorporeal flat-plate bioartificial liver in treating fulminant hepatic failure. J. Surg. Res. 2003;111:53. doi: 10.1016/s0022-4804(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 18.Shito M, Balis UJ, Tompkins RG, Yarmush ML, Toner M. A fulminant hepatic failure model in the rat: involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig. Dis. Sci. 2001;46:1700. doi: 10.1023/a:1010653504568. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N, Ishizuya T, Mori N. In-vitro preparation of experimental models of hepatitis with D-galactosamine and their modification by liver-repairing factors. Int. J. Tissue React. 1990;12:263. [PubMed] [Google Scholar]
- 20.Galun E, Axelrod JH. The role of cytokines in liver failure and regeneration: potential new molecular therapies. Biochim. Biophys. Acta. 2002;1592:345. doi: 10.1016/s0167-4889(02)00326-9. [DOI] [PubMed] [Google Scholar]
- 21.Desille M, Corcos L, L'Helgoualc'h A, Fremond B, Campion JP, Guillouzo A, Clement B. Detoxifying activity in pig livers and hepatocytes intended for xenotherapy. Transplantation. 1999;68:1437. doi: 10.1097/00007890-199911270-00002. [DOI] [PubMed] [Google Scholar]
- 22.Behnia K, Bhatia S, Jastromb N, Balis U, Sullivan S, Yarmush ML, Toner M. Xenobiotic metabolism by cultured primary porcine hepatocytes. Tissue Eng. 2000;6:467. doi: 10.1089/107632700750022125. [DOI] [PubMed] [Google Scholar]
- 23.Gregory PG, Connolly CK, Toner M, Sullivan SJ. In vitro characterization of porcine hepatocyte function. Cell Transplant. 2000;9:1. doi: 10.1177/096368970000900101. [DOI] [PubMed] [Google Scholar]
- 24.Fishman JA, Patience C. Xenotransplantation: infectious risk revisited. Am. J. Transplant. 2004;4:1383. doi: 10.1111/j.1600-6143.2004.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi Y, Patience C, Magre S, Weiss RA, Banerjee PT, Le Tissier P, Stoye JP. Host range and interference studies of three classes of pig endogenous retrovirus. J. Virol. 1998;72:9986. doi: 10.1128/jvi.72.12.9986-9991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 1997;3:282. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 27.Martin U, Winkler ME, Id M, Radeke H, Arseniev L, Takeuchi Y, Simon AR, Patience C, Haverich A, Steinhoff G. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation. 2000;7:138. doi: 10.1034/j.1399-3089.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 28.Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 29.Patience C, Patton GS, Takeuchi Y, Weiss RA, McClure MO, Rydberg L, Breimer ME. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 1998;352:699. doi: 10.1016/S0140-6736(98)04369-4. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham DA, Herring C, Fernandez-Suarez XM, Whittam AJ, Paradis K, Langford GA. Analysis of patients treated with living pig tissue for evidence of infection by porcine endogenous retroviruses. Trends Cardiovasc. Med. 2001;11:190. doi: 10.1016/s1050-1738(01)00104-9. [DOI] [PubMed] [Google Scholar]
- 31.Heneine W, Switzer WM, Soucie JM, Evatt BL, Shanmugam V, Rosales GV, Matthews A, Sandstrom P, Folks TM. Evidence of porcine endogenous retroviruses in porcine factor VIII and evaluation of transmission to recipients with hemophilia. J. Infect. Dis. 2001;183:648. doi: 10.1086/318540. [DOI] [PubMed] [Google Scholar]
- 32.Beck NB, Sidhu JS, Omiecinski CJ. Baculovirus vectors repress phenobarbital-mediated gene induction and stimulate cytokine expression in primary cultures of rat hepatocytes. Gene Ther. 2000;7:1274. doi: 10.1038/sj.gt.3301246. [DOI] [PubMed] [Google Scholar]
- 33.Kim DC, Reitz B, Carmichael DF, Bloedow DC. Kidney as a major clearance organ for recombinant human interleukin-1 receptor antagonist. J. Pharm. Sci. 1995;84:575. doi: 10.1002/jps.2600840511. [DOI] [PubMed] [Google Scholar]
- 34.Granowitz EV, Clark BD, Vannier E, Callahan MV, Dinarello CA. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood. 1992;79:2356. [PubMed] [Google Scholar]