Abstract
We have previously shown that the suppressive function of regulatory T cells (Tregs) from peripheral blood mononuclear cells (PBMCs) is enhanced in patients with prostate cancer when compared with healthy individuals. Two phase II studies using the PSA-TRICOM vaccine in patients with metastatic castration-resistant prostate cancer (mCRPC) showed evidence of patient benefit in terms of enhanced survival. The Halabi nomogram has been used to predict survival (HPS) of patients with mCRPC treated with conventional chemotherapy or second-line hormonal therapy. Tregs from PBMCs of patients (n = 23) with mCRPC were obtained pre- and post-three monthly vaccinations, and analyzed for number, phenotype, and suppressive function. Changes post- versus pre-vaccination in these parameters were compared with 3-year survival and HPS. No differences in Treg numbers were observed post- versus pre-vaccination. Trends (P = 0.029) were observed between overall survival (OS) and a decrease in Treg suppressive function post- versus pre-vaccination. Trends were also observed in analyzing effector:Treg (CD4+CD25+CD127−FoxP3+CTLA4+) ratio post- versus pre-vaccination with OS versus HPS. These data provide preliminary evidence for a possible association between improved OS and a decrease in Treg function when PBMCs are analyzed after three monthly vaccinations. Patients with an OS > HPS were more likely to have decreased Treg function following vaccine. Larger studies to confirm and extend these findings are warranted.
Keywords: Cancer vaccine, Prostate cancer, Immunotherapy, Regulatory T cells, Vaccination
Introduction
Prostate cancer (PCa) is the most common malignancy in men in USA and a leading cause of cancer death in North America. The American Cancer Society estimates that 217,730 men in USA are diagnosed yearly, resulting in approximately 32,050 deaths [1]. Treatment for localized disease includes radical prostatectomy or radiation therapy, but approximately 30% of patients will subsequently develop rising prostate-specific antigen (PSA). While androgen-deprivation therapy can control the disease temporarily, eventually the majority of patients develop metastatic castration-resistant disease. Docetaxel-based therapy has been shown to improve survival by approximately 2–3 months in this setting [2]. Immunotherapeutic approaches using peptide- or dendritic cell-based vaccines and poxviral-based vaccines have shown evidence of clinical benefit [3]. A recently reported phase III trial of sipuleucel-T vaccine in patients with metastatic castration-resistant PCa (mCRPC) was the first trial with overall survival (OS) as the primary endpoint to demonstrate a statistically significant improvement in OS with a cell-based therapeutic vaccine [4]. This trial has led to the FDA approval of spiuleucel-T (Provenge®) for the therapy of metastatic prostate cancer. A 43-center phase II randomized controlled trial of a poxviral-based PSA-targeted vaccine (PSA-TRICOM) in mCRPC also demonstrated an improvement in median OS of 8.5 months in this patient population [5]. These data provide additional evidence that therapeutic vaccines can lead to improved OS.
CD4+CD25highFoxP3+ regulatory T cells (Tregs) play an important role in immune homeostasis because of their ability to suppress the activation of T cells. An increase in the number or functionality of Tregs could thus favor tumor development. Increased levels of CD4+CD25highFoxP3+ Tregs have been detected in the peripheral blood mononuclear cells (PBMCs), tumor microenvironment, and draining lymph nodes of patients with PCa [6–8] and other solid tumors [9–21], as well as hematologic malignancies [22–24]. Clinical studies have demonstrated that Tregs can inhibit both antigen-specific and non-specific T-cell responses [25, 26], and that an increase in FoxP3+ Tregs is associated with high-risk factors [27–29]. Depletion of human Tregs with a recombinant interleukin (IL)-2 diphtheria toxin conjugated as DAB389IL-2 (denileukin diftitox; ONTAK) in carcinoma patients whose tumors expressed carcinoembryonic antigen (CEA) specifically enhanced antigen-specific immune responses to a dendritic cell-based cancer vaccine [30]. Another study in patients with ovarian cancer showed a direct correlation between tumor-infiltrating Tregs and OS. In that study, treatment with denileukin diftitox led to depletion of Tregs and improved antitumor responses [31]. Results of other studies have also suggested that CD4+CD25highFoxP3+ Tregs could reduce the efficacy of cancer immunotherapy [32], and that depletion of Tregs could augment vaccine-mediated antitumor immune responses [20]. In a recent study [7], we reported that although numbers of CD4+CD25highFoxP3+ Tregs in the PBMCs of patients with PCa were not significantly increased as compared to healthy donors, their suppressive function was significantly higher than Tregs from healthy donors.
Several investigators have reported that CTLA-4 plays an essential role in the function of Tregs [33, 34]. In a recent report, Miyara et al. [35] demonstrated that human FoxP3+CD4+ T cells can be separated into three populations: FoxP3lowCD45RA+, FoxP3highCD45RA−CD25+++, and FoxP3lowCD45RA−CD25++. FoxP3highCD45RA−CD25+++ cells express higher levels of intracellular CTLA-4, HLA-DR, and inducible co-stimulator (ICOS). CTLA-4 is a pivotal negative regulator of T-cell signaling in conventional T cells, but not much is known about how CTLA-4 modulates T-cell signaling in Tregs. A recent report demonstrated that treatment with anti-CTLA-4 antibody does not deplete CD4+FoxP3 Tregs, but expands effector T cells as well as functional Tregs in humans [36]. These results suggest that, similar to its action on effector T cells, CTLA-4 may inhibit Treg proliferation [36, 37].
The Halabi nomogram [38] has been shown to predict the survival (HPS) of over 1,100 patients with mCRPC who have been treated with chemotherapy or second-line hormonal therapy; the nomogram is based on seven baseline disease characteristics: visceral disease, Gleason score, performance status, baseline PSA, LDH, alkaline phosphatase, and hemoglobin. In a clinical trial at the National Cancer Institute (NCI), mCRPC patients were treated with a poxviral-based vaccine containing the transgenes for PSA and three costimulatory molecules (PSA-TRICOM) [39]. Vaccinated patients with a HPS >18 months had a significant improvement in OS above the HPS. Twelve of 15 patients with an HPS ≥18 months had an OS > HPS (P = 0.035); median OS for this group has not been reached, with 8/15 patients still alive at 37.3 months, an improvement of ≥16.4 months over the median HPS of 20.9 months. In contrast, of patients with an HPS of <18 months, an improvement of only 2.3 months over the HPS of 12.3 months was observed. In the study reported here, we evaluated the number, phenotype and function of Tregs pre- and post-vaccination, and analyzed Treg phenotype in relation to suppressive function. Results showed a correlation between improved OS over that predicted by the Halabi nomogram and a decrease in Treg function from PBMCs obtained after three PSA-TRICOM vaccinations. More comprehensive studies on larger populations will be required to validate this finding. To our knowledge, however, this is the first qualitative and quantitative analysis of Treg populations in patients with mCRPC pre- and post-vaccination.
Patients and methods
Patients
This clinical trial [39] enrolled 32 patients with mCRPC who had not received any radiotherapy or chemotherapy within 6 months prior to blood draw and 23 patients were evaluable in this analysis. Patients were given a primary vaccination with recombinant vaccinia (rV)-PSA-TRICOM, and then received monthly boosts of recombinant fowlpox (rF)-PSA-TRICOM until progression [39]. At enrollment all patients had evidence of disease progression as defined by (a) new metastatic findings on bone scan and/or (b) disease progression on computerized tomography scan, or (c) increased serum PSA as determined by PSA consensus criteria [40]. All patients signed a consent form approved by the NCI Institutional Review Board.
Collection of PBMCs
Apheresis was performed prior to vaccination and after three vaccinations (around day 85). PBMCs were isolated by Ficoll (Amersham Biosciences; Piscataway, NJ) density gradient separation, washed three times, and cryopreserved in liquid nitrogen at a concentration of 1–2 × 107 cells/mL until assayed.
Flow cytometry analysis
Cryopreserved PBMCs were analyzed by 5-color flow cytometry for phenotypic characterization of Tregs. Cells were resuspended in staining buffer (PBS containing 3% fetal bovine serum) and stained for 30 min at 4°C with FITC-conjugated anti-CD4, PECy7-conjugated anti-CD25, PE-conjugated or PerCp-Cy5.5-conjugated anti-CD127, and PE-conjugated anti-CTLA-4 (BD Biosciences; San Jose, CA, USA). FoxP3 intracellular staining was performed on cells stained with anti-CD4, anti-CD25, and anti-CD127. Cells were fixed and permeabilized using a fix/perm kit (eBioscience; San Diego, CA, USA) according to the manufacturer’s instructions, then labeled with APC-conjugated anti-FoxP3 antibody (236A/E7 clone) (eBioscience) or its isotype control antibody (eBioscience), as a negative control. Flow cytometry was done on a Becton–Dickinson LSRII (BD Biosciences); 1 × 105 cells were acquired and data were analyzed using DiVa software (BD Biosciences). To determine the percentage of Tregs, lymphocytes were gated by plotting forward versus side scatter. The CD4+ population was gated first, followed by the CD25+CD127− and FoxP3+ populations.
CD4+CD25high T-cell enrichment
CD4+CD25high T cells were enriched using a CD4+CD25+ Treg isolation kit (Miltenyi Biotec; Auburn, CA, USA), with modifications to the manufacturer’s instructions. CD4+CD25high T cells were enriched by a method described by Yokokawa et al. [7]. CD4+ T cells were negatively enriched by LD column, and positive selection for CD25+ T cells was done on the negatively selected CD4+ T cells. The CD25+ fraction was collected by eluting the cells twice through a magnetic separation column for further enrichment of CD4+CD25high T cells. In a representative experiment, 92.3% of Tregs isolated by this method were CD4+CD25high. By comparison, 93.0% of Tregs obtained by a cell-sorting procedure from PBMCs of the same donor were CD4+CD25high (data not shown), showing that Tregs isolated by MicroBeads had the same phenotypic profile as Tregs isolated by cell sorting.
Immunosuppression assay
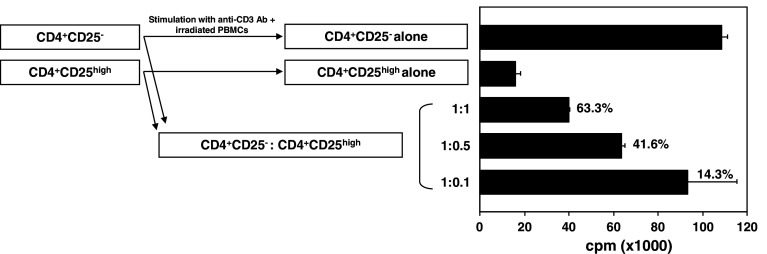
A modified version of the protocol described by Yokokawa et al. [7] was used for the immunosuppression assay. CD4+CD25− T cells (1 × 104 cells/well) were cultured alone or with CD4+CD25high T cells in three different ratios with 1 μg/mL of anti-CD3 antibody (OKT3; eBioscience) in the presence of irradiated (3,500 rad) T-depleted PBMCs (1 × 105 cells/well) in a 96-well flat-bottomed plate at 37°C and 5% CO2. Cells were cultured in RPMI 1640 (Mediatech; Manassas, VA, USA) supplemented with 10% heat-inactivated human AB serum (Gemini Bio-Products; West Sacramento, CA, USA), 100 units/mL of penicillin, 100 μg/mL of streptomycin (Mediatech), and 2 mmol/L of l-glutamine (Mediatech). Proliferation was measured by [3H]thymidine (PerkinElmer; Waltham, MA, USA) incorporation at 1 μCi (0.037 MBq) per well. Cells were pulsed on day 4 and quantified 18 h later using a liquid scintillation counter (PerkinElmer). All experiments were done in triplicate. Proliferation of CD4+CD25− T cells without co-culturing with Tregs constituted 100% proliferation.
Statistical analysis
Differences among groups were assessed using the paired Student’s t test. P < 0.05 was considered statistically significant. Spearman’s rank correlation coefficient (r) (–1.00 to +1.00) was used to calculate the correlation between two variables.
Results
Treg levels in peripheral blood
We investigated levels of Tregs in the peripheral blood of 23 patients pre- and post-vaccination with PSA-TRICOM. CD4 cells were first gated for expression of CD25+ and CD127− cells. The CD4+CD25+CD127− population was then gated for FoxP3+ cells (Fig. 1a). No significant differences in levels of CD4+CD25+CD127− FoxP3+ Tregs pre- and post-vaccination were seen among patients enrolled on study (4.6 and 4.1%, respectively; n = 23; P = 0.1) (Fig. 1b). In those patients with OS > HPS, there were no significant differences in levels of Tregs pre- and post-vaccination (4.9 and 4.3%, respectively; n = 16; P = 0.107) (Fig. 1c). In patients with OS < HPS, there were also no significant differences in levels of Tregs pre- and post-vaccination (3.8 and 3.7%, respectively; n = 7; P = 0.745) (Fig. 1d). In addition, the percentages of CD4+CD25− T cells pre- and post-vaccination were also similar between patients with OS > HPS (73.7 and 75.1%, respectively) and patients with OS < HPS (71.9 and 72.9%, respectively).
Fig. 1.
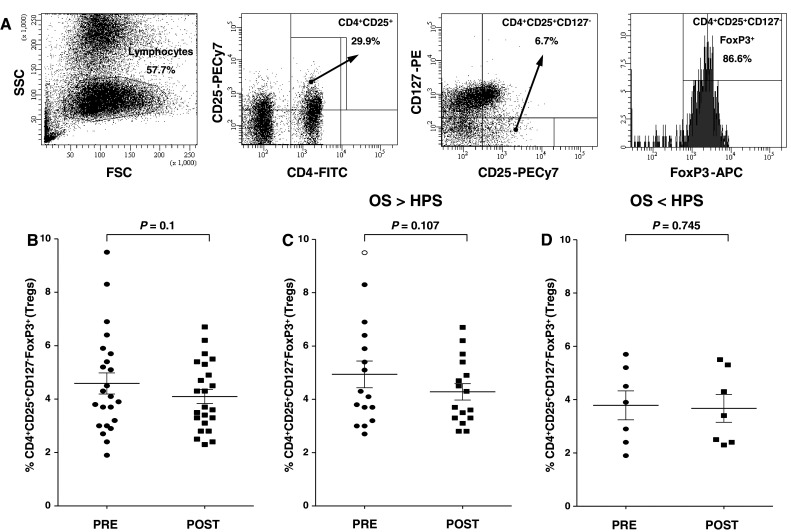
Expression of FoxP3 in CD4+CD25+CD127− Tregs in patients receiving the PSA-TRICOM vaccine. a Expression of FoxP3 in CD4+CD25+CD127− Tregs. PBMCs from a patient were analyzed by flow cytometry after cell surface staining with FITC-conjugated anti-CD4, phycoerythrin-conjugated anti-CD127, PECy7-conjugated anti-CD25, and intracellular staining with APC-conjugated anti-FoxP3. b Percentage of CD4+CD25+CD127−Foxp3+ Tregs in CD4 population pre- and post-vaccination in the peripheral blood of all patients enrolled on study. c Percentage of CD4+CD25+CD127−Foxp3+ Tregs in CD4 population pre- and post-vaccination in the peripheral blood of patients with OS > HPS. d Percentage of CD4+CD25+CD127−Foxp3+ Tregs in CD4 population pre- and post-vaccination in the peripheral blood of patients with OS < HPS. There were no significant differences in levels of Tregs pre- and post-vaccination among patients on study, patients with OS > HPS, and patients with OS < HPS (P = 0.1, P = 0.107, and P = 0.745, respectively)
Treg suppressive function
We analyzed the functionality of CD4+CD25highFoxP3+ Tregs from 23 patients pre- and post-vaccination by investigating their ability to suppress autologous CD4+CD25− T-cell proliferation upon stimulation with anti-CD3 monoclonal antibody. Figure 2 shows the functional activity of Tregs from a representative patient with OS > HPS. The suppressive activity of Tregs decreased in 12/15 patients (80%) in the OS > HPS group pre- and post-vaccination (Table 1), but only in 2/8 patients (25%) in the OS < HPS group (Table 1). For all 23 patients on study, there was a correlation between decreased suppressive activity of Tregs in PBMCs pre- and post-vaccination and OS (Spearman’s P = 0.029; r = −0.45). The suppressive activity of Tregs decreased in 9/10 patients in the longer than 30-month survival group, as compared to 5/13 patients in the shorter than 30-month survival group.
Fig. 2.
Suppression of CD4+CD25− T-cell proliferation by CD4+CD25high Tregs in a patient with PCa. Isolated effectors (CD4+CD25−) and Tregs (CD4+CD25high) were cultured alone or at three different ratios (1:1, 1:0.5, and 1:0.1) upon stimulation with anti-CD3 and irradiated T cell-depleted PBMCs. Experiments were performed in triplicate. Columns mean (cpm), bars SD. Percentages indicate level of inhibition
Table 1.
Treg suppressive activity pre- versus post-vaccination
| Patients | Patients with OS > HPS | Patients | Patients with OS < HPS | ||||
|---|---|---|---|---|---|---|---|
| % Treg suppression | ∆ Suppression | % Treg suppression | ∆ Suppression | ||||
| Pre | Post | Post − pre | Pre | Post | Post − pre | ||
| Patients with decreased suppression | Patients with decreased suppression | ||||||
| #1 | 72.1 | 56.4 | −15.7 | #2 | 65.0 | 45.0 | −20.0 |
| #3 | 83.9 | 73.0 | −10.9 | #11 | 55.8 | 30.8 | −25.0 |
| #6 | 25.7 | 24.4 | −1.3 | ||||
| #14 | 38.7 | 29.1 | −9.6 | ||||
| #15 | 76.9 | 64.2 | −12.7 | ||||
| #17 | 23.8 | 14.1 | −9.7 | ||||
| #19 | 50.6 | 40.1 | −10.5 | ||||
| #20 | 48.3 | 23.8 | −24.5 | ||||
| #23 | 66.3 | 43.0 | −23.3 | ||||
| #24 | 93.3 | 85.0 | −8.3 | ||||
| #25 | 52.9 | 22.3 | −30.6 | ||||
| #32 | 33.5 | 15.1 | −18.4 | ||||
| Mean | 55.5 | 40.9 | −14.6 | Mean | 60.4 | 37.9 | −22.5 |
| Patients with increased suppression | Patients with increased suppression | ||||||
| #5 | 37.8 | 55.3 | 17.5 | #4 | 37.0 | 55.3 | 18.3 |
| #13 | 39.0 | 59.0 | 20.0 | #8 | 40.2 | 63.0 | 22.8 |
| #26 | 63.3 | 90.6 | 27.3 | #12 | 28.4 | 39.3 | 10.9 |
| #18 | 51.7 | 64.6 | 12.9 | ||||
| #21 | 50.4 | 74.3 | 23.9 | ||||
| #28 | 61.9 | 75.0 | 13.1 | ||||
| Mean | 46.7 | 68.3 | 21.6 | Mean | 44.9 | 61.9 | 17.0 |
Results are expressed as % of Treg suppression of CD4+CD25− effector cell proliferation
We performed additional experiments to determine whether the levels of suppressive function seen in Tregs obtained from PCa patients pre- and post-vaccination were influenced by the patients’ effector T cells. Effector cells were obtained from a patient pre- and post-vaccination, then cocultured with autologous Tregs isolated post-vaccination and stimulated with anti-CD3 monoclonal antibody and autologous APCs. Suppression of proliferation of effector cells by post-vaccination Tregs was similar in the pre- and post-vaccination samples (19.2 and 20.6%, respectively, at a 1:1 effector:Treg ratio) (Fig. 3a, b). Next, effector cells from a patient pre- and post-vaccination were cocultured with Tregs isolated pre-vaccination from the same patient and stimulated with anti-CD3 monoclonal antibody and autologous APCs, with a similar outcome (40.2 and 39.4%, respectively, at a 1:1 effector:Treg ratio) (Fig. 3c, d). These results suggest that the difference in suppressive function pre- and post-vaccination was due to the difference in Treg function, not to the functional activity of effector cells.
Fig. 3.
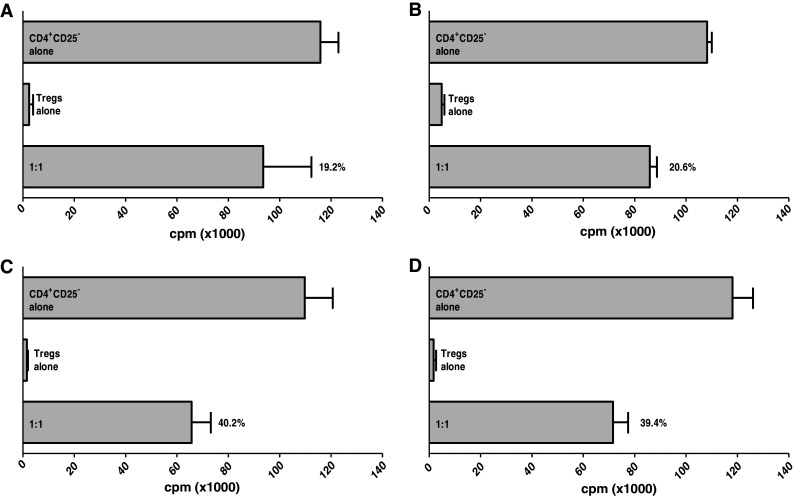
Treg suppressive function was not influenced by effector T cells. CD4+CD25− T cells from a PCa patient pre-vaccination (a) and post-vaccination (b) were cultured alone or at a 1:1 ratio with Tregs isolated from the same PCa patient post-vaccination, upon stimulation with anti-CD3 and irradiated T cell-depleted PBMCs. CD4+CD25− T cells from a PCa patient pre-vaccination (c) and post-vaccination (d) were cultured alone or at a 1:1 ratio with Tregs isolated from the same PCa patient pre-vaccination, upon stimulation with anti-CD3 and irradiated T cell-depleted PBMCs. Experiments were performed in triplicate. Columns means (cpm), bars SD. Percentages indicate level of inhibition
CTLA-4-expressing Tregs
Measuring suppressive function of Tregs is labor intensive and, more importantly, requires large-volume blood draws or leukapheresis to obtain enough cells for assay. We thus investigated if a more defined phenotype of Tregs would correlate with suppressive function and possibly OS of patients in this trial. We thus measured expression of CTLA-4 in CD4+CD25+CD127−FoxP3+ Tregs from patients pre- and post-vaccination with PSA-TRICOM. Results showed no differences in levels of CTLA-4 expression in Tregs pre- and post-vaccination among patients enrolled on study (n = 22, P = 0.380) (Fig. 4a). Results also showed no difference in the level of CTLA-4 expression in Tregs in patients with OS > HPS pre- and post-vaccination (P = 0.184) (Fig. 4b), but an apparent increase in CTLA-4 expression in Tregs from patients with OS < HPS post-vaccination (P = 0.019) (Fig. 4c). Moreover, only 4 of 11 patients (36%) with decreased suppressive functional activity had increased levels of CTLA-4+ Tregs, as compared to 7 of 8 patients (87.5%) with increased suppressive functional activity who had increased levels of CTLA-4+ Tregs (Table 2).
Fig. 4.
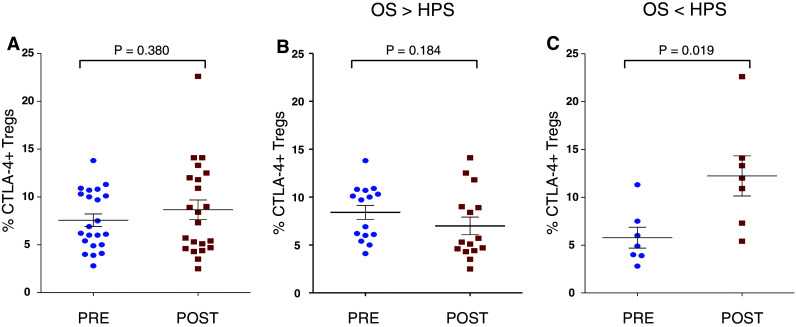
Expression of CTLA-4 on Tregs. a Levels of CTLA-4-expressing CD4+CD25+CD127−FoxP3+ Tregs in PBMCs of all PCa patients on study. No statistically significant differences were detected pre- and post-vaccination (P = 0.380). b Levels of CTLA-4-expressing CD4+CD25+CD127−FoxP3+ Tregs in PBMCs of PCa patients with OS > HPS. No statistically significant differences were detected pre- and post-vaccination (P = 0.184). c Levels of CTLA-4-expressing CD4+CD25+CD127−FoxP3+ Tregs in PBMCs of PCa patients with OS < HPS. Statistically significant differences were detected pre- and post-vaccination (P = 0.019)
Table 2.
Correlation between Treg suppressive activity pre- and post-vaccination and CTLA-4 expression on Tregs
| Patients with decreased Treg suppression | Patients with increased Treg suppression | ||||||
|---|---|---|---|---|---|---|---|
| Patients | % CTLA-4+ Tregs | Δ CTLA-4+ Tregs | Patients | % CTLA-4+ Tregs | Δ CTLA-4+ Tregs | ||
| Pre | Post | Post − pre | Pre | Post | Post − pre | ||
| Patients with decreased CTLA-4 + Tregs | Patients with decreased CTLA-4 + Tregs | ||||||
| #6 | 10.0 | 2.5 | −7.5 | #5 | 10.9 | 4.4 | −6.5 |
| #14 | 6.0 | 5.7 | −0.3 | ||||
| #15 | 9.7 | 8.9 | −0.8 | ||||
| #20 | 6.9 | 4.6 | −2.3 | ||||
| #23 | 10.7 | 5.3 | −5.4 | ||||
| #24 | 10.3 | 4.7 | −5.6 | ||||
| #32 | 5.0 | 3.5 | −1.5 | ||||
| Mean | 8.4 | 5.0 | −3.3 | Mean | 10.9 | 4.4 | −6.5 |
| Patients with increased CTLA-4 + Tregs | Patients with increased CTLA-4 + Tregs | ||||||
| #1 | 6.1 | 12.5 | 6.4 | #4 | 6.0 | 10.9 | 4.9 |
| #2 | 7.5 | 13.3 | 5.8 | #12 | 4.9 | 22.6 | 17.7 |
| #11 | 2.8 | 7.3 | 4.5 | #13 | 13.8 | 14.1 | 0.3 |
| #17 | 4.1 | 9.0 | 4.9 | #18 | 4.0 | 12.0 | 8.0 |
| #21 | 3.9 | 5.4 | 1.5 | ||||
| #26 | 10.1 | 11.8 | 1.7 | ||||
| #28 | 11.3 | 14.1 | 2.8 | ||||
| Mean | 5.1 | 10.5 | 5.4 | Mean | 7.7 | 13.0 | 5.3 |
Results are expressed as % of CTLA-4+ Tregs in CD4+CD25+CD127−FoxP3+ Tregs
We then analyzed the effector:CTLA-4+ Treg ratio in patients pre- and post-vaccination and found no differences in the effector:CTLA-4+ Treg ratio among patients enrolled on study pre- and post-vaccination (P = 0.894) (Fig. 5a). However, we found an increase in the post- versus pre-vaccination ratio in patients with OS > HPS (P = 0.029) (Fig. 5b) and a decrease in the post-vaccination ratio in patients with OS < HPS (P = 0.027) (Fig. 5c).
Fig. 5.
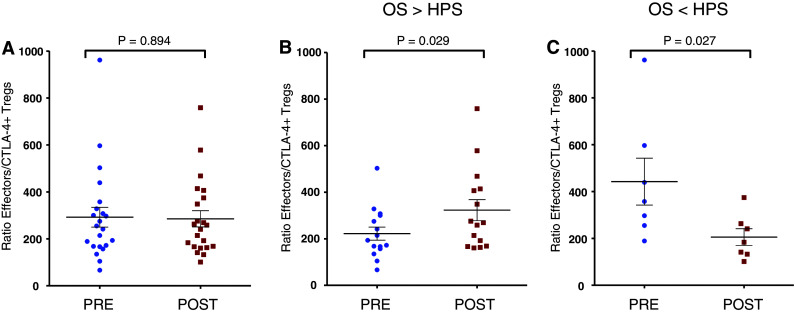
Effector:CTLA4+ Treg ratios post- versus pre-vaccination. a There were no significant differences in effector:CTLA-4+ Treg ratio pre- and post-vaccination among PCa patients enrolled on study (P = 0.894). b There was a statistically significant increase in effector:CTLA-4+ Treg ratio post-vaccination in PCa patients with OS > HPS (P = 0.029). c There was a statistically significant decrease in effector:CTLA-4+ Treg ratio post-vaccination in PCa patients with OS < HPS (P = 0.027)
Discussion
The analyses of Tregs and effector:Treg ratios reported here were carried out using PBMCs obtained from patients after three monthly vaccinations versus pre-vaccination, and results were compared with OS (median 26.6 months). While this is a retrospective analysis, and a relatively small trial, we believe the results are hypothesis generating and merit similar analyses in larger trials. If validated in such trials, one could employ these analyses to help define, early in the vaccination regimen (i.e., at 3 months), which patients are likely to benefit from further vaccination and, perhaps more importantly, which patients will most likely not benefit. These latter patients can thus go on to receive other therapies.
Tregs are heterogeneous, but are predominantly composed of two main subsets of CD4 cells: (a) thymus-derived natural CD4 Tregs (nTregs) that migrate to the periphery and (b) adaptive CD4+ Tregs (Tr1, Th3, and inducible CD4+CD25+FoxP3+) [41]. Inducible CD4+CD25+FoxP3+ Tregs (iTregs) are induced from CD4+CD25− precursors in the peripheral lymphoid organs [42]. In humans, nTregs express high levels of CD25 and the FoxP3 transcription factor [43–45]. iTregs are induced with IL-2 and transforming growth factor-β [46]. The present study investigated the levels and function of CD4+CD25+CD127−FoxP3+ Tregs in the peripheral blood of patients with mCRPC pre- and post-vaccination with a vector-driven PSA-specific vaccine. Tregs were identified as CD4+CD25+CD127−FoxP3+ (Fig. 1a). Approximately 86.6% of cells in the CD4+CD25+CD127− population were FoxP3+, which is consistent with FoxP3 expression largely confined to this population [47–49]. Studies of many different types of cancer have shown elevated levels of Tregs in PBMCs [6, 13, 16, 19, 50]. Previous studies have shown that the number of CD4+CD25+FoxP3+ Tregs correlates inversely with outcome in various types of cancers [15, 27, 51–53]. However, in most of these studies, the functional activity of Tregs was not evaluated. Furthermore, CD127 was not used as a negative surface marker for Tregs, and not all FoxP3+ T cells are functionally suppressive in humans [54]. In a previous study, we demonstrated that although levels of CD4+CD25highFoxP3+ Tregs in PBMCs of patients with PCa were not significantly higher than levels in healthy donors, Tregs in PCa patients had significantly greater suppressive functionality than Tregs from healthy donors [7]. In the absence of more specific cell surface markers for human Tregs, it is thus important to characterize human Tregs by analyzing both phenotype and inhibitory function.
In the study reported here, the functional activity of Tregs was significantly decreased in patients with OS > HPS after vaccination with PSA-TRICOM vaccine, indicating a correlation between decreased suppressive functional activity of Tregs post-vaccination and OS of these patients. To our knowledge, a trend in the correlation between Treg levels and functionality and disease outcome in patients with PCa has not been reported.
We analyzed the frequency of CTLA-4+ Tregs in PCa patients pre- and post-vaccination with PSA-TRICOM and found a significant correlation between the percentage of CTLA-4+ Tregs and functional activity. This finding agrees with other studies that have observed that CTLA-4 expression is linked to enhanced suppressive activity and higher expression of FoxP3 when compared with CTLA-4− Tregs in humans. Blockade of CTLA-4 in this subset of Tregs resulted in a significant, but not complete loss of Treg suppressive functional activity [55]. CTLA-4 may also mediate Treg suppressive activity through a direct interaction between CTLA-4 on Tregs and CD80 molecules on dendritic cells to induce tryptophan catabolism [56–58]. We investigated the CD4+CD25− effector:CTLA-4+ Treg ratio in PCa patients pre- and post-vaccination with PSA-TRICOM. Results indicate that a high effector:CTLA-4+ Treg ratio post-vaccination is associated with longer survival in most patients, and that a low effector:CTLA-4+ Treg ratio post-vaccination is associated with shorter survival.
Conclusion
This study investigated the level and functional activity of Tregs in mCRPC patients treated with PSA-TRICOM vaccine. It is the first report of a trend in correlation of Treg function and OS in mCRPC patients treated with a PCa vaccine. Results suggest that measuring Treg function may provide a useful parameter for monitoring PCa patients pre- and post-vaccination, and that measuring the frequency of CTLA-4-expressing Tregs may help to further characterize Tregs. Additional vaccine trials will be required to correlate expression of CTLA-4 in Tregs with patient survival. Overall, results suggest that protocols that combine Treg depletion strategies and vaccines may provide effective immunotherapy for PCa. These hypothesis-generating findings provide the impetus for larger validation studies looking at the prospective correlation of Treg function and clinical outcomes with PSA-TRICOM and other vaccines.
Acknowledgments
The authors thank Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript. Intramural research program of the Center for Cancer Research, National Cancer Institute, NIH.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
M. Vergati and V. Cereda contributed equally to this work.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA Cancer J Clin. 2010;60:1–24. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Arlen PM, Mohebtash M, Madan RA, Gulley JL. Promising novel immunotherapies and combinations for prostate cancer. Future Oncol. 2009;5:187–196. doi: 10.2217/14796694.5.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff P, Higano C, Shore N, Berger E, Small E, Penson D, Redfern C, Ferrari A, Dreicer R, Sims R, Xu Y, Frohlich M, Schellhammer P. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 7.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+CD25highFoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 8.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 10.Vardouli L, Lindqvist C, Vlahou K, Loskog A, Eliopoulos A. Adenovirus delivery of human CD40 ligand gene confers direct therapeutic effects on carcinomas. Cancer Gene Ther. 2009;16:848–860. doi: 10.1038/cgt.2009.31. [DOI] [PubMed] [Google Scholar]
- 11.Karagoz B, Bilgi O, Gumus M, Erikci AA, Sayan O, Turken O, Kandemir EG, Ozturk A, Yaylaci M. CD8+CD28− cells and CD4+CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol. 2010;27:29–33. doi: 10.1007/s12032-008-9165-9. [DOI] [PubMed] [Google Scholar]
- 12.Javia LR, Rosenberg SA. CD4+CD25+suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26:85–93. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 14.Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, Xue J, Zhang FM, Ge HL, Xu D. CD4(+)CD25(+)CD127(low/−) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131:109–118. doi: 10.1016/j.clim.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 18.DeLong P, Carroll RG, Henry AC, Tanaka T, Ahmad S, Leibowitz MS, Sterman DH, June CH, Albelda SM, Vonderheide RH. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther. 2005;4:342–346. doi: 10.4161/cbt.4.3.1644. [DOI] [PubMed] [Google Scholar]
- 19.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 20.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 22.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S, Hallek M, Schultze JL. Reduced frequencies and suppressive function of CD4+CD25high regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 23.Motta M, Rassenti L, Shelvin BJ, Lerner S, Kipps TJ, Keating MJ, Wierda WG. Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1788–1793. doi: 10.1038/sj.leu.2403907. [DOI] [PubMed] [Google Scholar]
- 24.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherji B, Guha A, Chakraborty NG, Sivanandham M, Nashed AL, Sporn JR, Ergin MT. Clonal analysis of cytotoxic and regulatory T cell responses against human melanoma. J Exp Med. 1989;169:1961–1976. doi: 10.1084/jem.169.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty NG, Twardzik DR, Sivanandham M, Ergin MT, Hellstrom KE, Mukherji B. Autologous melanoma-induced activation of regulatory T cells that suppress cytotoxic response. J Immunol. 1990;145:2359–2364. [PubMed] [Google Scholar]
- 27.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 28.Wolf AM, Rumpold H, Wolf D, Gastl G, Reimer D, Jenewein N, Marth C, Zeimet AG. Role of forkhead box protein 3 expression in invasive breast cancer. J Clin Oncol. 2007;25:4499–4500. doi: 10.1200/JCO.2007.13.2092. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Joshi K, Wig JD, Arora SK. Intratumoral FOXP3 expression in infiltrating breast carcinoma: its association with clinicopathologic parameters and angiogenesis. Acta Oncol. 2007;46:792–797. doi: 10.1080/02841860701233443. [DOI] [PubMed] [Google Scholar]
- 30.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 32.Antony PA, Restifo NP. Do CD4+CD25+ immunoregulatory T cells hinder tumor immunotherapy? J Immunother. 2002;25:202–206. doi: 10.1097/00002371-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/S1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 35.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Kavanagh B, O’Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, Caillat-Zucman S, Zitvogel L, Robert C. CTLA-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14:5242–5249. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 38.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, Levine EG, Blumenstein BA, Vogelzang NJ. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 39.Gulley J, Arlen P, Madan R, Tsang K, Pazdur M, Skarupa L, Jones J, Poole D, Higgins J, Hodge J, Cereda V, Steinberg S, Halabi S, Jones E, Chen C, Parnes H, Wright J, Dahut W, Schlom J. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castration-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 41.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 43.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+ CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 44.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 45.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+ CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 47.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, Bohe J, Lepape A, Ayala A, Monneret G. Increased circulating regulatory T cells [CD4(+)CD25(+)CD127(-)] contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–686. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de Fazekas St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3-expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 52.Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N, Tan B, Maraskovsky E, Miloradovic L, Hopkins W, Pan L, Venhaus R, Hoffman EW, Chen W, Cebon J. Regulatory T cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15:2166–2173. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 53.Griffiths RW, Elkord E, Gilham DE, Ramani V, Clarke N, Stern PL, Hawkins RE. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother. 2007;56:1743–1753. doi: 10.1007/s00262-007-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 55.Birebent B, Lorho R, Lechartier H, de Guibert S, Alizadeh M, Vu N, Beauplet A, Robillard N, Semana G. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34:3485–3496. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 56.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 57.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/S1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 58.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]