Abstract
Precise wiring of cortical circuits during development depends upon axon extension, guidance, and branching to appropriate targets. Motile growth cones at axon tips navigate through the nervous system by responding to molecular cues, which modulate signaling pathways within axonal growth cones. Intracellular calcium signaling has emerged as a major transducer of guidance cues but exactly how calcium signaling pathways modify the actin and microtubule cytoskeleton to evoke growth cone behaviors and axon branching is still mysterious. Axons must often pause their extension in tracts while their branches extend into targets. Some evidence suggests a competition between growth of axons and branches but the mechanisms are poorly understood. Since it is difficult to study growing axons deep within the mammalian brain, much of what we know about signaling pathways and cytoskeletal dynamics of growth cones comes from tissue culture studies, in many cases, of non-mammalian species. Consequently it is not well understood how guidance cues relevant to mammalian neural development in vivo signal to the growth cone cytoskeleton during axon outgrowth and guidance. In this review we describe our recent work in dissociated cultures of developing rodent sensorimotor cortex in the context of the current literature on molecular guidance cues, calcium signaling pathways, and cytoskeletal dynamics that regulate growth cone behaviors. A major challenge is to relate findings in tissue culture to mechanisms of cortical development in vivo. Toward this goal, we describe our recent work in cortical slices, which preserve the complex cellular and molecular environment of the mammalian brain but allow direct visualization of growth cone behaviors and calcium signaling. Findings from this work suggest that mechanisms regulating axon growth and guidance in dissociated culture neurons also underlie development of cortical connectivity in vivo.
Keywords: axon outgrowth, axon guidance, axon branching, calcium signaling, Wnt5a, CaMKII, corpus callosum, microtubules
Introduction
The development of appropriate connections is essential for the nervous system to function correctly. To achieve this motile growth cones at axon tips guide growing axons along appropriate pathways and into targets by responding to environmental guidance cues (Dickson, 2002). Thus intense interest has focused on the signaling mechanisms within the growth cone that link activation of surface guidance cue receptors to the actin and microtubule cytoskeleton that regulates growth cone motility and guidance behaviors (Dent and Gertler, 2003; Lowery and Van Vactor, 2009). It is well established, particularly in the mammalian central nervous system, that axons establish connections not only by activity of their growth cones but also by extending collateral branches from the shaft of the primary axon into the target (Kalil et al., 2000). This mechanism of interstitial branching (O’Leary et al., 1990) is especially important for the wiring of the cerebral cortex to distant targets in the spinal cord and contralateral cortex by efferent corticospinal and callosal axons. In cortical slice preparations direct visualization of cortical axons branching within the corpus callosum (Halloran and Kalil, 1994) and corticopontine tract (Bastmeyer and O’Leary, 1996) revealed that interstitial branching occurs from the axon shaft after the growth cone has extended past the target. Defining the signaling mechanisms that regulate axon growth, guidance, and branching is essential for understanding how the cerebral cortex becomes wired to appropriate targets during development. To address this question our laboratory used in vitro preparations of developing hamster sensorimotor cortex, which, in vivo, gives rise to the major efferent corticospinal and callosal pathways that contribute to motor, sensory, and cognitive functions. We identified guidance cues that promote cortical axon outgrowth, guidance, and branching, the intracellular calcium signaling pathways that evoke these axonal responses and the cytoskeletal mechanisms that regulate them. This review will focus on recent work from our laboratory and others showing how these cellular mechanisms shape the wiring of cortical connectivity during development.
Guidance Cues Promote or Inhibit Cortical Axon Branching by Cytoskeletal Reorganization
Cortical axons navigate over long distances by responses of their motile growth cones to attractive and inhibitory guidance cues in their environment. However, the growth cone at the tip of the primary axon does not typically grow directly into cortical and spinal targets. Instead branches arise interstitially from the axon shaft and then enter targets where they then form terminal arbors. Axon branching may be mechanistically linked to pausing behaviors of growth cones, which leave behind remnants on the axon at sites of future branching (Kalil et al., 2000; Dent et al., 2003). Growth cones consist of a central region which contains bundled microtubules and a thin expanded peripheral region dominated by actin filaments that form a meshwork in veil-like lamellipodia and bundles in fingerlike filopodia that protrude from highly motile lamellipodia (Figure 1). The growth cone responds to attractive cues by turning toward the source of the positive guidance cue whereas inhibitory guidance cues repel axons away from the repulsive guidance cue (Huber et al., 2003).
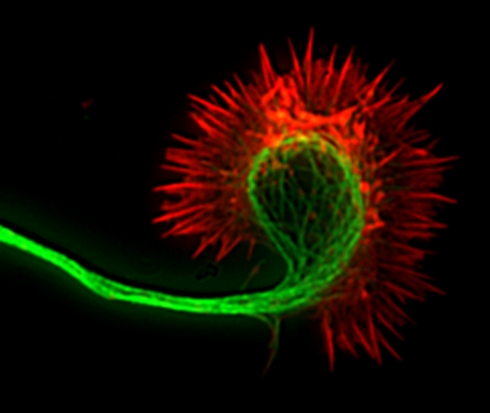
Figure 1.
Fluorescence image of a cortical axon and its growth cone. Actin filaments (indicated in red) are stained with phalloidin and occupy the lamellipodium and the spiky filopodia that protrude from its periphery. Microtubules (indicated in green) are stained with anti-tubulin antibodies and occupy the axon and central region of the growth cone. Dynamic microtubules can extend from the central region into the growth cone periphery to interact with actin filaments.
Guidance cues influence growth cone behaviors through cytoskeletal reorganization (Dent and Gertler, 2003; Kalil and Dent, 2005). Netrins, such as netrin-1, promote outgrowth of a wide variety of axons (Manitt and Kennedy, 2002) and attract growing cortical axons in explant cocultures (Metin et al., 1997; Richards et al., 1997). In contrast semaphorin 3A repels cortical axons in vitro (Bagnard et al., 1998; Polleux et al., 1998, 2000). However, little is known about effects of these attractive and repulsive guidance cues on cortical axon branching or the underlying cytoskeletal mechanisms. We therefore investigated effects of netrin-1 and semaphorin 3A on axon branching of cortical neurons in dissociated culture (Dent et al., 2004) and found that netrin-1 increased branching by almost 60%. Branches began as filopodia that grew to form stable branches within a few hours. Thus the effect of netrin-1 was to accelerate and increase branch formation. Local application of netrin-1 pulsed onto cortical axons evoked branching de novo from the axon shaft within minutes but had no effect on outgrowth of the primary axon, suggesting that axon outgrowth and branching are differentially regulated. Semaphorin 3A had opposing effects, reducing axon branching and branch length without affecting axon length. Using live cell imaging of cortical neurons microinjected with fluorescent markers for actin filaments or microtubules, we investigated the cytoskeletal mechanisms underlying increased branching by netrin-1 or decreased branching by semaphorin 3A. In growth cones and at branch points along the axon shaft of cortical neurons interactions between dynamic microtubules (i.e., those able to grow and shrink in dynamic instability) and actin filaments are necessary for branch formation (Dent and Kalil, 2001). Netrin-1 increased actin filament bundles in the growth cone and axon shaft thereby increasing the number of filopodia along the axon shaft. Dynamic splayed microtubules interacted with the newly polymerized actin bundles to initiate new directions of growth by axon branches. In growth cones treated with semaphorin 3A bundled actin filaments in the growth cone depolymerized, which reduced the motility and protrusion of filopodia and decreased microtubule exploration in the growth cone periphery. In collateral branches semaphorin 3A caused collapse of splayed microtubules and disappearance of actin bundles leading to a decrease in cortical axon branching. These results demonstrate that axon outgrowth and branching may in some cases be differentially regulated since netrin-1 and semaphorin 3A increased or decreased cortical axon branching respectively without affecting axon length. Moreover, development of axon branches involves changes at the growth cone as well as the axon shaft in cytoskeletal organization and dynamics. When actin/microtubule interactions are inhibited by guidance cues that depolymerize the cytoskeleton or inhibit their dynamics growth cones collapse and branching is inhibited.
Calcium Signaling Pathways Mediate Netrin-1 Induced Cortical Axon Branching
The intracellular mechanisms that regulate axon branching independent of axon outgrowth are not well understood. Calcium is an essential second messenger that transduces axon guidance signals to regulate growth cone motility and pathfinding (Gomez and Zheng, 2006; Wen and Zheng, 2006; Zheng and Poo, 2007). In cortical neurons spontaneous global calcium (Ca2+) fluctuations (transients) regulate axon outgrowth in a frequency dependent manner (Tang et al., 2003). Since netrin-1 induces extensive axon branching (Dent et al., 2004) and elevates Ca2+ levels in growth cones during steering events (Hong et al., 2000; Ming et al., 2002) we hypothesized that netrin-1 might promote axon branching through Ca2+ signaling events in the axon. Activation of specific signaling components is related to frequencies of Ca2+ transients. For example, calcium/calmodulin-dependent protein kinase II (CaMKII) functions as a spike-frequency detector (Hudmon and Schulman, 2002) and mitogen activated protein kinases (MAPKs) are also sensitive to intracellular Ca2+ changes (Cullen and Lockyer, 2002). Thus if repetitive Ca2+ transients are indeed involved in axon branching, CaMKII and MAPKs are likely candidates as downstream targets of Ca2+ signaling.
We investigated the role of calcium signaling in netrin-1 induced axon branching (Tang and Kalil, 2005) by loading cortical neurons with the membrane permeable calcium indicator dye Fluo-4AM, which detects changes in Ca2+ activity by changes in fluorescence intensity. Bath applied netrin-1 increased the average frequency of calcium transients fourfold either locally or globally in the axon. When netrin was withdrawn, Ca2+ activity declined to baseline levels. Reducing Ca2+ activity by blocking IP3 receptors on the endoplasmic reticulum to inhibit Ca2+ release from intracellular stores severely reduced branching. Local application of netrin-1 to the axon elicited large changes in Ca2+ in local regions of the axon. Remarkably, within minutes branches often protruded de novo from these regions of netrin-1 induced Ca2+ activity. Increased frequency of calcium transients by netrin-1 was strongly correlated with activation of CaMKII (as shown by increased phosphorylation). Whereas CaMKII inhibitors reduced cortical axon branching in the presence of netrin-1 and also reduced axon outgrowth, over expression of αCaMKII increased axon branching. Conversely, RNAi knockdown of αCaMKII prevented netrin-1 induced axon branching. MAPK over expression increased axon branching whereas MAPK inhibition prevented netrin-1 induced branching but did not affect axon length. These results demonstrate for the first time that the axon guidance cue netrin-1 evokes rapid cortical axon branching through repetitive Ca2+ transients that activate the downstream kinases CaMKII and MAPK, which are sensitive to changes in the frequency of Ca2+ transients.
How are these findings relevant to cortical axon branching by netrin-1 in vivo? Netrin-1 is not expressed in the developing rodent cortical plate. Instead, the DCC ligand netrin-4 (Qin et al., 2007) is expressed in sensorimotor cortex at P6 (Takemoto et al., 2011) when mouse layer 2–3 axons are first extending branches into this region (Wang et al., 2007). Strong netrin-1 expression is observed in the cortical plate of the developing human fetus by 14 weeks of gestation (Harter et al., 2010), the time when callosal axons cross the midline and project to the contralateral cortex (Ren et al., 2006). This suggests that netrin-1 may have a larger role in human cortical development compared to rodents. In addition, DCC-expressing cortical axons extend collateral branches into the striatum (Sheth et al., 1998), which expresses netrin-1 at high levels throughout development (Livesey and Hunt, 1997; Takemoto et al., 2011). Taken together with our results on netrin-1 induced axon branching (Dent et al., 2004; Tang and Kalil, 2005), these data strongly suggest that during embryonic development netrins may direct the extension of collateral branches into the targets of cortical projection neurons.
Calcium Transients Differentially Regulate Cortical Axon Outgrowth and Branching
In vivo and in vitro outgrowth of axons and the extension of branches are independently regulated such that branches can extend while their axons stall or retract (Luo and O’Leary, 2005). Thus, during the formation of corticospinal and callosal branches, axons have already completed their extension into efferent pathways (reviewed in Kalil et al., 2000). The mechanisms that regulate differential rates of outgrowth by axons and branches are poorly understood. One possibility, a competitive activity-dependent mechanism, regulates synaptic innervation at target sites. For example (Uesaka et al., 2006) in organotypic cocultures of thalamus and cortex neural activity enhanced the branch dynamics of thalamocortical axons that were biased toward more branch addition and elongation of branches in specific cortical target layers. The importance of electrical activity was further demonstrated in vivo where reducing neuronal activity in cortical neurons inhibited their morphological maturation including neurite elongation and branch development (Cancedda et al., 2007). Competition among neighboring axon arbors for synaptic space in the zebra fish visual system also favored arbors with higher levels of neural activity (Hua et al., 2005). Since electrical activity is reflected by changes in intracellular Ca2+ (Spitzer, 2006), we hypothesized that differences in levels of Ca2+ activity could also be a mechanism for regulating the differential growth of several processes of the same axon. This idea is supported by experiments with sympathetic neurons grown in divided chambers (Singh and Miller, 2005) which showed that one axon branch depolarized to increase Ca2+ activity grew longer at the expense of unstimulated branches in another chamber. This competition was dependent on calcium influx and downstream activation of CaMKII. However, these experiments could not distinguish between branches vs. axons from another cell and further investigation (Singh et al., 2008) suggested the involvement of neighboring axons.
To determine whether cortical neurons regulate axon outgrowth and branching by different levels of Ca2+ activity we measured spontaneous activity or manipulated calcium levels in an axon and its branches (Hutchins and Kalil, 2008). The frequencies of spontaneous Ca2+ transients were often different in a region of an axon vs. a branch or in one branch vs. another branch of the same axon (Figure 2). Higher frequency Ca2+ transients were correlated with more rapid outgrowth. Interestingly, differences in rates of outgrowth between processes of the same axon were dependent on relative differences in their Ca2+ transient frequencies. Processes with higher frequency transients were favored for growth while those with lower frequencies not only grew more slowly but retracted, suggesting a competitive effect. Moreover, the greatest differences in rates of outgrowth of two different processes of the same axon occurred when differences in frequencies were the highest. Global transients contributed relatively little to differential process outgrowth. To induce Ca2+ transients we applied BayK, which opens L-type voltage gated Ca2+ channels. BayK evoked localized calcium transients in cortical axons, which exhibited significant differences in outgrowth of processes belonging to the same axon. Over a 24-h time course both extension and retraction occurred in axons and their branches (Figure 3). Ca2+ imaging during the last 10–15 min revealed that higher frequency Ca2+ transients were associated with increased rates of outgrowth vs. association of lower frequency Ca2+ transients with retraction or slower rates of outgrowth. We also used photolysis of caged calcium to evoke repetitive transients in restricted regions of axons and their branches (Hutchins and Kalil, 2008). This approach releases caged calcium in a region restricted to a small spot (12–50 μm) and permits the choice of an optimal Ca2+ transient frequency by repetitive pulses of UV light. In most cases the stimulated process was favored for growth while unstimulated processes often retracted. Thus results from spontaneous and induced Ca2+ activity revealed similar competitive effects on the growth of one axonal process at the expense of another by differential frequencies of Ca2+ transients.
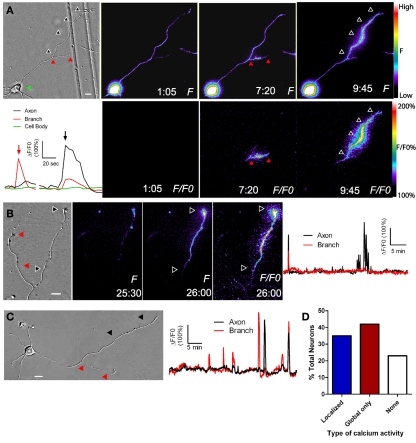
Figure 2.
Localized calcium transients occur in restricted regions of primary axons or their collateral branches. (A) DIC image at left and fluorescence images of calcium activity in a cortical neuron in which calcium transients are localized to the primary axon (at 9:45) or its collateral branch (7:20). At 1:05 there is no calcium activity. Black and red arrowheads correspond to regions of localized calcium activity. Panels F/FO show fluorescence change from baseline rather than raw fluorescence (lower panels). Pseudocolor calibration bars for images of raw fluorescence (F) and fluorescence normalized to baseline (F/FO) are shown at right. The graph at left shows calcium activity in the primary axon (black), branch (red), and cell body (green) during calcium transients shown in the fluorescence images at 7:20 (red arrow) and 9:45 (black arrow). (B) DIC image (left) and fluorescence images of localized calcium activity indicated by black arrowheads in the primary axon of a cortical neuron (right panels). As shown in the graph of calcium activity (far right), localized calcium transients occurred only in the primary axon (black), and not in the branch (red). One global calcium transient occurred at the beginning of the imaging session. (C) Both the primary axon (black arrowheads) and branch (red arrowheads) show localized calcium transients but the branch has a higher frequency (graph at right). (D) Distribution of total cortical neurons with different types of calcium activity. Times are in minutes and seconds. Scale bar, 10 μm. (Reprinted from Hutchins and Kalil, 2008; with permission from the Society for Neuroscience).
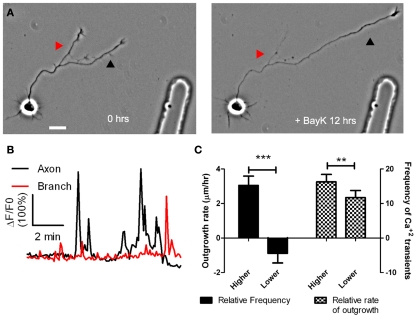
Figure 3.
BayK induced calcium transients, which persist throughout long term treatments, are correlated in their frequencies with process outgrowth. (A) Phase images of a cortical neuron treated with BayK in the bath for 12 h. At the end of the imaging session the process at right (black arrowhead) had extended, whereas the process at left (red arrowhead) retracted. (B) After phase imaging, cortical neurons were loaded with Fluo-4 and calcium activity was measured. The extending process in (A) (black) showed high levels of calcium activity compared with the retracting process (red). (C) Quantifications from 16 experiments imaging calcium activity after long term BayK treatments revealed correlations between BayK induced differential calcium activity and process outgrowth. At left comparisons between processes from the same axon showed that those with higher frequency calcium transients had higher rates of outgrowth, whereas those with lower frequencies retracted (n = 22 comparisons; ***p < 0.001, paired t test). At right processes that extended faster had higher frequencies of calcium transients (n = 26 comparisons; **p < 0.01, paired t test). Scale bar, 20 μm. (Reprinted from Hutchins and Kalil, 2008; with permission from the Society for Neuroscience).
How do results from these in vitro experiments shed light on mechanisms of cortical axon branching in vivo? Cortical axons in pathways such as the corpus callosum vs. their branches projecting into cortical target regions could encounter different guidance cues such as netrin-1 at varying concentrations that evoke different levels of Ca2+ activity. This could lead to competition among different processes of the same axon for growth such that the primary axon could stall or retract while a branch of the same axon could extend into targets. In living cortical slices (Halloran and Kalil, 1994), we observed these events directly in the developing corpus callosum. Different frequencies of Ca2+ transients could regulate the differential outgrowth of an axon vs. its branch by activation of calcium frequency dependent effectors such as CaMKII and MAPK in each axonal process. Effectors such as CaMKI and CaMKII promote neurite outgrowth (Borodinsky et al., 2003; Wayman et al., 2004, 2006) and CaMKII is preferentially activated in cortical axon branches with higher frequency Ca2+ transients (Tang and Kalil, 2005). CaMKIIβ binds to actin filaments and increases filopodial dynamics (Fink et al., 2003), consistent with the observation that direct elevation of Ca2+ activity by photolysis of caged calcium can rapidly evoke protrusion of new axonal filopodia by actin filament polymerization (Lau et al., 1999). Calcium transients of different frequencies may target different kinases and phosphatases such as CaMKII and calcineurin in the cytoplasm to evoke different cellular responses (Tomida et al., 2003). Events, such as extension and retraction of axonal processes, may be regulated by Ca2+ transients at optimal frequencies (Eshete and Fields, 2001). For rapid extension of cortical axons and branches the optimal frequencies of spontaneous and induced localized Ca2+ transients were similar across experiments (Hutchins and Kalil, 2008). Thus, one role for localized Ca2+ signaling may be to restrict Ca2+ transients at optimal frequencies to local regions of the axon. Local Ca2+ signaling would thereby regulate extension and retraction of axonal processes in response to environmental growth and guidance cues.
Although competition between different branches of a single axon has now been well-documented (Ruthel and Banker, 1999; Singh and Miller, 2005; Hutchins and Kalil, 2008), the competitive signaling mechanisms initiated by localized calcium activity remain mysterious. An early hypothesis of the mechanism of competitive axon growth proposed that actin ruffles flowing distally along the axon mediated competition among axonal processes (Ruthel and Banker, 1999). These actin-rich axon waves are important in promoting axon outgrowth and branching (Flynn et al., 2009). However, while disrupting actin dynamics with cytochalasin reduced rates of axon growth, the competition between processes remained intact (Ruthel and Hollenbeck, 2000). An alternative hypothesis that branches compete for mitochondria transported along the axon arose from the observation that mitochondria selectively invade axons showing robust growth (Morris and Hollenbeck, 1993). However, subsequent investigation showed that mitochondria follow, rather than precede, competitive outgrowth among axonal processes (Ruthel and Hollenbeck, 2003). More recently, it was shown that cAMP and cGMP promote or inhibit, respectively, axon formation and that these signals mutually inhibit one another (Shelly et al., 2010). This cAMP signaling in one neurite initiates a long-distance cGMP signal that hydrolyzes cAMP in all other neurites, leading to competition and the establishment of a single axon. Since calcium signaling can activate cAMP signaling (Gorbunova and Spitzer, 2002), one possibility is that the localized calcium transients that initiate competition among axonal processes (Hutchins and Kalil, 2008) do so through long-distance cyclic nucleotide signals. Supporting this possibility, recent experiments demonstrated that netrin-evoked calcium transients, of the type that promote growth cone advance, drive cAMP signals in the growth cone center (Nicol et al., 2011). Despite this promising avenue of research, it is still not known how such long-distance signals propagate or whether competitive outgrowth reflects competition for scarce resources or a purely signaling-based mechanism for restricting growth to certain branches. One possible mechanism for propagating long-distance signals is an inhibitory autocrine signal similar to that which mediates competition between neighboring axons (Singh et al., 2008). This mechanism could lead to signaling-based competition through mutual inhibition of growth between different branches from the same axon (Hutchins, 2010). However, another possibility is that these signaling events direct the transport of rate-limiting resources such as tubulin to the “winning” branch and thereby deprive other branches of the opportunity to grow (Butz et al., 2009; van Ooyen, 2011). The selective growth and retraction of branches is a fundamental mechanism of establishing connectivity in the mammalian CNS (McLaughlin and O’Leary, 2005). Elucidating the mechanisms of competitive axon growth will therefore be crucial for our understanding of the wiring principles of the cerebral cortex.
Wnt5a Promotes Cortical Axon Outgrowth and Repulsion by Differential Signaling
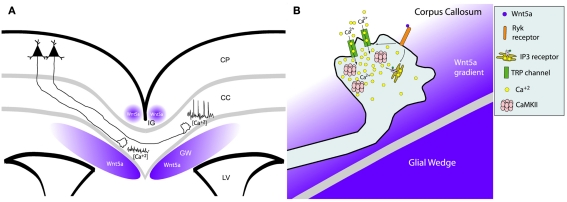
In addition to positive guidance cues that attract cortical axons and promote their branching, repulsive guidance cues that repel growing axons are equally important in shaping the formation of cortical connections. Recently, the morphogen Wnt5a was identified as an axon guidance cue (Salinas and Zou, 2008) that repels growing cortical axons during development (Zou and Lyuksyutova, 2007). Wnts play important roles in embryogenesis such as determination of cell fate, patterning, migration, and differentiation (Ciani and Salinas, 2005). Wnt5 was first identified in Drosophila as a repulsive guidance cue for commissural axons (Yoshikawa et al., 2003). In the mouse corticospinal pathway a high to low gradient of Wnt5a in vivo acts as a repulsive cue for cortical axons (Liu et al., 2005) through the Wnt5a receptor Ryk (an atypical tyrosine kinase receptor). In vitro a source of secreted Wnt5a inhibited axon outgrowth from cortical explants facing a Wnt5a gradient. Since Wnt5a is expressed in a high to low gradient surrounding the corticospinal tract, these results (Liu et al., 2005) suggested that cortical axons must navigate down the spinal cord through a high to low inhibitory Wnt5a gradient. In the embryonic mouse corpus callosum Keeble et al. (2006) also demonstrated the repulsive effects of Wnt5a on cortical axon outgrowth. In vitro axons extending from cortical explants were repelled away from a source of Wnt5a but this repulsive effect was abolished in cortex from mutant mice lacking the Ryk receptor. In vivo the corpus callosum is surrounded by a gradient of Wnt5a secreted by midline glial structures, the indusium griseum, and glial wedge (Keeble et al., 2006; Lindwall et al., 2007). In Ryk knockout mice callosal axons, which normally express Ryk receptors during development, were able to cross the midline but became misrouted after the midline and were thus unable to project away from it and formed abnormal bundles on the contralateral side. How a repulsive axon guidance cue could promote the growth of cortical axons down the spinal cord and across the corpus callosum was still a mystery.
To elucidate the mechanisms by which Wnt5a could regulate axon outgrowth of developing cortical pathways (Li et al., 2009) we bath applied Wnt5a to dissociated cortical neurons. This doubled axon outgrowth rates over 3 day in culture and in 1 h increased axon outgrowth fourfold. To determine whether a point source of Wnt5a could repel cortical axons, we used a turning assay in which axons were exposed to a gradient of Wnt5a. Control axons in BSA gradients showed no preference in turning toward or away from the BSA source. In contrast (Figure 4), axons consistently turned away from a source of Wnt5a by reorienting their growth cones in as little as 20 min. However, growth cones remained motile with no obvious collapse. Surprisingly, axons turning away from the Wnt5a source also increased their growth rates by 50%. This simultaneous repulsion and increased axon outgrowth in response to Wnt5a could explain how a Wnt5a gradient in vivo propels the extension of cortical axons by a repulsive mechanism.
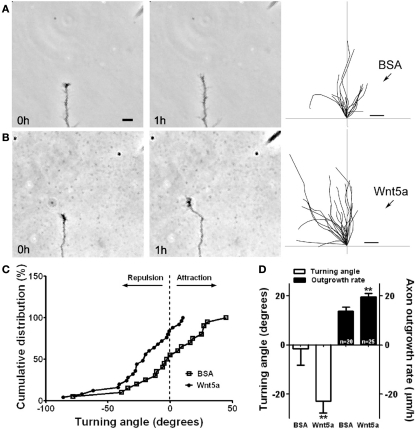
Figure 4.
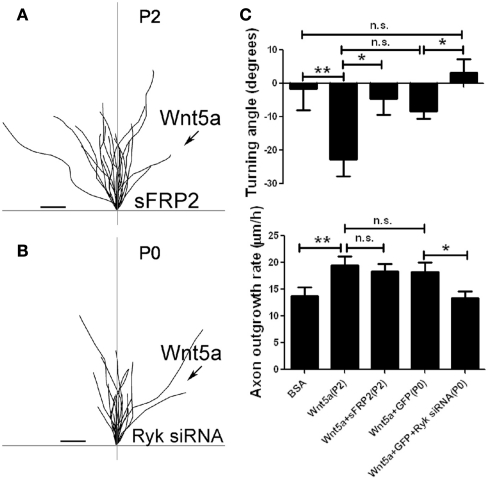
Wnt5a gradients simultaneously increase axon outgrowth and induce repulsive axon turning. (A,B) Time lapse images of a cortical axon and its growth cone at the beginning and end of a 1-h exposure to a point source of BSA (control) (A) or 10 μg/ml Wnt5a (B) emitted by the pipette at right. The axon extends straight in the BSA gradient but is repelled by the Wnt5a gradient. Right, tracings of trajectories of individual axons extending during 1 h exposure to BSA (n = 20 axons) or Wnt5a gradients (n = 25 axons) showing random turning in the presence of BSA and repulsive turning in response to Wnt5a. Note that axons repelled by Wnt5a are also significantly longer. (C) Cumulative distribution of turning angles of axons showing random turning in BSA and repulsive turning in Wnt5a gradients. (D) Bar graphs comparing average turning angles and average axon outgrowth rates in BSA and Wnt5a. In all experiments, neurons were obtained from P2 pups and cultured for 2–3 day before the turning assays. Scale bar, 5 μm. In all histograms, **p < 0.01. (Reprinted from Li et al., 2009; with permission from the Society for Neuroscience).
What signaling pathways mediate responses of cortical axons to Wnt5a? Wnts exert their effects through both Ryk and Frizzled (Fz) receptors (Salinas and Zou, 2008) which are expressed on developing hamster cortical neurons (Li et al., 2009) at P0–P3 when axons are projecting into corticospinal and callosal pathways (Reh and Kalil, 1981; Norris and Kalil, 1992). Early postnatal mouse cerebral cortex also expresses seven different Fz receptors (Shimogori et al., 2004). In Fz3 knockout mice cortical axon tracts including the corpus callosum and corticospinal pathway failed to develop (Wang et al., 2002) providing the first evidence that Fz signaling plays an important role in cortical axon development. We blocked or knocked down Ryk receptors which prevented increased cortical axon outgrowth by Wnt5a (Li et al., 2009). In contrast, blocking Wnt/Fz interactions (Rodriguez et al., 2005), did not prevent increased axon outgrowth (Figure 5). These results showed that Wnt5a increases in axon outgrowth are mediated by Ryk but not Fz receptors whereas knocking down Ryk and blocking Fz receptors prevents Wnt5a repulsive turning (Figure 5). Thus both Ryk and Fz receptors mediate repulsive cortical axon guidance by Wnt5a. Fz receptors are involved in Wnt/calcium signaling during morphogenic events and in cell motility (Kohn and Moon, 2005) but the involvement of Wnt/calcium signaling in axon growth and guidance are unknown. Further, the downstream components of Ryk signaling have not been identified. Wnt5a induced global or local Ca2+ transients involving both Ryk and Fz receptor types (Li et al., 2009). The source of intracellular calcium is important for its function (Henley and Poo, 2004; Gomez and Zheng, 2006) and Ca2+ release from intracellular stores regulates axon growth and guidance (Ooashi et al., 2005; Jacques-Fricke et al., 2006). To determine the role of this Ca2+ signaling mechanism in effects of Wnt5a we blocked IP3 receptors on the endoplasmic reticulum which blocked calcium release from intracellular stores. This partially attenuated Wnt5a induced Ca2+ activity. Calcium influx through TRP (transient receptor potential) channels also plays an important role during axon growth and guidance (Li et al., 2005; Shim et al., 2005; Wang and Poo, 2005). We therefore blocked TRP channels, which also partially inhibited Wnt5a induced calcium activity. Simultaneously blocking calcium release from stores and calcium influx through TRP channels virtually silenced Wnt5a induced calcium activity. Blocking Ca2+ release from stores prevented Wnt5a induced increase in axon outgrowth but the same axons showed repulsive turning away from the source of Wnt5a (Li et al., 2009). In contrast, blocking TRP channels prevented both increased axon outgrowth and repulsion by Wnt5a. As in netrin-1 calcium signaling (Tang and Kalil, 2005) CaMKII is an essential downstream component of Wnt5a/calcium signaling. These results suggest a model (Figure 6) in which increased axon outgrowth induced by Wnt5a involves activation of Ryk receptors, calcium release from intracellular stores and activation of CaMKII signaling. Growth cone repulsion by a gradient of Wnt5a is mediated by both Ryk and Fz receptors and requires calcium entry through TRP channels but not calcium release from intracellular stores. In support of our results that different calcium signaling mechanisms (perhaps in different regions of the growth cone) mediate Wnt5a axon growth vs. guidance behaviors, a recent study (Nicol et al., 2011) showed that in Xenopus spinal neurons different calcium and cyclic AMP signaling localized to central vs. filopodial growth cone regions mediate netrin-1 evoked axon outgrowth vs. steering. Thus Wnt5a can simultaneously activate different receptors and different calcium signaling pathways at the growth cone to elicit growth and guidance behaviors required for wiring of cortical circuits during development.
Figure 5.
Fz receptors mediate Wnt5a induced axon repulsion but not outgrowth, but Ryk receptors mediate both outgrowth and repulsion. (A) Tracings of trajectories of axons exposed to Wnt5a gradients in the presence of sFRP2 showing increased axon outgrowth but not repulsion. (B) Tracings of trajectories of P0 axons exposed to Wnt5a gradients following Ryk knockdown, which prevents increased axon outgrowth as well as repulsion by Wnt5a. (C) Bar graphs quantifying turning angles (top) and outgrowth rates (bottom) of axons in (A) and (B). Scale bar, 5 μm. In all histograms *p < 0.05, **p < 0.01; n.s., not significant. (Reprinted from Li et al., 2009; with permission from the Society for Neuroscience).
Figure 6.
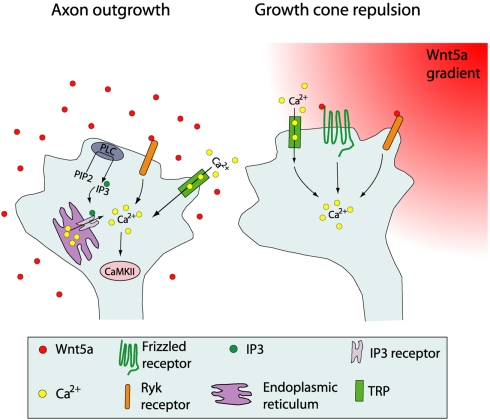
Schematic summary showing Wnt receptors and calcium signaling components involved in cortical growth cone behaviors in response to Wnt5a. At left, increased cortical axon outgrowth is induced by Wnt5a, which involves activation of Ryk receptors, calcium entry through TRP channels, calcium release from intracellular stores via IP3 receptors on the endoplasmic reticulum, and activation of PLC and CaMKII signaling. At right, growth cone repulsion by a Wnt5a gradient is mediated by both Ryk and Fz receptors and requires calcium entry through TRP channels. Symbols in the growth cone are shown in the box. (Reprinted from Li et al., 2009; with permission from the Society for Neuroscience).
Axon Growth and Guidance in the Corpus Callosum is Regulated by Wnt/Calcium Signaling
Thus far we have discussed mechanisms of axon growth and guidance in dissociated cortical neurons. However, it is important to test the relevance of these mechanisms in a more complex cellular environment that mimics development in vivo. For example, in dissociated cultures knocking down CaMKI decreased hippocampal and cortical axon elongation (Ageta-Ishihara et al., 2009; Davare et al., 2009; Neal et al., 2010) whereas knockdown of CaMKI in vivo during activity-dependent cortical wiring decreased callosal axon branching into cortex without affecting rates of callosal axon elongation (Ageta-Ishihara et al., 2009). To test the in vivo function of Wnt/calcium signaling mechanisms we used living slices of developing hamster sensorimotor cortex that contained the entire callosal pathway (Hutchins et al., 2011). This permitted live cell imaging of intact callosal axons extending along their entire trajectory (Halloran and Kalil, 1994). We electroporated plasmids, encoding the cytoplasmic fluorescent marker DsRed2, into one hemisphere of cortical slices from P0 brains and after 48 h used confocal microscopy to image fluorescently labeled callosal axons (Figure 7), which exhibited continual growth cone motility and forward advance during the 90-min imaging sessions. Importantly, axons before the midline (precrossing) advanced more slowly than those that have crossed the midline (postcrossing). To determine whether callosal axons expressed Ca2+ transients correlated with rates of axon outgrowth we electroporated a genetically encoded calcium indicator (GCaMP2) into one hemisphere and measured frequencies of Ca2+ transients in pre- and post-crossing axons for up to 30 min. (Figure 8). Calcium transients were expressed in axons and growth cones (Figure 8) in frequencies closely related to rates of callosal axon outgrowth. Thus higher frequencies of Ca2+ transients are well correlated with higher rates of callosal axon outgrowth, similar to our results for dissociated cortical neurons (Hutchins and Kalil, 2008).
Figure 7.
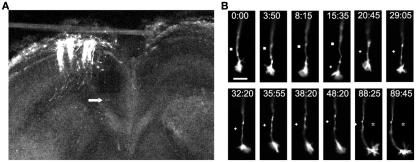
Imaging of individual callosal axons and their growth cones extending through the corpus callosum. (A) A low power confocal image of a cortical slice at 3DIV, after electroporation of cortical neurons with DsRed2 in the slice from P0 sensorimotor hamster cortex. Individual efferent axons are clearly visible. Arrow indicates location of the cortical growth cone imaged at higher power in the time lapse sequence in (B) which shows filopodia and lamellipodia of growth cones during outgrowth and turning. Times are in minutes and seconds. Scale bar, 10 μm. Symbols show reference points. (Reprinted from Hutchins et al., 2011).
Figure 8.
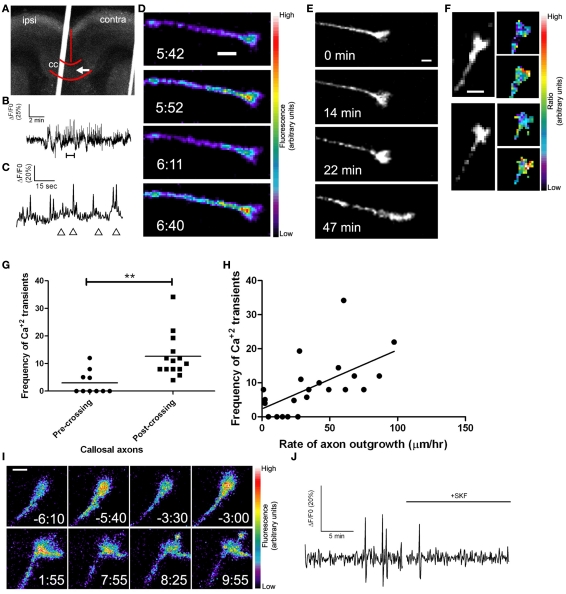
Callosal axons express spontaneous calcium transients positively correlated with rates of axon outgrowth. (A) A coronal cortical slice electroporated with GCaMP2 plasmids into the left cortex. Arrow indicates postcrossing (contra) position of the growth cone imaged in (D). Borders of the callosum (cc) and the midline are outlined in red. (B) Tracing of calcium activity measured by changes in GCaMP2 fluorescence over baseline. Calcium activity increases after a few min. (C) Tracing of calcium activity from (B) zoomed in to the time shown in the bracket. (D) Fluorescence images of the growth cone measured in (B,C) at time points indicated by the arrowheads in (C). (E) Within 20 min of the onset of calcium activity shown in (B) the axon begins to advance rapidly through the contralateral callosum. (F) Examples of single calcium transients measured by ratiometric imaging of growth cones co-expressing DsRed2 and GCaMP2. (G) Plot of frequencies of calcium transients in pre- or post-crossing callosal axons, **p < 0.01, t test. All frequencies are in transients/h. (H) Scatter plot of the frequency of calcium transients vs. the rate of axon outgrowth in individual callosal axons. The line represents the least-squares linear regression (slope significantly non-zero, p < 0.01). (I) An example of spontaneous calcium transients (top row) which are attenuated by application of SKF at time 0.00 (bottom row). (J) Tracing of calcium activity in the growth cone shown in (I) before and after application of SKF. Scale bars 10 μm except (I) which is 5 μm. Pseudocolor calibration bars indicate fluorescence intensity (D) or ratio of GCaMP2 to DsRed2 fluorescence intensities (F) in arbitrary units. (Reprinted from Hutchins et al., 2011).
The corpus callosum is surrounded by a Wnt5a gradient secreted by midline glial structures (Keeble et al., 2006). Since callosal axons express Ca2+ transients correlating with rates of axon outgrowth we reasoned that in the corpus callosum the Wnt5a gradient could mediate axon growth and guidance by calcium signaling mechanisms. To determine whether Ca2+ release from stores plays a role in regulating callosal axon outgrowth we blocked IP3 receptors (on the endoplasmic reticulum) pharmacologically to inhibit calcium release from stores. Using live cell imaging, we measured growth of postcrossing axons since a recent study (Wang et al., 2007) showed that in vivo suppression of spontaneous electrical activity in mouse callosal axons decreased rates of axon outgrowth on the postcrossing but not the precrossing side of the callosum. Treatment with blockers to IP3 receptors slowed axon outgrowth of postcrossing axons but their normal rates were restored after washout of the blocking reagent. However, blocking Ca2+ release from stores had no effect on guidance of callosal axons whose trajectories (measured from images obtained at the beginning and end of the 3-h experiments) after crossing the midline were similar to those of untreated controls. In contrast, pharmacologically blocking Ca2+ entry through TRP channels not only slowed axon outgrowth but also severely misrouted postcrossing callosal axons, which turned prematurely toward the cortical plate or turned ventrally toward the septum. We verified the involvement of calcium activity by blocking TRP channels and then measuring effects on Ca2+ transients in individual postcrossing callosal axons. As shown in Figure 8I, blocking TRP channels attenuated frequencies of Ca2+ transients to about 3 h compared with 12 h for controls, showing directly that the growth and guidance defects resulted from decreased Ca2+ activity. Furthermore, as we found in dissociated cultures, calcium signaling by store release or by Ca2+ entry through TRP channels respectively mediates axon outgrowth or both axon outgrowth and guidance in the corpus callosum.
Although these results demonstrate the requirement for calcium signaling in outgrowth and guidance of callosal axons, it was important to show the specific involvement of Wnt5a signaling. In vivo knockout mice lacking the Wnt5a Ryk receptor showed guidance errors in callosal axons only after they crossed the midline (Keeble et al., 2006). This is consistent with the finding that mouse callosal axons do not respond to Wnt5a until the age when they cross the midline (E 16) and begin to express the Ryk receptor (Keeble et al., 2006). However, these experiments did not address signaling mechanisms downstream of Ryk receptors. Therefore, we knocked down the Ryk receptor by electroporation of Ryk siRNA in a small number of axons and then analyzed effects on DsRed2 labeled callosal axons in living cortical slices (Hutchins et al., 2011). Ryk knockdown slowed the growth rate of postcrossing callosal axons to half their normal growth rate, but had no effect on precrossing axon growth rates, consistent with Ryk expression on callosal axons only after they cross the midline (Keeble et al., 2006). Importantly, Ryk knockdown also caused severe guidance errors in about a third of labeled postcrossing axons that deviated inappropriately toward the septum or prematurely turned toward the overlying cortex. Since we found previously (Li et al., 2009) that knocking down the Ryk receptor reduced the proportion of neurons expressing Ca2+ transients in response to Wnt5a, we asked whether growth and guidance defects in callosal axons in which Ryk was knocked down resulted from interference with Wnt5a evoked calcium signaling. To address this question we coelectroporated the calcium biosensor GCaMP2 with Ryk siRNA to measure calcium activity in neurons in which RyK/Wnt signaling was disrupted. In such neurons frequencies of Ca2+ transients were reduced fourfold in postcrossing axons. Remarkably, in growth cones projecting aberrantly toward the septum, Ca2+ transients were undetectable. Taken together, these results suggest that callosal axon growth and guidance errors caused by Ryk knockdown result from attenuation of calcium activity. Since CaMKII is also a component of the Wnt/calcium signaling pathway (Li et al., 2009), we investigated its role in callosal axon growth and guidance by cortical transfection of plasmids encoding an inhibitory CaMKII protein (Tang and Kalil, 2005). For postcrossing but not precrossing axons, this treatment slowed axon outgrowth by about half and caused dramatic axon guidance errors in which some axons projected aberrantly while others looped backward in the corpus callosum. Do these defects represent a failure of axon repulsion mechanisms mediated by Wnt5a? Using turning assays in which dissociated neurons were exposed to Wnt5a gradients, we found that neurons transfected with the CaMKII inhibitor were unable to increase their growth rates or show repulsive turning. These results suggest that CaMKII, downstream of Wnt/calcium signaling, is necessary for the outgrowth and repulsive guidance mechanisms by which Wnt5a regulates development of callosal axons.
Taken together these results, summarized in a cortical slice model of the developing corpus callosum (Figure 9), show that Wnt/calcium signaling pathways are essential for regulating callosal axon outgrowth and guidance. Nevertheless, additional growth and guidance factors are known to regulate development of the corpus callosum (Lindwall et al., 2007). For example, in addition to Wnt5a, sources of netrins, semaphorins, and Slit 2 surround the corpus callosum and their role in callosal axon guidance across the midline has been well characterized (Shu and Richards, 2001; Shu et al., 2003; Niquille et al., 2009; Piper et al., 2009). Although direct evidence for netrins in guiding callosal axons to the midline is lacking, callosal axons express the netrin-1 receptor DCC and netrin-1 knockout mice lack all forebrain commissural projections (Serafini et al., 1996). Slits are chemorepellent proteins expressed in glial structures adjacent to the callosal midline in a pattern similar to that of Wnt5a. Knockout mice lacking the Slit receptor Robo 1 display aberrant projections of callosal axons that are misdirected at the midline, suggesting that Slits may channel callosal axons into the correct pathway (Lindwall et al., 2007). Mutant mice whose axons are unable to bind semaphorins also show callosal malformations. However, our finding that inhibiting calcium signaling only affected growth of axons after but not before the midline suggests the involvement of Wnt5a signaling because cortical axons do not respond to Wnt5a until the age at which they cross the midline (Keeble et al., 2006). Although Slit 2 affects both pre- and post-crossing axons (Shu and Richards, 2001; Shu et al., 2003) our results show that interfering with Wnt/calcium/CaMKII signaling only affects postcrossing axon outgrowth.
Figure 9.
Wnt/calcium signaling guides axon growth through the corpus callosum. (A) Schematic of a cortical slice showing cortical axons extending through the corpus callosum (cc) surrounded by Wnt5a secreted by the indusium griseum (IG) and glial wedge (GW). Axons in the contralateral callosum have high frequencies of calcium transients evoked by Wnt5a signaling, which accelerates rates of axon outgrowth. In contrast, ipsilateral axons not yet responsive to Wnt5a, have low levels of calcium activity and thus grow more slowly. LV, lateral ventricle; CP, cortical plate. (B) Schematic of intracellular signaling in the postcrossing axon from (A) showing that Wnt5a gradient from the glial wedge activates RyK receptors to open IP3 receptors and TRP channels causing release of calcium from intracellular stores and calcium influx through the plasma membrane. These calcium transients activate CaMKII to accelerate axon extension and repel growth cones away from the glial wedge toward the contralateral cortex. (Reprinted from Hutchins et al., 2011).
Microtubule Dynamics Regulate Wnt5a Evoked Axon Outgrowth and Guidance
Growth cone behaviors are regulated by reorganization and dynamics of the actin and microtubule (MT) cytoskeleton (Dent and Gertler, 2003; Lowery and Van Vactor, 2009), which can be modulated by extracellular guidance cues (Luo, 2002; Huber et al., 2003; Kalil and Dent, 2005). Stable microtubules occupy the central region of the growth cone while dynamic MTs that grow and shrink in dynamic instability actively explore the periphery (Dent et al., 1999; Dent and Kalil, 2001; Schaefer et al., 2002, 2008; Lowery and Van Vactor, 2009). Inhibiting MT dynamics can abolish growth cone turning and localized MT stabilization and de-stabilization can induce attractive turning and repulsion (Buck and Zheng, 2002), suggesting an instructive role for MTs in axon guidance. Wnt signaling increases MT stability and Wnt3a and Wnt7a specifically decrease axon length and increase growth cone size of DRG and cerebellar mossy fiber terminals (Lucas and Salinas, 1997; Hall et al., 2000) by reorganization of MTs into stable loops (Ciani and Salinas, 2005; Salinas, 2007; Purro et al., 2008). This occurs through changes in MT directionality across the growth cone rather than toward the leading edge (Purro et al., 2008). In contrast we showed that Wnt5a increases cortical axon outgrowth and induces growth cone repulsion (Li et al., 2009) but the cytoskeletal mechanisms are unknown. Our preliminary results (Li et al., unpublished observations) suggest that reorganization of dynamic MTs plays a major role in increased axon outgrowth and repulsive growth cone turning induced by Wnt5a.
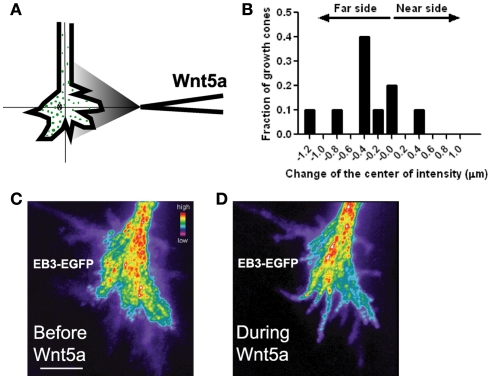
We first confirmed that dynamic MTs are required for the growth promoting and guidance effects of Wnt5a by applying nocodazole, an inhibitor of MT dynamics (Dent and Kalil, 2001), which prevented Wnt5a induced increases in axon outgrowth and repulsive turning in Wnt5a gradients. To determine the relationship between directionality of MTs and the direction of growth cone advance induced by Wnt5a, we used high resolution fluorescence (total internal reflection fluorescence, TIRF) microscopy to image growth cones of early postnatal cortical neurons transfected with an EGFP-EB3 construct to label dynamic MT plus ends and DsRed2 to label the cytoplasm. EB3 is a +TIP protein that binds to the growing plus ends of MTs (Nakagawa et al., 2000) and EGFP-EB3 has been used to image MT dynamics in neurons (Stepanova et al., 2003). Dynamic growing MT tips were visualized as EB3 “comets” in cortical growth cones before and after bath application of Wnt5a. Wnt5a oriented dynamic MTs in the direction of axon elongation, suggesting that increased axon outgrowth by Wnt5a occurs by reorientation of dynamic MTs. We then applied Wnt5a as a gradient to investigate the possible redistribution of dynamic MTs in growth cones during repulsive turning behaviors. Preliminary results (Figure 10) showed that before application of Wnt5a EB3 comets were randomly distributed throughout the growth cone but during 30 min exposure of growth cones to Wnt5a gradients EB3 comets redistributed to the far side of the growth cone away from the Wnt5a source. These results suggest that in Wnt5a gradients asymmetric redistribution of dynamic MTs may be a first step in repulsive turning.
Figure 10.
Wnt5a gradients induce redistribution and reorganization of dynamic microtubules (MTs) in growth cones. (A) Schematic of measurement of the distribution of EB3 comets on MT plus ends in the presence of a pipette gradient of Wnt5a locally applied at the right side of the growth cone. Individual EB3 comets are detected by fluorescence (green dots) and their center of intensity (cross) is determined. (B) Localization of the center of intensity of EB3 comets evoked by a Wnt5a gradient in 10 growth cones in independent experiments including the growth cone shown in (C,D). Negative numbers indicate EB3 localization to the side of the growth cone farthest from the Wnt5a source. Change of the center of intensity = −0.35 ± 0.14 μm (p < 0.05, Wilcoxon signed rank test). (C,D) Composite images of the same growth cone showing redistribution of EB3 comets over time 30 min before and during 30 min of application of the Wnt5a gradient (n = 601 images before Wnt5a and 601 images during Wnt5a application). The maximum intensity was computed for each pixel from the entire image stack by using algorithms in Metamorph. Using these values stacks of all images were superimposed and pixels assigned values in pseudocolor to indicate fluorescence intensity.
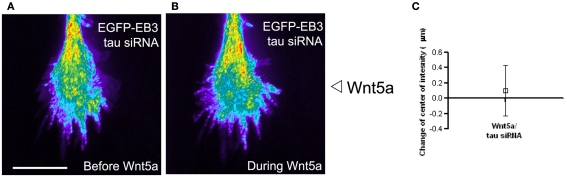
Gradients of guidance cues can evoke asymmetries in calcium activity (Gomez and Zheng, 2006) that are typically highest on the side of the growth cone facing a source of either an attractive or repulsive guidance cue gradient. Thus it was surprising to find that Wnt5a can evoke Ca2+ transients localized to the far side of the growth cone facing away from the Wnt5a source. The coincidence of MT and Ca2+ asymmetries led us to investigate a possible relationship between asymmetries in Ca2+ transients and the redistribution of dynamic MTs in growth cones exposed to Wnt5a gradients. One candidate link is the MT associated protein tau which binds to MTs to increase their stability (Dehmelt and Halpain, 2005). CaMKII phosphorylates tau at the MT binding site (Litersky et al., 1996; Sironi et al., 1998). This leads to detachment of tau from MTs and thus decreased MT stability and increased MT dynamics (Biernat et al., 1993). Furthermore, Wnt5a is known to activate CaMKII (Kuhl et al., 2000). Together these findings led us to investigate tau as a possible link between calcium signaling and dynamic MTs. To determine whether tau is required for the growth promoting effects of Wnt5a on cortical axons, we knocked down tau with siRNAs which prevented Wnt5a from increasing rates of cortical axon outgrowth. Importantly, this treatment also prevented growth cone repulsion in Wnt5a gradients. We then co-transfected cortical neurons with EGFP-EB3 and tau siRNA, and imaged dynamic MTs by following EB3 comets in growth cones. Remarkably, we found that tau knockdown prevented the redistribution of dynamic MTs toward the far side of the growth cone facing away from the Wnt5a gradient. As shown in Figure 11 dynamic MTs remain randomly distributed throughout the growth cones as in untreated controls. Since calcium activity is required for the growth and guidance effects of Wnt5a on cortical axons in vitro (Li et al., 2009) and within the corpus callosum (Hutchins et al., 2011) these results suggest a relationship, possibly mediated by tau, between Wnt5a evoked calcium signaling and the redistribution of dynamic MTs during Wnt5a evoked cortical growth cone repulsion.
Figure 11.
The microtubule associated protein tau is required for Wnt5a induced redistribution of dynamic MTs in growth cones. (A,B) Composite images showing the redistribution of EB3 comets in the same growth cone over time 30 min before and during 30 min of application of the Wnt5a gradient (n = 361 images before Wnt5a and 601 images during Wnt5a application). The maximum intensity was computed for each pixel from the entire image stack by using algorithms in Metamorph. Using these values stacks of all images were superimposed and pixels assigned values in pseudocolor to indicate fluorescence intensity. Wnt5a was locally applied as a gradient at the right side of the growth cone. In this growth cone tau was knocked down with siRNA, which prevented the asymmetric redistribution of EB3 comets during exposure of the growth cone to Wnt5a gradients. (C) Localization of the center of intensity of EB3 comets evoked by Wnt5a gradients in four growth cones in independent experiments including the growth cone shown in (A,B). Quantitative analysis shows that Wnt5a fails to induce redistribution of EB3 comets to the far side of the growth cone after tau knockdown with siRNA (change in center of intensity not different from 0. p > 0.05, Wilcoxon signed rank test).
Future Directions
This review has emphasized the central role of intracellular calcium signaling in transducing the effects of guidance molecules on growth and guidance of cortical axons during development. However, many questions remain unanswered. First, the mechanisms by which guidance cues evoke calcium transients localized to small regions of axons and growth cones are not well understood. Second, how competitive mechanisms, mediated by calcium signaling, favor the growth of one cortical axon process over another requires further study. Third, the relationship between calcium activity and cytoskeletal dynamics leading to cortical axon branching, outgrowth and guidance remains obscure. Fourth, effects of guidance cues on cytoskeletal reorganization leading to specific growth cone behaviors are only beginning to be addressed. Finally, it is not always clear that axon growth and guidance mechanisms identified in tissue culture are applicable to development in vivo. Thus a major challenge will be to elucidate how axons of neurons in the complex in vivo environment of the cerebral cortex can integrate multiple extracellular guidance cues and intracellular signaling pathways necessary for appropriate axon outgrowth, guidance, and branching during wiring of cortical circuits.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work from our laboratory was funded by National Institutes of Health Grant NSO14428 and grants from the Whitehall Foundation and the University of Wisconsin Graduate School (to Katherine Kalil), a Herman Shapiro fellowship (to Li Li), and National Research Service Award GM801642 (to Bruce Ian Hutchins). Bruce Ian Hutchins is currently supported by the Intramural Research Program of NINDS and the Pharmacology Research Associate Program of NIGMS.
References
- Ageta-Ishihara N., Takemoto-Kimura S., Nonaka M., Adachi-Morishima A., Suzuki K., Kamijo S., Fujii H., Mano T., Blaeser F., Chatila T. A., Mizuno H., Hirano T., Tagawa Y., Okuno H., Bito H. (2009). Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade. J. Neurosci. 29, 13720–13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnard D., Lohrum M., Uziel D., Puschel A. W., Bolz J. (1998). Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development 125, 5043–5053 [DOI] [PubMed] [Google Scholar]
- Bastmeyer M., O’Leary D. D. (1996). Dynamics of target recognition by interstitial axon branching along developing cortical axons. J. Neurosci. 16, 1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J., Gustke N., Drewes G., Mandelkow E. M., Mandelkow E. (1993). Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11, 153–163 10.1016/0896-6273(93)90279-Z [DOI] [PubMed] [Google Scholar]
- Borodinsky L. N., O’Leary D., Neale J. H., Vicini S., Coso O. A., Fiszman M. L. (2003). GABA-induced neurite outgrowth of cerebellar granule cells is mediated by GABA(A) receptor activation, calcium influx and CaMKII and erk1/2 pathways. J. Neurochem. 84, 1411–1420 10.1046/j.1471-4159.2003.01638.x [DOI] [PubMed] [Google Scholar]
- Buck K. B., Zheng J. Q. (2002). Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci. 22, 9358–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz M., Worgotter F., van Ooyen A. (2009). Activity-dependent structural plasticity. Brain Res. Rev. 60, 287–305 10.1016/j.brainresrev.2008.12.023 [DOI] [PubMed] [Google Scholar]
- Cancedda L., Fiumelli H., Chen K., Poo M. M. (2007). Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J. Neurosci. 27, 5224–5235 10.1523/JNEUROSCI.5169-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L., Salinas P. C. (2005). WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6, 351–362 10.1038/nrg1601 [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Lockyer P. J. (2002). Integration of calcium and Ras signaling. Nat. Rev. Mol. Cell Biol. 3, 339–348 10.1038/nrm808 [DOI] [PubMed] [Google Scholar]
- Davare M. A., Fortin D. A., Saneyoshi T., Nygaard S., Kaech S., Banker G., Soderling T. R., Wayman G. A. (2009). Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J. Neurosci. 29, 9794–9808 10.1523/JNEUROSCI.1544-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L., Halpain S. (2005). The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 6, 204. 10.1186/gb-2004-6-1-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Barnes A. M., Tang F., Kalil K. (2004). Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J. Neurosci. 24, 3002–3012 10.1523/JNEUROSCI.4963-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Callaway J. L., Szebenyi G., Baas P. W., Kalil K. (1999). Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J. Neurosci. 19, 8894–8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Gertler F. B. (2003). Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40, 209–227 10.1016/S0896-6273(03)00633-0 [DOI] [PubMed] [Google Scholar]
- Dent E. W., Kalil K. (2001). Axon branching requires interactions between dynamic microtubules and actin filaments. J. Neurosci. 21, 9757–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Tang F., Kalil K. (2003). Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms. Neuroscientist 9, 343–353 10.1177/1073858403252683 [DOI] [PubMed] [Google Scholar]
- Dickson B. J. (2002). Molecular mechanisms of axon guidance. Science 298, 1959–1964 10.1126/science.1075882 [DOI] [PubMed] [Google Scholar]
- Eshete F., Fields R. D. (2001). Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons. J. Neurosci. 21, 6694–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C. C., Bayer K. U., Myers J. W., Ferrell J. E., Jr., Schulman H., Meyer T. (2003). Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron 39, 283–297 10.1016/S0896-6273(03)00428-8 [DOI] [PubMed] [Google Scholar]
- Flynn K. C., Pak C. W., Shaw A. E., Bradke F., Bamburg J. R. (2009). Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev. Neurobiol. 69, 761–779 10.1002/dneu.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez T. M., Zheng J. Q. (2006). The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 7, 115–125 10.1038/nrn1844 [DOI] [PubMed] [Google Scholar]
- Gorbunova Y. V., Spitzer N. C. (2002). Dynamic interactions of cyclic AMP transients and spontaneous Ca(2+) spikes. Nature 418, 93–96 10.1038/nature00835 [DOI] [PubMed] [Google Scholar]
- Hall A. C., Lucas F. R., Salinas P. C. (2000). Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525–535 10.1016/S0092-8674(00)80689-3 [DOI] [PubMed] [Google Scholar]
- Halloran M. C., Kalil K. (1994). Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J. Neurosci. 14, 2161–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter P. N., Bunz B., Dietz K., Hoffmann K., Meyermann R., Mittelbronn M. (2010). Spatio-temporal deleted in colorectal cancer (DCC) and netrin-1 expression in human foetal brain development. Neuropathol. Appl. Neurobiol. 36, 623–635 10.1111/j.1365-2990.2010.01100.x [DOI] [PubMed] [Google Scholar]
- Henley J., Poo M. M. (2004). Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 14, 320–330 10.1016/j.tcb.2004.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Nishiyama M., Henley J., Tessier-Lavigne M., Poo M. (2000). Calcium signaling in the guidance of nerve growth by netrin-1. Nature 403, 93–98 10.1038/47507 [DOI] [PubMed] [Google Scholar]
- Hua J. Y., Smear M. C., Baier H., Smith S. J. (2005). Regulation of axon growth in vivo by activity-based competition. Nature 434, 1022–1026 10.1038/nature03409 [DOI] [PubMed] [Google Scholar]
- Huber A. B., Kolodkin A. L., Ginty D. D., Cloutier J. F. (2003). Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26, 509–563 10.1146/annurev.neuro.26.010302.081139 [DOI] [PubMed] [Google Scholar]
- Hudmon A., Schulman H. (2002). Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 71, 473–510 10.1146/annurev.biochem.71.110601.135410 [DOI] [PubMed] [Google Scholar]
- Hutchins B. I. (2010). Competitive outgrowth of neural processes arising from long-distance cAMP signaling. Sci. Signal. 3, jc1. 10.1126/scisignal.3118jc1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins B. I., Kalil K. (2008). Differential outgrowth of axons and their branches is regulated by localized calcium transients. J. Neurosci. 28, 143–153 10.1523/JNEUROSCI.4548-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins B. I., Li L., Kalil K. (2011). Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev. Neurobiol. 71, 269–283 10.1002/dneu.20846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Fricke B. T., Seow Y., Gottlieb P. A., Sachs F., Gomez T. M. (2006). Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J. Neurosci. 26, 5656–5664 10.1523/JNEUROSCI.0675-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K., Dent E. W. (2005). Touch and go: guidance cues signal to the growth cone cytoskeleton. Curr. Opin. Neurobiol. 15, 521–526 10.1016/j.conb.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Kalil K., Szebenyi G., Dent E. W. (2000). Common mechanisms underlying growth cone guidance and axon branching. J. Neurobiol. 44, 145–158 [DOI] [PubMed] [Google Scholar]
- Keeble T. R., Halford M. M., Seaman C., Kee N., Macheda M., Anderson R. B., Stacker S. A., Cooper H. M. (2006). The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 26, 5840–5848 10.1523/JNEUROSCI.1175-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. D., Moon R. T. (2005). Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 38, 439–446 10.1016/j.ceca.2005.06.022 [DOI] [PubMed] [Google Scholar]
- Kuhl M., Sheldahl L. C., Malbon C. C., Moon R. T. (2000). Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275, 12701–12711 10.1074/jbc.M001321200 [DOI] [PubMed] [Google Scholar]
- Lau P. M., Zucker R. S., Bentley D. (1999). Induction of filopodia by direct local elevation of intracellular calcium ion concentration. J. Cell Biol. 145, 1265–1275 10.1083/jcb.145.6.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Hutchins B. I., Kalil K. (2009). Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J. Neurosci. 29, 5873–5883 10.1523/JNEUROSCI.3517-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Jia Y. C., Cui K., Li N., Zheng Z. Y., Wang Y. Z., Yuan X. B. (2005). Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898 10.1038/nature03477 [DOI] [PubMed] [Google Scholar]
- Lindwall C., Fothergill T., Richards L. J. (2007). Commissure formation in the mammalian forebrain. Curr. Opin. Neurobiol. 17, 3–14 10.1016/j.conb.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Litersky J. M., Johnson G. V., Jakes R., Goedert M., Lee M., Seubert P. (1996). Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II within its microtubule-binding domains at Ser-262 and Ser-356. Biochem. J. 316(Pt 2), 655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Shi J., Lu C. C., Wang Z. B., Lyuksyutova A. I., Song X. J., Zou Y. (2005). Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 8, 1151–1159 10.1038/nn1522 [DOI] [PubMed] [Google Scholar]
- Livesey F. J., Hunt S. P. (1997). Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol. Cell. Neurosci. 8, 417–429 10.1006/mcne.1997.0598 [DOI] [PubMed] [Google Scholar]
- Lowery L. A., Van Vactor D. (2009). The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10, 332–343 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas F. R., Salinas P. C. (1997). WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 192, 31–44 10.1006/dbio.1997.8734 [DOI] [PubMed] [Google Scholar]
- Luo L. (2002). Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18, 601–635 10.1146/annurev.cellbio.18.031802.150501 [DOI] [PubMed] [Google Scholar]
- Luo L., O’Leary D. D. (2005). Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 10.1146/annurev.neuro.28.061604.135632 [DOI] [PubMed] [Google Scholar]
- Manitt C., Kennedy T. E. (2002). Where the rubber meets the road: netrin expression and function in developing and adult nervous systems. Prog. Brain Res. 137, 425–442 [DOI] [PubMed] [Google Scholar]
- McLaughlin T., O’Leary D. D. (2005). Molecular gradients and development of retinotopic maps. Annu. Rev. Neurosci. 28, 327–355 10.1146/annurev.neuro.28.061604.135714 [DOI] [PubMed] [Google Scholar]
- Metin C., Deleglise D., Serafini T., Kennedy T. E., Tessier-Lavigne M. (1997). A role for netrin-1 in the guidance of cortical efferents. Development 124, 5063–5074 [DOI] [PubMed] [Google Scholar]
- Ming G. L., Wong S. T., Henley J., Yuan X. B., Song H. J., Spitzer N. C., Poo M. M. (2002). Adaptation in the chemotactic guidance of nerve growth cones. Nature 417, 411–418 10.1038/nature745 [DOI] [PubMed] [Google Scholar]
- Morris R. L., Hollenbeck P. J. (1993). The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 104(Pt 3), 917–927 [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Koyama K., Murata Y., Morito M., Akiyama T., Nakamura Y. (2000). EB3, a novel member of the EB1 family preferentially expressed in the central nervous system, binds to a CNS-specific APC homologue. Oncogene 19, 210–216 10.1038/sj.onc.1203308 [DOI] [PubMed] [Google Scholar]
- Neal A. P., Molina-Campos E., Marrero-Rosado B., Bradford A. B., Fox S. M., Kovalova N., Hannon H. E. (2010). CaMKK-CaMKI signaling pathways differentially control axon and dendrite elongation in cortical neurons. J. Neurosci. 30, 2807–2809 10.1523/JNEUROSCI.5984-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X., Hong K. P., Spitzer N. C. (2011). Spatial and temporal second messenger codes for growth cone turning. Proc. Natl. Acad. Sci. U.S.A. 108, 13776–13781 10.1073/pnas.1100247108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquille M., Garel S., Mann F., Hornung J. P., Otsmane B., Chevalley S., Parras C., Guillemot F., Gaspar P., Yanagawa Y., Lebrand C. (2009). Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS Biol. 7, e1000230. 10.1371/journal.pbio.1000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C. R., Kalil K. (1992). Development of callosal connections in the sensorimotor cortex of the hamster. J. Comp. Neurol. 326, 121–132 10.1002/cne.903260111 [DOI] [PubMed] [Google Scholar]
- O’Leary D. D., Bicknese A. R., De Carlos J. A., Heffner C. D., Koester S. E., Kutka L. J., Terashima T. (1990). Target selection by cortical axons: alternative mechanisms to establish axonal connections in the developing brain. Cold Spring Harb. Symp. Quant. Biol. 55, 453–468 [DOI] [PubMed] [Google Scholar]
- Ooashi N., Futatsugi A., Yoshihara F., Mikoshiba K., Kamiguchi H. (2005). Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. J. Cell Biol. 170, 1159–1167 10.1083/jcb.200503157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M., Plachez C., Zalucki O., Fothergill T., Goudreau G., Erzurumlu R., Gu C., Richards L. J. (2009). Neuropilin 1-Sema signaling regulates crossing of cingulate pioneering axons during development of the corpus callosum. Cereb. Cortex 19(Suppl. 1), i11–i21 10.1093/cercor/bhp027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F., Giger R. J., Ginty D. D., Kolodkin A. L., Ghosh A. (1998). Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science 282, 1904–1906 10.1126/science.282.5395.1904 [DOI] [PubMed] [Google Scholar]
- Polleux F., Morrow T., Ghosh A. (2000). Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 404, 567–573 10.1038/35007001 [DOI] [PubMed] [Google Scholar]
- Purro S. A., Ciani L., Hoyos-Flight M., Stamatakou E., Siomou E., Salinas P. C. (2008). Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J. Neurosci. 28, 8644–8654 10.1523/JNEUROSCI.2320-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Yu L., Gao Y., Zhou R., Zhang C. (2007). Characterization of the receptors for axon guidance factor netrin-4 and identification of the binding domains. Mol. Cell. Neurosci. 34, 243–250 10.1016/j.mcn.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Reh T., Kalil K. (1981). Development of the pyramidal tract in the hamster. I. A light microscopic study. J. Comp. Neurol. 200, 55–67 10.1002/cne.902000105 [DOI] [PubMed] [Google Scholar]
- Ren T., Anderson A., Shen W. B., Huang H., Plachez C., Zhang J., Mori S., Kinsman S. L., Richards L. J. (2006). Imaging, anatomical, and molecular analysis of callosal formation in the developing human fetal brain. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288, 191–204 [DOI] [PubMed] [Google Scholar]
- Richards L. J., Koester S. E., Tuttle R., O’Leary D. D. (1997). Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J. Neurosci. 17, 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J., Esteve P., Weinl C., Ruiz J. M., Fermin Y., Trousse F., Dwivedy A., Holt C., Bovolenta P. (2005). SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat. Neurosci. 8, 1301–1309 10.1038/nn1547 [DOI] [PubMed] [Google Scholar]
- Ruthel G., Banker G. (1999). Role of moving growth cone-like “wave” structures in the outgrowth of cultured hippocampal axons and dendrites. J. Neurobiol. 39, 97–106 [DOI] [PubMed] [Google Scholar]
- Ruthel G., Hollenbeck P. J. (2000). Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J. Neurosci. 20, 2266–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G., Hollenbeck P. J. (2003). Response of mitochondrial traffic to axon determination and differential branch growth. J. Neurosci. 23, 8618–8624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas P. C. (2007). Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol. 17, 333–342 10.1016/j.tcb.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Salinas P. C., Zou Y. (2008). Wnt signaling in neural circuit assembly. Annu. Rev. Neurosci. 31, 339–358 10.1146/annurev.neuro.31.060407.125649 [DOI] [PubMed] [Google Scholar]
- Schaefer A. W., Kabir N., Forscher P. (2002). Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 158, 139–152 10.1083/jcb.200203038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A. W., Schoonderwoert V. T., Ji L., Mederios N., Danuser G., Forscher P. (2008). Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev. Cell 15, 146–162 10.1016/j.devcel.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T., Colamarino S. A., Leonardo E. D., Wang H., Beddington R., Skarnes W. C., Tessier-Lavigne M. (1996). Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 10.1016/S0092-8674(00)81795-X [DOI] [PubMed] [Google Scholar]
- Shelly M., Lim B. K., Cancedda L., Heilshorn S. C., Gao H., Poo M. M. (2010). Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science 327, 547–552 10.1126/science.1179735 [DOI] [PubMed] [Google Scholar]
- Sheth A. N., McKee M. L., Bhide P. G. (1998). The sequence of formation and development of corticostriate connections in mice. Dev. Neurosci. 20, 98–112 10.1159/000017306 [DOI] [PubMed] [Google Scholar]
- Shim S., Goh E. L., Ge S., Sailor K., Yuan J. P., Roderick H. L., Bootman M. D., Worley P. F., Song H., Ming G. L. (2005). XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat. Neurosci. 8, 730–735 10.1038/nn1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T., VanSant J., Paik E., Grove E. A. (2004). Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J. Comp. Neurol. 473, 496–510 10.1002/cne.20135 [DOI] [PubMed] [Google Scholar]
- Shu T., Richards L. J. (2001). Cortical axon guidance by the glial wedge during the development of the corpus callosum. J. Neurosci. 21, 2749–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T., Sundaresan V., McCarthy M. M., Richards L. J. (2003). Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J. Neurosci. 23, 8176–8184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. K., Miller F. D. (2005). Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron 45, 837–845 10.1016/j.neuron.2005.01.049 [DOI] [PubMed] [Google Scholar]
- Singh K. K., Park K. J., Hong E. J., Kramer B. M., Greenberg M. E., Kaplan D. R., Miller F. D. (2008). Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat. Neurosci. 11, 649–658 10.1038/nn.2114 [DOI] [PubMed] [Google Scholar]
- Sironi J. J., Yen S. H., Gondal J. A., Wu Q., Grundke-Iqbal I., Iqbal K. (1998). Ser-262 in human recombinant tau protein is a markedly more favorable site for phosphorylation by CaMKII than PKA or PhK. FEBS Lett. 436, 471–475 10.1016/S0014-5793(98)01185-5 [DOI] [PubMed] [Google Scholar]
- Spitzer N. C. (2006). Electrical activity in early neuronal development. Nature 444, 707–712 10.1038/nature05300 [DOI] [PubMed] [Google Scholar]
- Stepanova T., Slemmer J., Hoogenraad C. C., Lansbergen G., Dortland B., De Zeeuw C. I., Grosveld F., van Cappellen G., Akhmanova A., Galjart N. (2003). Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. Neurosci. 23, 2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M., Hattori Y., Zhao H., Sato H., Tamada A., Sasaki S., Nakajima K., Yamamoto N. (2011). Laminar and areal expression of unc5d and its role in cortical cell survival. Cereb. Cortex 21, 1925–1934 10.1093/cercor/bhq265 [DOI] [PubMed] [Google Scholar]
- Tang F., Dent E. W., Kalil K. (2003). Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. J. Neurosci. 23, 927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Kalil K. (2005). Netrin-1 induces axon branching in developing cortical neurons by frequency-dependent calcium signaling pathways. J. Neurosci. 25, 6702–6715 10.1523/JNEUROSCI.1331-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida T., Hirose K., Takizawa A., Shibasaki F., Iino M. (2003). NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 22, 3825–3832 10.1093/emboj/cdg381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N., Ruthazer E. S., Yamamoto N. (2006). The role of neural activity in cortical axon branching. Neuroscientist 12, 102–106 10.1177/1073858405281673 [DOI] [PubMed] [Google Scholar]
- van Ooyen A. (2011). Using theoretical models to analyse neural development. Nat. Rev. Neurosci. 12, 311–326 10.1038/nrn3031 [DOI] [PubMed] [Google Scholar]
- Wang C. L., Zhang L., Zhou Y., Zhou J., Yang X. J., Duan S. M., Xiong Z. Q., Ding Y. Q. (2007). Activity-dependent development of callosal projections in the somatosensory cortex. J. Neurosci. 27, 11334–11342 10.1523/JNEUROSCI.4488-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. X., Poo M. M. (2005). Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434, 898–904 10.1038/nature03478 [DOI] [PubMed] [Google Scholar]
- Wang Y., Thekdi N., Smallwood P. M., Macke J. P., Nathans J. (2002). Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J. Neurosci. 22, 8563–8573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman G. A., Impey S., Marks D., Saneyoshi T., Grant W. F., Derkach V., Soderling T. R. (2006). Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50, 897–909 10.1016/j.neuron.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Wayman G. A., Kaech S., Grant W. F., Davare M., Impey S., Tokumitsu H., Nozaki N., Banker G., Soderling T. R. (2004). Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J. Neurosci. 24, 3786–3794 10.1523/JNEUROSCI.3294-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Zheng J. Q. (2006). Directional guidance of nerve growth cones. Curr. Opin. Neurobiol. 16, 52–58 10.1016/j.conb.2005.12.005 [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., McKinnon R. D., Kokel M., Thomas J. B. (2003). Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature 422, 583–588 10.1038/nature01522 [DOI] [PubMed] [Google Scholar]
- Zheng J. Q., Poo M. M. (2007). Calcium signaling in neuronal motility. Annu. Rev. Cell Dev. Biol. 23, 375–404 10.1146/annurev.cellbio.23.090506.123221 [DOI] [PubMed] [Google Scholar]
- Zou Y., Lyuksyutova A. I. (2007). Morphogens as conserved axon guidance cues. Curr. Opin. Neurobiol. 17, 22–28 10.1016/j.conb.2007.01.006 [DOI] [PubMed] [Google Scholar]