Abstract
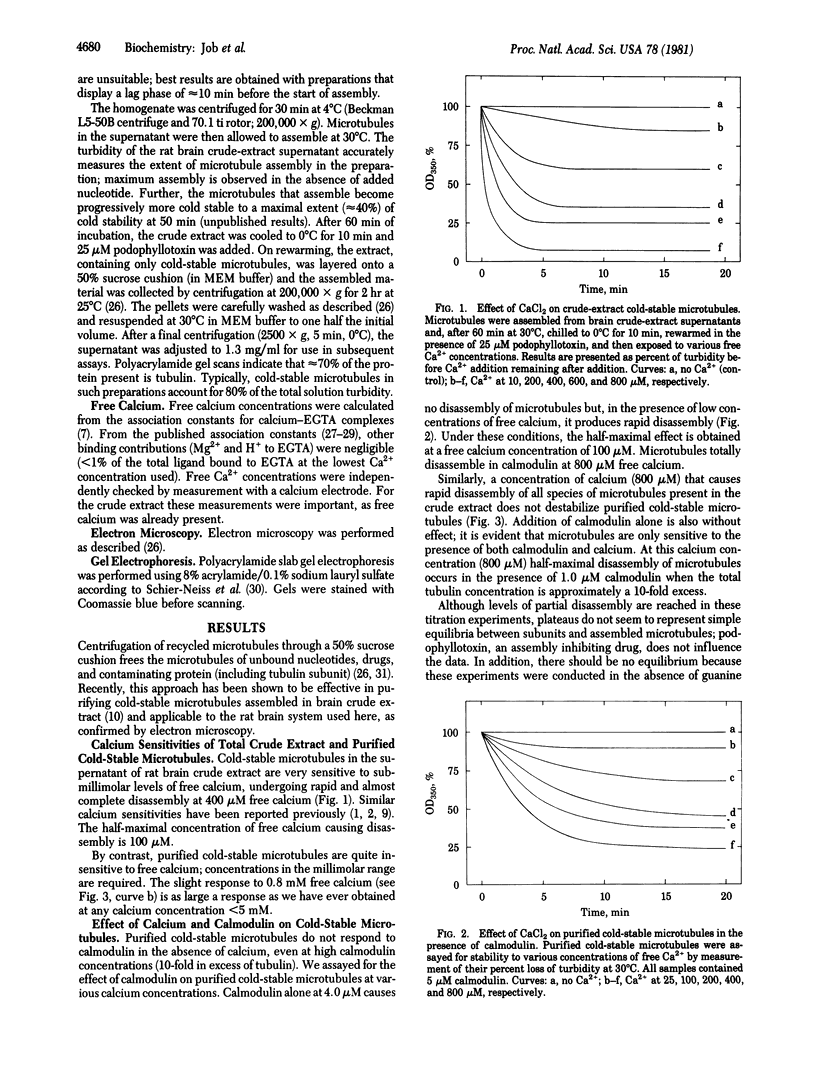
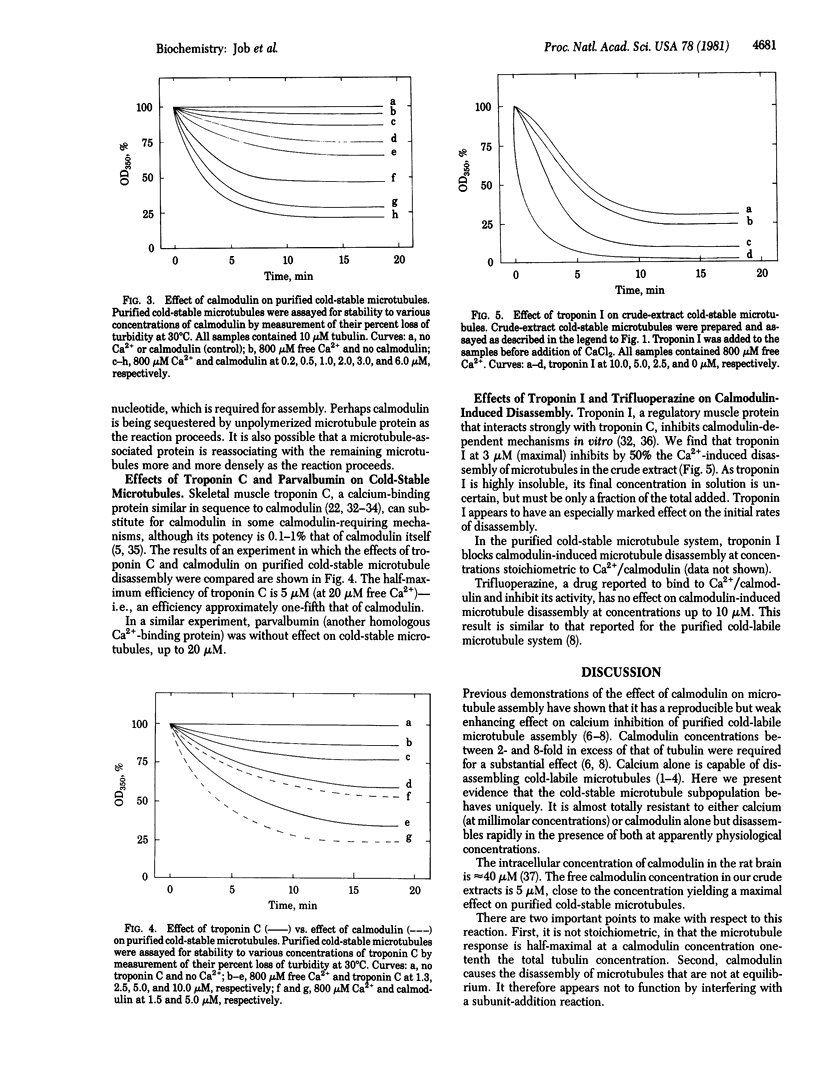
Purified cold-stable microtubules from the rat brain are insensitive to podophyllotoxin and to millimolar concentrations of free calcium. However, in the presence of calmodulin at concentrations substoichiometric to that of tubulin, calcium causes rapid microtubule disassembly. The half-maximal effective calcium concentration in the presence of calmodulin is 100 microM. With 800 microM free calcium, the half-maximal effective concentration of calmodulin is 1.0 microM (or one-tenth the tubulin concentration). Calmodulin is without effect in the absence of calcium. Troponin C is approximately one-fifth as effective as calmodulin, and parvalbumin is totally ineffective. Troponin I partially inhibits the calcium/calmodulin-induced disassembly of microtubules in the crude extract and blocks the calcium/calmodulin effect on purified cold-stable microtubules. A 5-fold excess of trifluoperazine does not inhibit the calcium/calmodulin-induced disassembly.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Vanaman T. C., Perry S. V. Effect of the troponin C-like protein from bovine brain (brain modulator protein) on the Mg2+-stimulated ATPase of skeletal muscle actinomyosin. FEBS Lett. 1976 Dec 15;72(1):163–168. doi: 10.1016/0014-5793(76)80836-8. [DOI] [PubMed] [Google Scholar]
- Andersen B., Osborn M., Weber K. Specific visualization of the distribution of the calcium dependent regulatory protein of cyclic nucleotide phosphodiesterase (modulator protein) in tissue culture cells by immunofluorescence microscopy: mitosis and intercellular bridge. Cytobiologie. 1978 Aug;17(2):354–364. [PubMed] [Google Scholar]
- Brinkley B. R., Cartwright J., Jr Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann N Y Acad Sci. 1975 Jun 30;253:428–439. doi: 10.1111/j.1749-6632.1975.tb19218.x. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Means A. R. Biological cross-reactivity of rat testis phosphodiesterase activator protein and rabbit skeletal muscle troponin-C. J Biol Chem. 1977 Apr 10;252(7):2437–2440. [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Euteneuer U., McIntosh J. R. Structural polarity of kinetochore microtubules in PtK1 cells. J Cell Biol. 1981 May;89(2):338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuseler J. W. Temperature dependence of anaphase chromosome velocity and microtubule depolymerization. J Cell Biol. 1975 Dec;67(3):789–800. doi: 10.1083/jcb.67.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Biochemical studies on the in vitro assembly and disassembly of microtubules. Ann N Y Acad Sci. 1975 Jun 30;253:133–146. doi: 10.1111/j.1749-6632.1975.tb19197.x. [DOI] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1977 Jun;73(3):601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973 Mar 25;248(6):2125–2133. [PubMed] [Google Scholar]
- Heidemann S. R., McIntosh J. R. Visualization of the structural polarity of microtubules. Nature. 1980 Jul 31;286(5772):517–519. doi: 10.1038/286517a0. [DOI] [PubMed] [Google Scholar]
- Inoué S., Ritter H., Jr Dynamics of mitotic spindle organization and function. Soc Gen Physiol Ser. 1975;30:3–30. [PubMed] [Google Scholar]
- Karr T. L., Kristofferson D., Purich D. L. Calcium ion induces endwise depolymerization of bovine brain microtubules. J Biol Chem. 1980 Dec 25;255(24):11853–11856. [PubMed] [Google Scholar]
- Kerrick W. G., Donaldson S. K. The effects of Mg 2+ on submaximum Ca 2+ -activated tension in skinned fibers of frog skeletal muscle. Biochim Biophys Acta. 1972 Jul 12;275(1):117–122. doi: 10.1016/0005-2728(72)90030-8. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Rauch C. T., Wilson L. Mechanism of colchicine-dimer addition to microtubule ends: implications for the microtubule polymerization mechanism. Biochemistry. 1980 Nov 25;19(24):5550–5557. doi: 10.1021/bi00565a014. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L., Keifer B. I. Mitotic mechanism based on intrinsic microtubule behaviour. Nature. 1978 Mar 30;272(5652):450–452. doi: 10.1038/272450a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Nishida E., Kumagai H., Ohtsuki I., Sakai H. The interactions between calcium-dependent regulator protein of cyclic nucleotide phosphodiesterase and microtubule proteins. I. Effect of calcium-dependent regulator protein on the calcium sensitivity of microtubule assembly. J Biochem. 1979 May;85(5):1257–1266. [PubMed] [Google Scholar]
- Nishida E., Sakai H. Calcium-sensitivity of the microtubule reassembly system. Difference between crude brain extract and purified microtubular proteins. J Biochem. 1977 Jul;82(1):303–306. doi: 10.1093/oxfordjournals.jbchem.a131685. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975 Jul;14(13):2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Stevens F. C., Walsh M., Ho H. C., Teo T. S., Wang J. H. Comparison of calcium-binding proteins. Bovine heart and brain protein activators of cyclic nucleotide phosphodiesterase and rabbit skeletal muscle troponin C. J Biol Chem. 1976 Aug 10;251(15):4495–4500. [PubMed] [Google Scholar]
- Stull J. T., Buss J. E. Calcium binding properties of beef cardiac troponin. J Biol Chem. 1978 Sep 10;253(17):5932–5938. [PubMed] [Google Scholar]
- Teshima Y., Kakiuchi S. Membrane-bound forms of Ca2+-dependent protein modulator: Ca2+-dependent and independent binding of modulator protein to the particulate fraction from brain. J Cyclic Nucleotide Res. 1978 Jun;4(3):219–231. [PubMed] [Google Scholar]
- Van Eerd J. P., Kawasaki Y. Effect of calcium(II) on the interaction between the subunits of troponin and tropomyosin. Biochemistry. 1973 Nov 20;12(24):4972–4980. doi: 10.1021/bi00748a024. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Watterson D. M., Mendel P. A., Vanaman T. C. Comparison of calcium-modulated proteins from vertebrate brains. Biochemistry. 1980 Jun 10;19(12):2672–2676. doi: 10.1021/bi00553a020. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- Webb B. C., Wilson L. Cold-stable microtubules from brain. Biochemistry. 1980 Apr 29;19(9):1993–2001. doi: 10.1021/bi00550a041. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Calcium-dependent regulator protein: localization in mitotic apparatus of eukaryotic cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1867–1871. doi: 10.1073/pnas.75.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Tubulin and calmodulin. Effects of microtubule and microfilament inhibitors on localization in the mitotic apparatus. J Cell Biol. 1979 Jun;81(3):624–634. doi: 10.1083/jcb.81.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]



