Abstract
Background
Adipose tissue (AT) is the body’s largest free cholesterol (FC) reservoir and abundantly expresses ATP binding cassette transporter A1 (ABCA1), a key cholesterol transporter for HDL biogenesis. However, the extent to which AT ABCA1 expression contributes to HDL biogenesis in vivo is unknown.
Methods and Results
Adipocyte-specific ABCA1 knockout mice (ABCA1−A/−A) were generated by crossing ABCA1floxed mice with aP2 cre transgenic mice. AT from ABCA1−A/−A mice had <10% of wild type (WT) ABCA1 protein expression, but normal hepatic and intestinal expression. Deletion of adipocyte ABCA1 resulted in a significant decrease in plasma HDL cholesterol (~15%) and apoA-I (~13%) concentrations. AT from ABCA1−A/−A mice had a two-fold increase in FC content, compared to WT mice, and failed to efflux cholesterol to apoA-I. However, cholesterol efflux from AT to plasma HDL was similar for both genotypes of mice. Incubation of WT AT explants with apoA-I resulted in formation of multiple discrete-sized nascent HDL particles ranging in diameter from 7.1–12 nm; similar incubations with ABCA1−A/−A AT explants resulted in nascent HDL <8 nm. Plasma decay and tissue uptake of WT 125I-HDL tracer was similar in both genotypes of recipient mice, suggesting that adipocyte ABCA1 deficiency reduces plasma HDL concentrations solely by reducing nascent HDL particle formation.
Conclusions
We provide in vivo evidence that AT ABCA1-dependent cholesterol efflux and nascent HDL particle formation contribute to systemic HDL biogenesis and that AT ABCA1 expression plays an important role in adipocyte cholesterol homeostasis.
Keywords: apolipoproteins, cholesterol, lipids, lipoproteins
Adipose tissue (AT) represents 10–35% of total body mass in humans 1 and plays a central role in integrating energy metabolism and glucose homeostasis 2. Although AT is the major site of fatty acid storage as triglyceride, it is also the body’s largest free cholesterol (FC) reservoir 3. Due to the lack of acyl-coenzyme A:cholesterol acetyltransferase (ACAT) enzyme activity in AT, nearly all cholesterol (>95%) exists as FC 4 and resides in the plasma membrane or at the cytosolic interface of lipid droplets 5. There is accumulating evidence that adipose cholesterol imbalance is closely associated with adipocyte dysfunction and obesity-mediated metabolic complications, including low levels of HDL cholesterol and insulin resistance 6, 7. Therefore, FC efflux from AT is likely to influence whole body cholesterol metabolism and homeostasis. However, the pathways by which FC exits from AT and its potential impact on plasma high density lipoprotein (HDL) cholesterol levels have not been fully investigated.
ATP-binding cassette transporter A1 (ABCA1) is an essential membrane protein for the initial step of HDL biogenesis by facilitating the efflux of cellular FC and phospholipid to extracellular lipid-free apolipoprotein A-I (apoA-I), forming nascent HDL particles 8. Loss of functional mutations in ABCA1 results in Tangier disease, with <5% the normal concentration of plasma HDL 9. Using human embryonic kidney (HEK)-293 cells stably overexpressing ABCA1, we previously demonstrated that ABCA1 expression in a non-hepatic cell line is necessary and sufficient to produce distinct, heterogeneous-sized nascent HDLs, ranging in diameter from 7–12 nm, which subsequently undergo intravascular metabolism to become mature HDL in plasma 10–12. Although ABCA1 is expressed to varying degrees in many different cell types13, not all ABCA1-expressing cells equally contribute in vivo to plasma HDL formation. For example, macrophage ABCA1 expression is abundant, but it has no significant impact on plasma HDL levels 14. On the other hand, hepatic and intestinal ABCA1 expression is estimated to contribute 70–80% and 15–20% of the plasma HDL pool, respectively 15, 16. Intriguingly, deletion of both hepatic and intestinal ABCA1 resulted in ~90% reduction in plasma HDL 16, whereas global ABCA1 knockout mice have <5% of normal levels of HDL cholesterol, similar to subjects with Tangier disease 9. These results suggest that ABCA1 expression in cells other than hepatocytes and intestinal epithelial cells would minimally affect plasma HDL formation. However, formal testing of a cell type’s contribution to the plasma HDL pool requires specific deletion of ABCA1 in that cell.
AT is a major storage pool of FC, which expands during obesity, and abundantly expresses ABCA1, but the role of adipocyte ABCA1 expression in FC efflux and HDL formation in vivo is unknown 3, 7, 17. AT has been proposed as important in plasma HDL production based on indirect evidence, such as an inverse association between adipose cholesterol content and plasma cholesterol concentrations 3. A recent study provided in vivo evidence that adipocytes support the transfer of FC to plasma HDL 17. In that study, mouse embryonic fibroblasts (from WT, ABCA1 KO, and SR-BI KO mice) were differentiated into adipocytes, radiolabeled with 3H-cholesterol, and injected into the peritoneal cavity of mice. Reverse cholesterol transport of 3H-cholesterol from adipocytes to the feces depended on adipocyte expression of ABCA1 and SR-BI 17. However, direct in vivo evidence for the role of adipose ABCA1 in plasma HDL formation remains lacking, and the extent to which adipose ABCA1 expression contributes to plasma HDL, particularly in the obese state, has not been investigated.
The purpose of this study was to directly test the role of adipocyte ABCA1 in the formation of nascent HDL particles and its contribution to the plasma HDL pool in vivo. To accomplish this, we generated adipocyte-specific ABCA1 knockout (ABCA1−A/−A) mice and investigated their plasma lipid and lipoprotein phenotypes during chow and high fat (HF) diet feeding. We also determined the role of ABCA1 in adipocyte cholesterol homeostasis and nascent HDL particle formation using AT explants from these mice. Our results suggest a significant role for AT ABCA1 expression in nascent HDL particle formation and AT cholesterol homeostasis.
Methods
Animals
Adipocyte specific ABCA1 knockout mice (ABCA1−A/−A) were generated by crossing ABCA1floxed mouse 15 (backcrossed into the >99% C57BL/6 background) with fatty acid binding protein 4 (FABP4; aP2) Cre transgenic mouse (Jackson Laboratories). For all experiments, 10- to 16-wk-old male mice were used. To expand AT mass, mice were switched from a commercial chow diet at 8 wks of age to a commercial HF diet (42% Cal from fat, 0.2% cholesterol; TD 88137, Harlan™) for an additional 8–16 wks, depending on the experiment. The mice were housed in the Wake Forest University Health Sciences animal care facilities with a 12 h light/12 h dark cycle. All protocols and procedures were approved by the Wake Forest University Health Sciences Animal Care and Use Committee.
Plasma cholesterol determination and FPLC
Plasma was collected by tail bleeding of mice fasted for 4 h. Plasma from 4 mice was pooled and size fractionated by FPLC using a Superose 6 TM column (Amersham Biosciences). Plasma cholesterol was determined using an enzymatic colorimetric assay kit (Roche Applied Science).
qPCR and Western blot analysis
Total RNA was extracted by Trizol (Invitrogen) according to the manufacturer’s protocol. cDNA preparation and qPCR were conducted as described previously 14. To prepare tissue lysate, ~0.1 g of snap frozen tissue was homogenized in RIPA buffer. For AT, the lysate was incubated on ice for 10 min to remove the solidified fat cake on top. Immunoblotting of ABCA1 was conducted as previously described using 4–16% SDS-PAGE 15. Monoclonal antibodies directed against PPARγ and GAPDH were purchased from Sigma. Polyclonal antibodies against apoE, apoA-I (Biodesign), and anti-SRB-I (Novartis) were also used for Western blotting.
Adipose cholesterol determination
AT cholesterol was determined by gas liquid chromatography (GLC) after extraction of total lipid from 0.1 g of AT with chloroform:methanol (2:1) 14. Data were normalized to tissue wet weight or protein, measured by the Lowry assay 18.
Preparation of ex vivo cultures of AT
To establish the ex vivo cultures of AT, freshly isolated epididymal fat (0.1~0.2 g) was minced into ~2 mm pieces, moved into 10 cm cell culture dishes, and incubated overnight in 1.5 ml of serum-free DMEM F/12 Ham (Sigma). The next day, AT cultures were washed thoroughly with caution to remove floating cells without aspirating the minced tissue explants.
Cholesterol efflux from AT
Measurement of cholesterol efflux was conducted according to standard methods, except ex vivo AT cultures were used 17, 19. Briefly, ex vivo cultures of AT (pooled from n=4 mice per genotype; n=4 replicates) were incubated with 5 μCi of [3H]-cholesterol (complexed to BSA) for 24 h. AT explants were then washed carefully and 1 ml of fresh serum-free medium was added. Cholesterol efflux was initiated by adding either 20 μg protein/ml of human apoA-I (ABCA1-dependent efflux) or 50 μg protein/ml of mouse HDL (ABCA1-independent efflux) as acceptors. After 4 h of additional incubation, cholesterol efflux was quantified by measuring [3H]-cholesterol in 500 μl of medium and in the cells, after isopropanol extraction. Percentage efflux was calculated as dpm [3H]-cholesterol in medium/(medium + cells) times 100%.
Nascent HDL formation
All the procedures for nascent formation were similar to our previous study 20. Briefly, 10 μg/ml of lipid-free [125I]-apoA-I (105 cpm/μg) was incubated with AT explants for 24 h. One ml of conditioned medium was then fractionated by high resolution fast protein liquid chromatography size-exclusion chromatography using three Superdex 200 HR columns in series. 125I radioactivity in each fraction was quantified using a gamma counter (Beckman). Electrophoretic mobility of adipose-derived nascent HDLs was analyzed using a Paragon lipoprotein agarose gel electrophoresis system (Beckman) according to the manufacturer’s instructions. Electrophoretic mobilities (α, β, preβ) were determined after lipid staining of plasma from LDLr knockout mice. The size distribution of nascent HDL particles in conditioned medium was also determined on a 4–30% non-denaturating gradient gel (NDGGE) as described previously20.
HDL catabolism and tissue specific uptake
125I-radiolabeled tyramine cellobiose (TC)-HDL ([125I]-TC-HDL) was prepared as previously described except HDL was isolated from wild type (WT) mouse plasma 15, 21. Recipient mice (n=4 per genotype) were anesthetized with isoflurane and [125I]-TC-HDL (500,000 cpm/animal) was injected via a jugular vein catheter. Blood samples (~30μl) were drawn at 5 min, 30 min, and 1, 3, 6 and 24 h after tracer injection by retro-orbital bleeding, and 15μl of plasma were used for quantification of [125I] radioactivity. Data were expressed as a percentage of the 5 min plasma radioactivity remaining in plasma after injection of the tracer. The plasma die-away curves were generated using a bi-exponential curve-fitting program (GraphPad Prism 5). For the determination of tissue-specific uptake of HDL, mice were sacrificed 24 h after [125I]-TC-HDL injection and liver, kidney and epididymal fat pads were removed to quantify 125I radioactivity.
Statistics
Results are presented as mean ± standard error of the mean. Data were statistically analyzed using multiple tests. A general linear regression model was used to assess relationships between FC and ABCA1 within and across fat depots while controlling for intra-animal correlations. A Student’s t test was used to test for differences in adipose tissue ABCA1 expression and radiolabeled HDL tracer tissue uptake between wild type and ABCA1−A/−A mice. For comparison of variables (i.e., total plasma cholesterol, epididymal fat cholesterol content) among all three genotypes of mice or more than two treatments (cholesterol efflux), one-way analysis of variance, followed by Tukey’s multiple comparison test to identify individual group differences was used. Two-way ANOVA with repeat measures (0–16 week time course) was used to compare total plasma cholesterol concentrations during HF diet feeding between WT and ABCA1−A/−A mice. Statistical tests were performed using GraphPad software (San Diego, CA) or SAS software.
Results
ABCA1 expression varies among fat depots
AT exhibits regional heterogeneity with regard to cellular composition, response to hormonal signals, and metabolic function22. To gain insight into the role of fat depot-specific cholesterol regulation by ABCA1, C57BL/6 mice (n=3) were fed a high fat (HF/HC)-diet (42% fat and 0.2% cholesterol) to increase fat mass. After the mice had consumed the diet for 8 wks, we measured cholesterol content by gas-liquid chromatography (GLC) and relative ABCA1 expression by Western blot in different fat depots. There was a sixfold variation in FC content (no detectable cholesteryl ester) among fat depots (epididymal > mesenteric > pericardial ≈ inguinal (subcutaneous) > brown fat). ABCA1 expression was equally variable among depots, with a strongly positive correlation (r2=0.34; 0.01) between fat depot FC content and ABCA1 expression, but not SR-BI or apoE expression (Fig 1A, B).
Figure 1. Adipocytes abundantly express ABCA1 and efficiently produce nascent HDL particles.
A. ABCA1, SR-BI, and apoE protein expression by Western blot analysis in different fat depots after 8 wks of HF diet in C57BL/6 mice (n=2 for each depot). GAPDH was used as a load control. B. Association between ABCA1 expression and FC content among six fat depots in C57BL/6 mice fed a HF diet for 8 wks (n=3 mice for each depot). Across depots, the line of best fit (r2=0.34), determined by regression analysis for all data points controlling for intra-animal correlations, is shown (p<0.01). Within depots, the overall association did not reached statistical significance due to one outlier value in retrorenal fat. C. ABCA1 expression in adipose tissue (AT), adipocytes (Adi), and stromal vascular (SV) cells. Collagenase-treated epididymal fat was fractionated into floating adipocytes (Adi) and pelleted stromal vascular cells (SV) for Western blot analysis of ABCA1 and aP2 expression. β-actin was used as a load control. D. ABCA1 expression at 0, 3, and 10 days after differentiation of SV cells into adipocytes. β-actin was used as a load control. E. Electrophoretic mobility of [125I]-apoA-I and conditioned medium from epididymal fat tissue (Epi Fat) incubated with [125I]-apoA-I for 24 hr on a Paragon lipoprotein agarose gel electrophoresis system. After electrophoresis, [125I]-apoA-I mobility was visualize using a phosphorimager. α, preβ and β positions on the gel are shown for reference. F. Nascent HDL formation from epididymal tissue explants. [125I]-apoA-I was incubated with either HEK293-ABCA1 cells (■) or 0.2 g of epididymal fat explants (○) for 24 h. Conditioned medium was then fractionated by high resolution FPLC and radiolabel in each fraction determined by gamma counting. Nascent HDL particles of increasing size are designated as preβ 1–5 based on previous studies. Elution position of [125I]-apoA-I is shown for reference.
Next, we examined AT ABCA1 expression using epididymal fat depots from C57BL/6 mice. There are conflicting results in the literature regarding the extent to which 3T3-L1 fibroblasts express ABCA1 before they are differentiated into adipocytes 17, 19. To determine whether ABCA1 is expressed in non-adipocyte cells residing in AT, stromal vascular (SV) cells were fractionated from floating mature adipocytes using methods previously described 23. ABCA1 expression was almost exclusively confined to adipocytes, with little expression in SV cells (Fig 1C). To establish a primary adipocyte culture system from mouse AT, isolated SV cells were incubated with a differentiation cocktail 23. After 10 days, ~60% of cells were converted into lipid-laden adipocytes by oil red-O staining (Suppl. Fig 1A). ABCA1 expression levels were also increased during adipogenesis (Fig 1D). To determine the cellular localization of ABCA1, adipocyte cultures were immunostained with ABCA1 monoclonal antibody. ABCA1 staining was observed in the plasma membrane and cytosol of adipocytes, suggesting that ABCA1 may traffic between the plasma membrane and cytosol (Suppl Fig 1B).
Adipose tissue generates nascent HDL particles of multiple sizes
ABCA1-dependent FC efflux has been reported for 3T3-L1 adipocytes 19, 24 and mouse embryonic fibroblast derived-adipocytes 17. However, ABCA1-dependent nascent HDL particle formation by unilocular adipocytes in AT has not been investigated. Thus, we established ex vivo cultures of mouse epididymal AT and incubated the freshly-isolated AT explants with 125I-apoA-I for 24 h, after which nascent HDL subpopulations in the conditioned medium were size-fractionated by high resolution FPLC 10, 11. Consistent with our previous studies using HEK293 cells stably expressing human ABCA1 10, 11, AT from C57BL/6 mice generated heterogeneously-sized nascent HDL particles that migrated in the pre-β position on agarose gels (Fig 1E). More importantly, the size distribution of nascent HDL species from AT followed a similar pattern as that from HEK293-ABCA1 expressing cells (Fig 1F) as well as mouse peritoneal macrophages and McArdle 7777 hepatoma cells 11, with five distinct subfractions ranging in size from 7–17 nm in diameter.
Deletion of adipocyte ABCA1 decreases plasma HDL levels
To investigate the role of adipocyte ABCA1 in AT cholesterol mobilization and its impact on HDL plasma level in vivo, we generated adipocyte-specific ABCA1 knockout mice (ABCA1−A/−A). Adipocyte-specific ABCA1 inactivation was achieved by crossing C57BL/6 ABCA1flox/flox mice with aP2 (fatty acid binding protein 4)-Cre transgenic mice backcrossed in the C57BL/6 background (Jackson Labs). ABCA1−A/−A mice were fertile and pups were born at the expected Mendelian frequency. Recombination of the floxed ABCA1 allele resulted in >90% decrease in ABCA1 protein expression in ABCA1−A/−A mouse epididymal fat and 87% reduction of ABCA1 mRNA in epididymal fat (Fig 2A). Inactivation of adipose ABCA1 had little effect on ABCA1 expression in liver and intestine, which are the main organs known to be important for HDL biogenesis (Fig 2B). However, ABCA1 expression was noticeably reduced in resident peritoneal macrophages (Fig 2C), thioglycolate-elicited macrophages (Fig 2D), and, to a lesser extent, in bone marrow-derived macrophages (Fig 2C). ABCA1 expression in WT epididymal and mesenteric AT was abundant and comparable to that in liver and macrophages, but its expression was marked blunted in ABCA1−A/−A mouse epididymal, brown and mesenteric fat (Figure 2D). Thus, deletion of ABCA1 expression was highly efficient in AT and somewhat variable in macrophages. However, macrophage ABCA1 expression has minimal impact on plasma HDL levels 14, 25–27, so we proceeded with studies on the role of adipocyte ABCA1 expression on HDL metabolism and adipocyte cholesterol balance.
Figure 2. Tissue-specific expression of ABCA1 in adipocyte-specific ABCA1 knockout mice.
A. ABCA1 expression in epididymal AT from WT (+/+), heterozygous (−A/+), and homozygous (−A/−A) ABCA1 knockout mice. Left panel: Western blot analysis of ABCA1, PPARγ 2 expression. ns=non-specific band. Right panel: mRNA expression of ABCA1 normalized to cyclophilin, determined by quantitative real time PCR. Mean ± SEM (n=3). B. Western blot analysis of hepatic and intestinal ABCA1 expression. ***=p<0.001. C. Western blot analysis of macrophage ABCA1 expression in resident peritoneal and bone marrow-derived macrophages (Mφ). D. Relative expression of ABCA1, aP2, and SR-BI in different tissues (i.e., liver, thioglycolate-elicited peritoneal macrophages, and 3 fat depots) of WT and ABCA11A/−A mice. Male mice were used in all assays and each lane represents for individual mouse. GAPDH was used as a load control in each blot.
Next, we evaluated the contribution of adipose ABCA1 expression on plasma cholesterol concentrations and distribution using WT and ABCA1−A/−A mice consuming chow or a HF diet. There was step-wise reduction in total plasma cholesterol with successive deletion of ABCA1 alleles (Figure 3A: WT, 83 ± 3; heterozygotes, 70 ± 6; homozygotes, 62 ± 4 mg/dl, respectively) that reached statistical significance for male WT vs. ABCA1−A/−A mice (p<0.0084 by ANOVA with Tukey’s post hoc test). In addition, Western blot analysis of plasma suggested a reduction in apoA-I (KO/WT ratio= 0.72) and apoE (KO/WT ratio= 0.86) concentration, but not LCAT (KO/WT ratio= 1.03) (Figure 3B). The lower plasma cholesterol levels for ABCA1−A/−A mice persisted throughout the 16 wks of HF-diet (2-way ANOVA with repeated measures; time effect, p<0.0001, genotype effect, p<0.001; n=4 each), suggesting that deletion of adipose ABCA1 results in decreased plasma cholesterol pool size despite expanding AT mass (Figure 3C). FPLC fractionation of plasma revealed that the decreased plasma cholesterol in ABCA1−A/−A mice was mainly in the HDL region of the column (Figure 3D). These data provide evidence that AT ABCA1 expression contributes a small, but significant, fraction of the plasma HDL pool.
Figure 3. Deletion of adipocyte ABCA1 reduces plasma cholesterol and HDL concentration.
A. Total plasma cholesterol levels of chow-fed WT (+/+), heterozygous (−A/+) and homozygous (−A/−A) adipocyte-specific ABCA1 knockout mice. B. Plasma levels of apoA-I, apoE, and LCAT determined by Western blot analysis of 1 ul of plasma from four chow-fed WT and ABCA1−A/−A mice. C. Total plasma cholesterol concentration of WT (+/+; ○) and ABCA1−A/−A (A/−A; ●) mice over 16 wks of HF-diet feeding (2-way ANOVA with repeated measures; time effect, p<0.0001, genotype effect, p<0.001; n=4 of each genotype). D. FPLC separation of plasma lipoproteins from WT (+/+; ○) and ABCA1−A/−A (−A/−A; ●) mice before (chow) and after 2 and 16 wks of HF diet feeding. Each profile represents a separation of a plasma pool from four mice of each genotype. Lipoprotein elution was monitored by enzymatic assay of cholesterol in individual fractions (Fr); elution position of VLDL, LDL, and HDL are indicated in the left panel.
To exclude the possibility that decreased plasma HDL concentration in ABCA1−A/−A mice was due to increased hepatic uptake and degradation of HDL, we measured plasma clearance and tissue uptake of HDL apoA-I. Because HDL from WT and ABCA1−A/−A mice was similar in chemical composition (data not shown), HDL was isolated from plasma of WT mice and radiolabeled with tyramine cellobiose (TC), a tissue residualizing compound that allows quantification of tissue uptake of radiolabeled tracer during turnover studies 15, 21. The plasma removal of [125I]-TC-HDL tracer in ABCA1−A/−A mice was indistinguishable from that of WT mice over the 24 h turnover study (Figure 4A). There also was no difference in tissue uptake of HDL tracer by liver, kidney or AT (Figure 4B), indicating that selective inactivation of adipocyte ABCA1 did not affect HDL catabolism in vivo. These results suggested that the decreased plasma HDL levels in ABCA1−A/−A mice were due to decreased HDL production by adipose tissue.
Figure 4. Deletion of adipocyte ABCA1 does not affect HDL catabolism.
A. HDL isolated from WT mice was radiolabeled with 125I-tyramine cellobiose (TC) and injected into the jugular vein of +/+ (WT; n=4) and −A/−A (ABCA1−A/−A; n=4) recipient mice. Periodic blood samples over a 24 h time period after injection were withdrawn to quantify the radioactivity remaining in plasma, normalized to percentage of injected tracer. B. Twenty-four hr after 125I-TC-HDL tracer injection, mice were sacrificed and liver, kidney, and epididymal fat (adipose) were removed to quantify tracer uptake by each organ. Data were normalized to liver uptake in wild type mice.
Deletion of adipocyte ABCA1 blunted ABCA1-dependent cholesterol efflux and large nascent HDL formation in adipose tissue
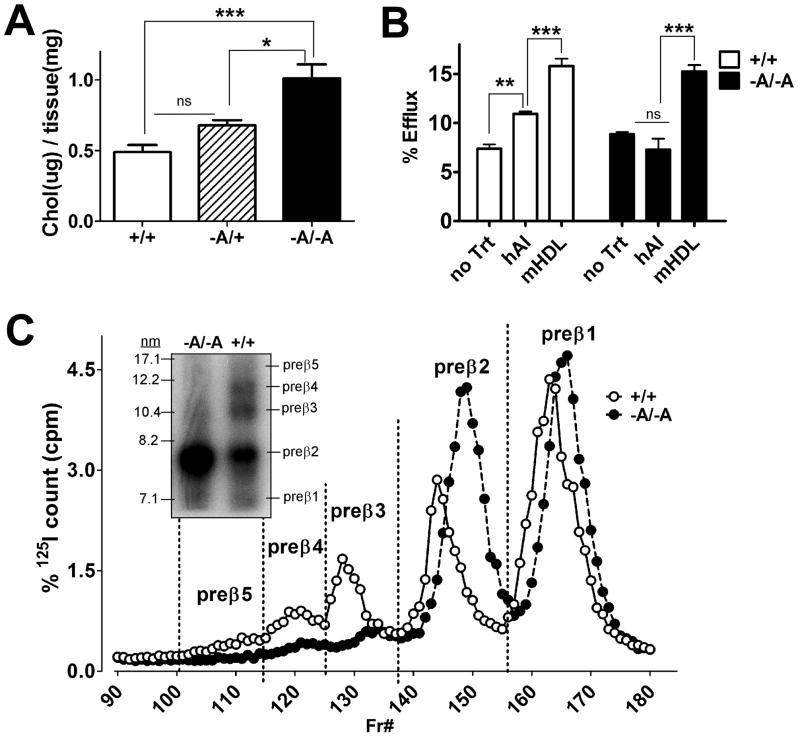
To determine whether deletion of ABCA1 in adipocytes resulted in increased FC content, similar to what we observed in macrophages from macrophage-specific ABCA1 knockout mice 14, we measured TC and FC in epididymal and brown fat from 3–4 mice after 16 wks of HF/HC diet feeding. There was a gene dose-related increase in FC content (p<0.001) in epididymal fat that reached statistical significance in ABCA1−A/−A mice (Figure 5A). CE was low to undetectable in the samples (data not shown). These results suggested that FC efflux from adipose explants was severely compromised in the absence of functional ABCA1. To address this question, we incubated AT explants from chow-fed mice with 3H-cholesterol for 24 h to radiolabel the cells, washed the cells, and incubated them for 4 h with apoA-I (20μg/ml) to measure ABCA1-dependent efflux, or with HDL (50 μg/ml) to measure ABCA1-independent efflux. ABCA1-dependent 3H-FC efflux to apoA-I was completely eliminated in ABCA1−A/−A epididymal fat compared with WT. However, HDL-mediated FC efflux (ABCA1-independent) was unaffected by deletion of adipose ABCA1 expression (Figure 5B). To determine whether the ablation of ABCA1-dependent efflux to apoA-I in ABCA1−A/−A epididymal fat was accompanied by loss of nascent HDL formation, we incubated [125I]-apoA-I with ex vivo cultures of WT or ABCA1−A/−A epididymal fat and monitored nascent HDL formation by non-denaturating gradient gel electrophoresis and high resolution FPLC (Figure 5C). WT epididymal fat generated multiple, heterogeneous-sized HDL subfractions that ranged in size from 7–12 nm diameter, which we have designated as preβ 1–5 in order of increasing particle size. We have also observed similar nascent HDL particle size heterogeneity in cultures of HEK293 cells stably expressing ABCA1, mouse peritoneal macrophages, and rat McArdle 7777 hepatoma cells 11, 20. In contrast, epididymal fat from ABCA1−A/−A mice generated only particles <10 nm in diameter (i.e., preβ 2).
Figure 5. Adipocyte ABCA1 generates heterogeneous-sized nascent HDL particles.
A. Free cholesterol accumulation in epididymal AT with progressive inactivation of adipocyte ABCA1 alleles. WT (+/+), heterozygote (−A/+), and homozygote (−A/−A) of adipocyte-specific ABCA1 knockout mice. B. Cholesterol efflux to apoA-I (ABCA1 dependent) and HDL (ABCA1 independent). Ex vivo cultures of epididymal fat from +/+ and −A/−A mice were radiolabeled with 3H-cholesterol for 24 h, washed, and then incubated with no addition (no Trt), apoA-I (hAI; 20 ug/ml), or mouse HDL (mHDL; 50 ug/ml) for 4 hr to measure 3H-FC efflux into the medium. For panels A and B, mean ± SEM, n=4. * p<0.05, ** p<0.01, ***p<0.001, ns= not significant at p=0.05. C. Nascent HDL formation in the presence of 125I-apoA-I in ex vivo cultures of epididymal AT from +/+ and −A/−A mice. After a 24 h incubation, conditioned medium was fractionated by high resolution FPLC and radiolabeled apoA-I in each fraction was quantified using a gamma counter. Elution regions for different-sized nascent pre-β HDL particles are denoted by the vertical dashed lines. Inset shows the size of AT derived-nascent HDLs separated by non-denaturating gradient gel electrophoresis (4–30%). Migration positions of the radiolabeled nascent HDL particles were visualized using a phosphorimager.
Discussion
This study was designed to elucidate the role of adipocyte ABCA1 expression in nascent HDL particle formation and its contribution to the plasma HDL pool in vivo. To address this issue, we generated ABCA1−A/−A mice and studied them while they were consuming a chow or HF diet. Several novel observations were made in this study. First, AT ABCA1 expression varied over a five-fold range among different fat depots in C57BL/6 mice and was significantly associated with FC content among different adipose tissues, suggesting an important role for ABCA1 expression in AT sterol homeostasis. Second, ABCA1−A/−A mice had <10% expression of ABCA1 protein in AT compared to WT mice, with a concomitant two-fold increase in AT cholesterol content. Although FC efflux from AT explants to HDL was similar between WT and ABCA1−A/−A mice, FC efflux to apoA-I was eliminated in ABCA1−A/−A AT, suggesting an important and specific role for ABCA1 in AT FC homeostasis. Third, WT AT explants were capable of generating multiple, discrete-sized nascent HDL subfractions from apoA-I, ranging in diameter from 7–12 nm, similar to other cell types. However, ABCA1−A/−A AT generated nascent HDL particles <10 nm, suggesting a considerable loss of function in the ability to generate nascent HDL. The decreased ability to generate nascent HDL was reflected in vivo as a 15% reduction in plasma HDL concentration in ABCA1−A/−A vs. WT mice that was attributed to HDL production, since HDL catabolism was similar between the two genotypes of mice. Collectively, these results establish a novel role for adipocyte ABCA1 in AT cholesterol mobilization and cholesterol balance and in vivo HDL production.
AT is the main depot for triglyceride and is used to meet systemic energy needs 3. It is also the body’s largest cholesterol storage depot; uniquely, >95% of AT cholesterol is FC, due to low esterification activity by ACAT 3. As adipocytes expand in size during obesity, FC and lipid droplet FC increase 3, but plasma membrane FC decreases 28. This redistribution of FC increases plasma membrane fluidity, decreases insulin signaling, and increases adipocyte cholesterol biosynthesis 28. Thus, maintaining the optimal amount and intracellular distribution of FC in adipocytes appears functionally important in AT. Since cholesterol biosynthesis is relatively low in AT 29, FC efflux may be an important regulator of sterol homeostasis. Recent studies indicate that ABCA1 and SR-BI are important regulators of cholesterol efflux from adipocytes 17. Initially, adipocytes were thought to have low ABCA1 expression 13, but subsequent studies using 3T3L1 adipocytes 17, 28, adipose tissue 30, and our data in isolated adipocytes (Figure 1C, D) demonstrate that ABCA1 is abundantly expressed. Furthermore, we observed that ABCA1 expression varies over a five-fold range among AT depots and that this variation is highly correlated with AT FC content (Figure 1A, B). These data suggest that as metabolically active visceral fat stores enlarge during TG accretion, FC also increases; this in turn upregulates expression of ABCA1 to rebalance cellular cholesterol homeostasis. ABCA1 gene transcription is highly regulated by liver X receptor expression in some tissues 31, 32, but whether this pathway is responsible for variable ABCA1 protein expression among AT depot sites is unknown. Nevertheless, these data establish a critical association between AT ABCA1 expression and FC content, which may be important in the modulation of adipocyte metabolic activity.
To determine the role of AT ABCA1 expression on plasma HDL concentrations, we generated ABCA1−A/−A mice by crossing ABCA1flox/flox mice with aP2 Cre recombinase transgenic mice. ABCA1 protein expression in epididymal fat decreased in proportion to the number of inactivated ABCA1 alleles (Figure 1A). Recombination of the ABCA1 gene was efficient in all AT depots examined, with >90% elimination of ABCA1 protein expression in ABCA1−A/−A mice. Expression of SR-BI, another protein important in adipocyte FC efflux 17, was unaffected (Figure 1D). ABCA1−A/−A mice had lower plasma total and HDL cholesterol concentrations compared to WT mice, whether they were fed chow or a HF diet (Figure 3C, D). To determine whether this phenotype was due directly to deletion of AT ABCA1 or indirectly due to changes in ABCA1 expression in other tissues, specificity of the recombination was investigated. ABCA1 expression in liver and intestine, two tissues previously shown to be important in determining plasma HDL concentrations 15, 16, was similar in WT vs. ABCA1−A/−A mice and could not account for the differences in plasma HDL concentrations. aP2 Cre recombinase has been reported to be active in macrophages 33, so we also examined ABCA1 expression in resident, elicited peritoneal, and bone marrow-derived macrophages. ABCA1 expression was variably reduced in macrophages from ABCA1−A/−A mice, confirming previous results that aP2 Cre recombinase was active in macrophages 34. However, evidence from our lab 14 and others 25–27 using transplantation of total ABCA1 knockout bone marrow has demonstrated that macrophage ABCA1 expression has no significant impact on plasma HDL concentrations. Taken together, these data suggest that AT ABCA1 expression plays a significant role in determining plasma HDL concentrations.
Although our data suggested that HDL production by AT was reduced in the absence of adipocyte ABCA1 expression, deletion of adipocyte ABCA1 may have indirectly increased HDL catabolism, resulting in reduced plasma HDL levels. Previous studies in humans suggest that HDL catabolism plays an important role in determining plasma HDL concentrations 35. To formally examine this possibility, we performed HDL turnover studies using radiolabeled WT HDL, since HDL composition was similar between WT and ABCA1−A/−A mice (data not shown). Plasma decay of the HDL tracer and uptake of radiolabel by the liver and kidney, the two most important tissues in HDL protein uptake and catabolism 15, 21, was similar for both genotypes of mice (Figure 4). Based on these data, we conclude that decreased plasma HDL concentrations in ABCA1−A/−A mice were due to decreased HDL production by AT, relative to WT mice, and not to differences in HDL catabolism.
Although our study as well as others 17 implicate AT in HDL production, direct evidence for the assembly of nascent HDL particles by AT has not been demonstrated. In this study, we show that epididymal fat from ABCA1−A/−A mice had a doubling of FC content, was defective in FC efflux to apoA-I, and was defective in production of nascent HDL particles >10 nm in diameter (Figure 5). Zhang et al 17 have shown that both ABCA1 and SR-BI, but not ABCG1, are important for FC efflux from adipocytes, differentiated from mouse embryonic fibroblasts. Our study confirms and extends their results by showing that ABCA1 is critical for maintaining FC content and efflux to ABCA1 in primary AT explants. Furthermore, the two-fold increase in AT FC content suggests that SR-BI cannot compensate for the loss of ABCA1 expression. This was confirmed by the similar expression of SR-BI among AT depots in WT and ABCA1−A/−A mice (Figure 2D). Previous studies have shown that ectopic expression of ABCA1 in cell lines is necessary and sufficient to generate several discrete-sized nascent HDL particles 10. We have designated these particles as preβ 1–5 on the basis of increasing particle size on non-denaturing gradient gels and FPLC columns and mobility on agarose or two-dimensional gels. These nascent preβ HDL particles appear to be formed simultaneously in cell culture systems, with no evidence for a precursor-product dependency (i.e., small particles → larger particles) 10, 36–38. Similar discrete, nascent HDL particle sizes have previously been observed for McArdle 7777 hepatoma cells, mouse peritoneal macrophages 11, and primary hepatocytes (unpublished data), in addition to transfected cell lines, suggesting that ABCA1 expression is the necessary and sufficient condition for nascent HDL size heterogeneity among these varied cell types. Epididymal fat explants from WT mice also generated nascent HDL particles with apoA-I that were strikingly similar in size to those reported for other cell culture systems (Figure 1F). However, epididymal fat explants from ABCA1−A/−A mice did not generate nascent HDL >10 nm (Figure 5C). We speculate that the formation of preβ 2 HDL resulted from the residual (<10%) ABCA1 protein expression in AT of ABCA1−A/−A mice (Figure 2D). Support for this idea comes from a study in which siRNA silencing of ABCA1 in McArdle 7777 hepatoma cells resulted in ~70–80% reduction of ABCA1 protein expression and decreased formation of nascent HDL >10 nm 20. Overall, our studies provide evidence that AT ABCA1 expression directly contributes to the formation of multiple, discrete-sized nascent HDL particles.
Obesity is associated with chronic low-grade inflammation, adipocyte dysfunction (i.e., adipokine secretion, impaired lipolysis, insulin resistance), and low plasma HDL 39. Adipocyte ABCA1 expression may represent a compensatory mechanism to limit inflammation and adipocyte dysfunction that accompanies obesity and metabolic syndrome. Since adipocyte FC content increases with obesity and adipocyte hypertrophy, we speculate that ABCA1 is upregulated in adipocytes to maintain optimal FC balance between the lipid droplet and plasma membrane, and adipocyte function. With development of obesity, accretion of FC during adipocyte expansion may sequester FC on lipid droplets, shunting it away from ABCA1-mediated HDL particle formation, resulting in decreased plasma HDL concentrations. In contrast, rapid weight loss may result in FC mobilization to the adipocyte plasma membrane, resulting in increased ABCA1-mediated HDL particle formation, and increased plasma HDL concentrations. Thus, adipocyte ABCA1 may be a critical negative regulator of obesity and metabolic syndrome, preventing adipocyte dysfunction and abnormal plasma membrane FC accumulation by facilitating FC efflux and nascent HDL formation.
Supplementary Material
Acknowledgments
We gratefully acknowledge Karen Klein (Research Support Core, Wake Forest School of Medicine) for editing the manuscript and Dr. Mark Espeland, Wake Forest School of Medicine, for assistance with statistical analysis and interpretation.
Funding Sources
This work was supported by National Institutes of Health Grants P01HL049373, R01 HL094525, P50 AT0027820 and by an American Heart Association Mid-Atlantic Affiliate Postdoctoral Fellowship 0825445E (to S. C.).
Footnotes
Disclosures
None
Reference List
- 1.Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21:681–689. doi: 10.1079/bjn19670070. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. J Lipid Res. 1984;25:97–110. [PubMed] [Google Scholar]
- 4.Schreibman PH, Dell RB. Human adipocyte cholesterol. Concentration, localization, synthesis, and turnover. J Clin Invest. 1975;55:986–993. doi: 10.1172/JCI108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prattes S, Horl G, Hammer A, Blaschitz A, Graier WF, Sattler W, Zechner R, Steyrer E. Intracellular distribution and mobilization of unesterified cholesterol in adipocytes: triglyceride droplets are surrounded by cholesterol-rich ER-like surface layer structures. J Cell Sci. 2000;113 ( Pt 17):2977–2989. doi: 10.1242/jcs.113.17.2977. [DOI] [PubMed] [Google Scholar]
- 6.Yu BL, Zhao SP, Hu JR. Cholesterol imbalance in adipocytes: a possible mechanism of adipocytes dysfunction in obesity. Obes Rev. 2010;11:560–567. doi: 10.1111/j.1467-789X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 7.Le LS, Ferre P, Dugail I. Adipocyte cholesterol balance in obesity. Biochem Soc Trans. 2004;32:103–106. doi: 10.1042/bst0320103. [DOI] [PubMed] [Google Scholar]
- 8.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- 9.Assman G, von Eckardstein A, Brewer HB., Jr . Familial Analphalipoproteinemia: Tangier Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Volkman BF, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw-Hill; 2001. pp. 2937–60. [Google Scholar]
- 10.Mulya A, Lee JY, Gebre AK, Thomas MJ, Colvin PL, Parks JS. Minimal Lipidation of Pre-β HDL by ABCA1 Results in Reduced Ability to Interact with ABCA1. Arterioscler Thromb Vasc Biol. 2007;27:1828–1836. doi: 10.1161/ATVBAHA.107.142455. [DOI] [PubMed] [Google Scholar]
- 11.Mulya A, Lee JY, Gebre AK, Boudyguina EY, Chung SK, Smith TL, Colvin PL, Jiang Xc, Parks JS. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J Lipid Res. 2008;49:2390–2401. doi: 10.1194/jlr.M800241-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Lanningham-Foster L, Boudyguina EY, Smith TL, Young ER, Colvin PL, Thomas MJ, Parks JS. Pre-β high density lipoprotein has two metabolic fates in human apolipoprotein A-I transgenic mice. J Lipid Res. 2004;45:716–728. doi: 10.1194/jlr.M300422-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Wellington CL, Walker EK, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, Francone O, McManus BM, Hayden MR. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest. 2002;82:273–283. doi: 10.1038/labinvest.3780421. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased Cellular Free Cholesterol in Macrophage-specific Abca1 Knock-out Mice Enhances Pro-inflammatory Response of Macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, McGillicuddy FC, Hinkle CC, O’Neill S, Glick JM, Rothblat GH, Reilly MP. Adipocyte Modulation of High-Density Lipoprotein Cholesterol. Circulation. 2010;121:1347–1355. doi: 10.1161/CIRCULATIONAHA.109.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Le LS, Robichon C, Le LX, Dagher G, Ferre P, Dugail I. Regulation of ABCA1 expression and cholesterol efflux during adipose differentiation of 3T3-L1 cells. J Lipid Res. 2003;44:1499–1507. doi: 10.1194/jlr.M200466-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Chung S, Gebre AK, Seo J, Shelness GS, Parks JS. A novel role for ABCA1-generated large pre-beta migrating nascent HDL in the regulation of hepatic VLDL triglyceride secretion. J Lipid Res. 2010;51:729–742. doi: 10.1194/jlr.M900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Timmins JM, Mulya A, Smith TL, Zhu Y, Rubin EM, Chisholm JW, Colvin PL, Parks JS. HDLs in apoA-I transgenic Abca1 knockout mice are remodeled normally in plasma but are hypercatabolized by the kidney. J Lipid Res. 2005;46:2233–2245. doi: 10.1194/jlr.M500179-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 23.Chung S, Brown JM, Provo JN, Hopkins R, McIntosh MK. Conjugated linoleic acid promotes human adipocyte insulin resistance through NFkappaB-dependent cytokine production. J Biol Chem. 2005;280:38445–38456. doi: 10.1074/jbc.M508159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verghese PB, Arrese EL, Soulages JL. Stimulation of lipolysis enhances the rate of cholesterol efflux to HDL in adipocytes. Mol Cell Biochem. 2007;302:241–248. doi: 10.1007/s11010-007-9447-0. [DOI] [PubMed] [Google Scholar]
- 25.Van Eck M, Bos IS, Kaminski WE, Orso E, Rothe G, Twisk J, Bottcher A, Van Amersfoort ES, Christiansen-Weber TA, Fung-Leung WP, Van Berkel TJC, Schmitz G. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. PNAS. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased Atherosclerosis in Hyperlipidemic Mice With Inactivation of ABCA1 in Macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 27.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le LS, Krief S, Farnier C, Lefrere I, Le LX, Bazin R, Ferre P, Dugail I. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J Biol Chem. 2001;276:16904–16910. doi: 10.1074/jbc.M010955200. [DOI] [PubMed] [Google Scholar]
- 29.Farkas J, Angel A, Avigan MI. Studies on the compartmentation of lipid in adipose cells. II. Cholesterol accumulation and distribution in adipose tissue components. J Lipid Res. 1973;14:344–356. [PubMed] [Google Scholar]
- 30.Zhang C, Yin W, Liao D, Huang L, Tang C, Tsutsumi K, Wang Z, Liu Y, Li Q, Hou H, Cai M, Xiao J. NO-1886 upregulates ATP binding cassette transporter A1 and inhibits diet-induced atherosclerosis in Chinese Bama minipigs. J Lipid Res. 2006;47:2055–2063. doi: 10.1194/jlr.M600226-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Wagner BL, Valledor AF, Shao G, Daige CL, Bischoff ED, Petrowski M, Jepsen K, Baek SH, Heyman RA, Rosenfeld MG, Schulman IG, Glass CK. Promoter-Specific Roles for Liver X Receptor/Corepressor Complexes in the Regulation of ABCA1 and SREBP1 Gene Expression. Molecular and Cellular Biology. 2003;23:5780–5789. doi: 10.1128/MCB.23.16.5780-5789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamehiro N, Shigemoto-Mogami Y, Kakeya T, Okuhira Ki, Suzuki K, Sato R, Nagao T, Nishimaki-Mogami T. Sterol Regulatory Element-binding Protein-2- and Liver X Receptor-driven Dual Promoter Regulation of Hepatic ABC Transporter A1 Gene Expression: MECHANISM UNDERLYING THE UNIQUE RESPONSE TO CELLULAR CHOLESTEROL STATUS. J Biol Chem. 2007;282:21090–21099. doi: 10.1074/jbc.M701228200. [DOI] [PubMed] [Google Scholar]
- 33.Wang ZV, Deng Y, Wang QA, Sun K, Scherer PE. Identification and Characterization of a Promoter Cassette Conferring Adipocyte-Specific Gene Expression. Endocrinology. 2010;151:2933–2939. doi: 10.1210/en.2010-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakrabarti P. Promoting Adipose Specificity: The Adiponectin Promoter. Endocrinology. 2010;151:2408–2410. doi: 10.1210/en.2010-0316. [DOI] [PubMed] [Google Scholar]
- 35.Brinton EA, Eisenberg S, Breslow JL. Increased apo A-I and apo A-II fractional catabolic rate in patients with low high density lipoprotein-cholesterol levels with or without hypertriglyceridemia. J Clin Invest. 1991;87:536–544. doi: 10.1172/JCI115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duong PT, Collins HL, Nickel M, Lund-Katz S, Rothblat GH, Phillips MC. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J Lipid Res. 2006;47:832–843. doi: 10.1194/jlr.M500531-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Bortnick AE, Nickel M, Dhanasekaran P, Subbaiah PV, Lund-Katz S, Rothblat GH, Phillips MC. Effects of Apolipoprotein A-I on ATP-binding Cassette Transporter A1-mediated Efflux of Macrophage Phospholipid and Cholesterol: FORMATION OF NASCENT HIGH DENSITY LIPOPROTEIN PARTICLES. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- 38.Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J., Jr Molecular and Cellular Physiology of Apolipoprotein A-I Lipidation by the ATP-binding Cassette Transporter A1 (ABCA1) J Biol Chem. 2004;279:7384–7394. doi: 10.1074/jbc.M306963200. [DOI] [PubMed] [Google Scholar]
- 39.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.