Abstract
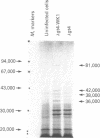
A segment of DNA containing cya, the gene for adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1], has been isolated from Salmonella typhimurium. The phage lambda gt4 was used as a cloning vector and adenylate cyclase-positive hybrid phages were isolated that complemented adenylate cyclase-negative bacteria. The cloned DNA fragment encodes a polypeptide of molecular weight 81,000 that gives rise to adenylate cyclase activity. This protein represents a functional mutant of the bacterial adenylate cyclase. When the cya gene was amplified by inserting into a multicopy plasmid, the enzyme activity was overproduced 20-fold, but the cyclic AMP level increased only 60%, suggesting several probable regulatory mechanisms. Overproduction of enzymes by recombinant DNA techniques can be a useful probe of relationships in the metabolizing organism in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Koshland D. E., Jr Multiple methylation in processing of sensory signals during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1980 May;77(5):2429–2433. doi: 10.1073/pnas.77.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. Transposition of the lac region of Escherichia coli. IV. Escape from repression in bacteriophage-carried lac genes. J Mol Biol. 1967 Dec 28;30(3):529–543. doi: 10.1016/0022-2836(67)90366-x. [DOI] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Phosphorylation of Isocitrate dehydrogenase of Escherichia coli. Science. 1979 Mar 16;203(4385):1111–1112. doi: 10.1126/science.34215. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., LeCam A., Bukowski M., Pastan I. Isolation of multiple classes of mutants of CHO cells resistant to cyclic AMP. Somatic Cell Genet. 1980 Jan;6(1):45–61. doi: 10.1007/BF01538695. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homcy C., Wrenn S., Haber E. Affinity purification of cardiac adenylate cyclase: dependence on prior hydrophobic resolution. Proc Natl Acad Sci U S A. 1978 Jan;75(1):59–63. doi: 10.1073/pnas.75.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Specialized transducing phages for ribosomal protein genes of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):6–10. doi: 10.1073/pnas.72.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Wierman B. M., Storm D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry. 1980 Aug 5;19(16):3814–3819. doi: 10.1021/bi00557a025. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeCam A., Gottesman M. M., Pastan I. Mechanism of cyclic AMP effect on nutrient transport in Chinese hamster ovary cells. A genetic approach. J Biol Chem. 1980 Sep 10;255(17):8103–8108. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. The Escherichia coli adenylate cyclase complex: activation by phosphoenolpyruvate. J Supramol Struct. 1978;9(2):219–230. doi: 10.1002/jss.400090207. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Larson T. J., Dowhan W. Gene cloning for the isolation of enzymes of membrane lipid synthesis: phosphatidylserine synthase overproduction in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1412–1416. doi: 10.1073/pnas.74.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Mutation in the crp gene of Salmonella typhimurium which interferes with inducer exclusion. J Bacteriol. 1980 Feb;141(2):751–757. doi: 10.1128/jb.141.2.751-757.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. A physical, genetic and transcriptional map of the cloned his3 gene region of Saccharomyces cerevisiae. J Mol Biol. 1980 Jan 25;136(3):309–332. doi: 10.1016/0022-2836(80)90376-9. [DOI] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyhach R. J., Hawrot E., Satre M., Kennedy E. P. Increased synthesis of phosphatidylserine decarboxylase in a strain of Escherichia coli bearing a hybrid plasmid. Altered association of enzyme with the membrane. J Biol Chem. 1979 Feb 10;254(3):627–633. [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr Evidence for protein kinase activities in the prokaryote Salmonella typhimurium. J Biol Chem. 1978 Nov 10;253(21):7605–7608. [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr The identification of distinct protein kinases and phosphatases in the prokaryote Salmonella typhimurium. J Biol Chem. 1981 May 10;256(9):4640–4648. [PubMed] [Google Scholar]
- Westcott K. R., La Porte D. C., Storm D. R. Resolution of adenylate cyclase sensitive and insensitive to Ca2+ and calcium-dependent regulatory protein (CDR) by CDR-sepharose affinity chromatography. Proc Natl Acad Sci U S A. 1979 Jan;76(1):204–208. doi: 10.1073/pnas.76.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]