Abstract
To assess the availability of Ca2+ in the lumen of the thylakoid membrane that is required to support the assembly of the oxygen-evolving complex of photosystem II, we have investigated the mechanism of 45Ca2+ transport into the lumen of pea (Pisum sativum) thylakoid membranes using silicone-oil centrifugation. Trans-thylakoid Ca2+ transport is dependent on light or, in the dark, on exogenously added ATP. Both light and ATP hydrolysis are coupled to Ca2+ transport through the formation of a transthylakoid pH gradient. The H+-transporting ionophores nigericin/K+ and carbonyl cyanide 3-chlorophenylhydrazone inhibit the transport of Ca2+. Thylakoid membranes are capable of accumulating up to 30 nmol Ca2+ mg−1 chlorophyll from external concentrations of 15 μm over the course of a 15-min reaction. These results are consistent with the presence of an active Ca2+/H+ antiport in the thylakoid membrane. Ca2+ transport across the thylakoid membrane has significant implications for chloroplast and plant Ca2+ homeostasis. We propose a model of chloroplast Ca2+ regulation whereby the activity of the Ca2+/H+ antiporter facilitates the light-dependent uptake of Ca2+ by chloroplasts and reduces stromal Ca2+ levels.
The physiology of Ca2+ in the plant cell and its distribution among various organelles, gradients, and transient fluxes continues to be the subject of research and the focus of several recent reviews (Evans et al., 1991; Bush, 1993, 1995; Gilroy et al., 1993). However, little attention has been given to the problem of Ca2+ transport across the thylakoid membrane. In fact, Ca2+ is required for several essential processes inside the chloroplast thylakoid lumen. In particular, Ca2+ ions are essential for the function of the OEC, a multimeric complex in the thylakoid lumen responsible for light-dependent oxygen evolution in plants. Functional assembly of PSII and the OEC requires that all essential polypeptides and cofactors are present in the stroma, thylakoid membrane, or thylakoid lumen.
The assembly of the OEC inside the thylakoid lumen requires the assembly of the OE33, OE23, and OE17 polypeptides and the essential inorganic ions Mn2+, Ca2+, and Cl− to the OEC in a light-dependent process (Ghanotakis et al., 1984; Becker et al., 1985; Miller and Brudvig, 1989). Additionally, in saturating light the reaction center D1 protein of PSII rapidly becomes damaged in a process known as photoinhibition (Mattoo et al., 1989). Damage resulting from photoinhibition is repaired by the proteolytic degradation of the D1 protein, followed by the disassembly of the remaining PSII proteins and OEC polypeptides, the resynthesis of D1, and the reassembly of a new PSII core and OEC from existing polypeptides and ions (Broussac et al., 1990; Hundal et al., 1990a, 1990b; Virgin et al., 1990). Therefore, both the initial assembly of PSII and its subsequent reassembly after photoinhibition require Ca2+ availability in the chloroplast thylakoid lumen. Furthermore, Ca2+ in the thylakoid lumen has been implicated in the formation and maintenance of localized proton domains (Dilley and Chiang, 1989) and the stabilization of the high redox potential form of Cyt b559 (McNamera and Gounaris, 1995). All of these processes require the availability of Ca2+ in the thylakoid lumen.
Ca2+ transported to the thylakoid lumen must originate from the cytosol and be transported through the chloroplast envelope membranes, the stroma, and the thylakoid membrane. It is well documented that Ca2+ is actively transported across the chloroplast envelope membranes in the light (Nobel and Packer, 1965; Nobel, 1967, 1969; Muto et al., 1982; Kreimer et al., 1985a, 1985b, 1988). Trans-envelope Ca2+ movement from the cytosol to the stroma is believed to be energetically coupled to a significant membrane potential across the inner envelope membrane through an electrogenic Ca2+ pump (Kreimer et al., 1985b).
Although it is conceivable that Ca2+ transport across the thylakoid membrane may be dependent on simple diffusion, the diffusion of a such a large divalent ion across the lipid bilayer of the thylakoid membrane could be a rate-limiting process of OEC biogenesis. Moreover, measurements of transthylakoid membrane potential in the light indicate that there is a 15- to 30-mV positive membrane potential across the thylakoid membrane (Bulychev et al., 1972; Hangarter and Good, 1982). According to the Nernst equation, neither simple nor facilitated diffusion of Ca2+ across the thylakoid membrane would be thermodynamically spontaneous unless the ratio of free Ca2+ were greater than about 3:1 (stromal:luminal). However, the low-affinity Ca2+-binding site for the photoactivation process of the OEC is reported to have a Kd of 0.3 mm (Miller and Brudvig, 1989; see also Debus, 1992). This suggests that significantly higher Ca2+ concentrations are required in the thylakoid lumen for PSII assembly than the 2 to 6 μm free Ca2+ in the stroma (Kreimer et al., 1988; Johnson et al., 1995).
In this study we investigated how the essential inorganic ion Ca2+ was translocated across the thylakoid membrane to the thylakoid lumen. Using isolated, intact thylakoid membranes we have demonstrated that a significant amount of 45Ca2+ is translocated across the thylakoid membrane in an energy- and time-dependent process. Light (or ATP in the dark) is necessary to support 45Ca2+ transport. Furthermore, the activity of the transport process was shown to be sensitive to proton-translocating uncouplers such as CCCP and nigericin/K+. On the basis of our results we propose that a Ca2+/H+ antiport actively moves Ca2+ from the stroma into the thylakoid lumen in the light. The antiporter provides the concentrations of luminal Ca2+ required for the assembly of the OEC and supports other Ca2+-requiring reactions in the thylakoid lumen.
MATERIALS AND METHODS
Materials
We purchased 45Ca2+ from New England Nuclear. Sigma provided ATP, Percoll, nigericin, CCCP, DCPIP, DCCD, AMP-PNP, tentoxin, and A23187. All other chemicals and reagents were of the highest quality commercially available.
Chloroplast and Thylakoid Isolation
We soaked pea (Pisum sativum L. cv Laxton's Progress) seeds in deionized water and planted them 1-cm deep in potting soil. The plants grew in the laboratory under fluorescent lights (18-h days and 6-h nights) and watered as needed with deionized water. Intact chloroplasts and thylakoids were isolated from 12- to 16-d-old pea seedlings essentially as described previously (Cline et al., 1985; Theg et al., 1986). Seedlings were homogenized in a grinding buffer containing 0.05 m potassium-Hepes (pH 7.3), 0.33 m sorbitol, 1 mm MgCl2, 1 mm MnCl2, 2 mm Na2EDTA, and 0.1% BSA. Intact chloroplasts were isolated by density-gradient centrifugation of the homogenate through a linear Percoll gradient. We isolated the thylakoid membranes from the chloroplasts osmotically. First, they were lysed in 10 mm potassium-Hepes (pH 6.5), and 5 mm MgCl2 on ice for 5 min. The thylakoids were then separated from the stroma by centrifugation and resuspended in an import buffer containing 0.05 m potassium-Tricine (pH 8.0), 0.33 m sorbitol, and 5 mm MgCl2. Chlorophyll concentration was determined by the method of Arnon (1949).
Measurement of 45Ca Transport
We completed the standard Ca2+ import reactions in 0.05 m potassium-Tricine (pH 8.0), 0.33 m sorbitol, and 5 mm MgCl2. Thylakoid membranes were added to a final concentration of 0.33 mg chlorophyll mL−1. Other additions to the reactions (ATP, nigericin, and ATPase inhibitors) are indicated in the figure legends. We initiated the reactions by adding 1.5 or 15 μm 45Ca2+ (12.6 Ci g−1) to the mixture containing import buffer and thylakoids. Reactions run in the light were placed 20 cm from a 60-W incandescent light filtered through 10 cm of a 5% CuSO4 solution at 25°C; dark reactions were placed inside a drawer at 25°C. Reactions were terminated by centrifugation of 60-μL aliquots (20 μg of chlorophyll) through 100 μL of silicone oil, 65% AR-200, and 35% AR-20 (Wacker Silicones, Adrian, MI) layered over 100 μL of 1.5 m perchloric acid in a 0.4-mL microfuge tube. Thylakoid membranes sedimented through the silicone oil layer into the perchloric acid, while the reaction buffer and unincorporated 45Ca2+ remained in the aqueous layer above the silicone oil (Heldt, 1980). The tubes were then frozen in liquid nitrogen. The frozen tubes were cut through the middle silicone oil layer, and the thylakoid membranes in the lower portion were suspended in a scintillation cocktail (Ecolume, Beckman) before counting. The results were converted from counts per minute to micromoles of Ca2+ per milligram of chlorophyll using an average 86% 45Ca-counting efficiency.
RESULTS
Energy-Dependent Transport of Ca2+ across the Thylakoid Membrane
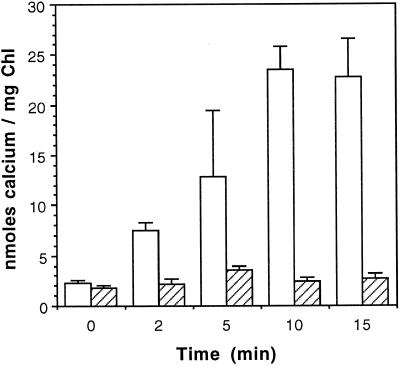
We sought to determine the mechanism of 45Ca2+ transport across the thylakoid membrane by using intact, isolated thylakoids and silicone oil centrifugation. We determined that Ca2+ transport proceeds at a significant rate when thylakoids are exposed to light but that the rate is very low in the dark (Fig. 1). The transport reaction reached a plateau after approximately 15 min. Analysis of the rate of Ca2+ transport under these conditions indicated that thylakoids are capable of accumulating approximately 2 nmol Ca2+ min−1 mg−1 chlorophyll from external concentrations of 15 μm. There was also a low level of 45Ca2+ uptake by the membranes that was independent of time or the state-of-energization of the membranes. This is consistent with Ca2+ binding to, or simply diffusing across, the membranes through non-energy-dependent processes.
Figure 1.
Light-dependent accumulation of 45Ca2+ across intact, isolated thylakoid membranes. Duplicate transport reactions were initiated by adding 45Ca2+ (15 μm final concentration) to thylakoid membranes maintained in the light (open bars) or dark (hatched bars). After mixing and at the intervals indicated, 60-μL aliquots were removed from the reactions and the transport reactions were stopped by centrifugation of the membranes through silicone oil. Chl, Chlorophyll.
Light or ATP Hydrolysis Can Facilitate Ca2+ Transport
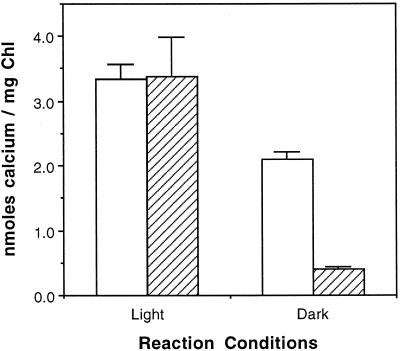
Predominant models of energy-dependent Ca2+ transport across membranes rely on either ATP (Ca2+-ATPase) or a ΔpH (Ca2+/H+ antiport) to support Ca2+ transport against its electrical or chemical potentials. The action of light on isolated thylakoid membranes can generate both a ΔpH and ATP through the combined actions of the photosystems and ATP-synthase. Therefore, we tried to determine the means by which light stimulated the uptake of 45Ca2+ by assessing the ability of ATP to drive the transport reaction in the dark. As shown in Figure 2, 3 mm ATP supported approximately 60% of the import amount in the dark as was achieved in the light in the absence of exogenously added ATP. In the light, added ATP had no significant effect on Ca2+ import.
Figure 2.
Light- or ATP-dependent accumulation of 45Ca2+. Transport reactions were initiated by adding 45Ca2+ (1.5 μm final concentration) to thylakoid membranes suspended in import buffer containing 5 mm MgCl2 and 3 mm ATP. Duplicate reactions were maintained in the dark or light. Reactions were terminated after 15 min. White bars, +ATP; hatched bars, −ATP. Chl, Chlorophyll.
ΔpH Is Necessary for Ca2+ Transport
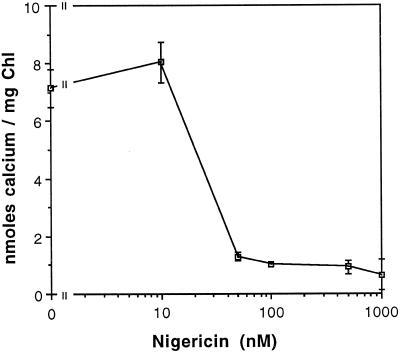
Exogenously added ATP could drive Ca2+ transport directly, through a Ca2+-ATPase, or indirectly, through a Ca2+/H+ antiport coupled to a transmembrane ΔpH generated by ATP hydrolysis by the thylakoid ATP-synthase. A Ca2+/H+-antiport mechanism would require that ATP hydrolysis be energetically coupled to the Ca2+/H+ antiport through the formation of a ΔpH. A Ca2+-ATPase would have no such dependence on a ΔpH. Inhibition of the ATP-dependent transport reaction by H+-translocating uncouplers suggest that Ca2+ transport is mediated by a Ca2+/H+ antiport. Nigericin catalyzes the electroneutral exchange of H+ and K+ across membranes and dissipates the thylakoid proton gradient as it transports K+ into the thylakoid lumen (Shavit et al., 1968). As demonstrated in Figure 3, nigericin/K+ inhibited the light-dependent import of 45Ca2+ at concentrations greater than approximately 50 nm. Therefore, the dependence of the reaction on ΔpH strongly suggests that ΔpH was the primary energy source driving Ca2+ import through a Ca2+/H+ antiporter. These results also suggest that the hydrolysis of ATP by the thylakoid ATP-synthase provided the ΔpH to support Ca2+ import in the dark.
Figure 3.
The light-dependent 45Ca2+ transport reaction is sensitive to nigericin. Reactions were initiated by adding 45Ca2+ (1.5 μm final concentration) to thylakoid membranes suspended in import buffer in the light. Nigericin was added from an ethanolic stock to the concentration indicated. Potassium, approximately 25 mm, was present in the reaction as the counterion to the Tricine buffer. An equal volume of ethanol (1 μL per 60-μL reaction) was added to all reactions. Reactions were terminated after 15 min. Chl, Chlorophyll.
Light Energy Provides the ΔpH Required for Ca2+ Import
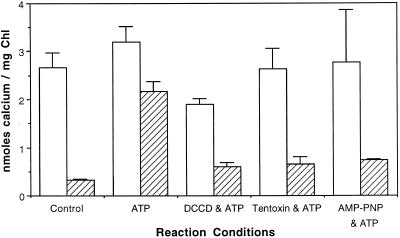
If low levels of residual ADP and Pi were trapped in the thylakoid membranes during isolation, and if ADP was subsequently phosphorylated by ATP-synthase in the light, then it is possible that small amounts of ATP could be formed in the light in our standard reactions. To rule out the direct involvement of ATP in the light-dependent transport reaction, we performed the reaction in the presence of various inhibitors of the thylakoid ATP-synthase. Inhibition of either the CF1 or CFo portion of ATP-synthase should prevent the formation of ATP in the light. If light-driven Ca2+ transport was not altered by the addition of ATPase inhibitors, then these results would confirm that the sole energy source for Ca2+ import is ΔpH and not ATP hydrolysis. DCCD blocked the Cfo channel of the thylakoid H+-ATPase (Nelson et al., 1977). Tentoxin stopped ATP hydrolysis or synthesis by H+-ATPase (Steele et al., 1976). AMP-PNP is an ATP analog that inhibited ATP binding to ATPases (Robinson and Wiskich, 1977). We used these inhibitors of ATP-synthase in the light to demonstrate that neither ATP synthesis nor hydrolysis was required for light-dependent 45Ca2+ import (Fig. 4). These inhibitors stopped ATP-dependent import in the dark, which was expected since they blocked ATP-synthase-dependent ΔpH formation. The slight decrease in the activity of the Ca2+/H+ antiporter in the presence of DCCD may have been due to the reported sensitivity of the tonoplast Ca2+/H+ antiporter to DCCD (Schumaker and Sze, 1986). Furthermore, these results suggest that a Ca2+-ATPase is not responsible for import, as it likely would have been inhibited by AMP-PNP. These results are consistent with a thylakoid-localized Ca2+/H+ antiporter.
Figure 4.
ATP-dependent 45Ca2+ transport is sensitive to inhibitors of the H+-ATP synthase. Thylakoid membranes were preincubated with DCCD (100 μm) for 10 min and the membranes were repurified by centrifugation before use. Tentoxin (4 μm) was added to intact chloroplasts, which were then incubated on ice for 1 h before thylakoid membranes were isolated. AMP-PNP was added to a final concentration of 3 mm from an aqueous stock. Transport reactions were initiated by adding 45Ca2+ (1.5 μm final concentration) to thylakoid membranes suspended in import buffer containing 5 mm MgCl2 and 3 mm ATP in the light (white bars) or in the dark (hatched bars). Reactions were terminated after 15 min. Chl, Chlorophyll.
Characterization of the Ca2+/H+ Antiporter
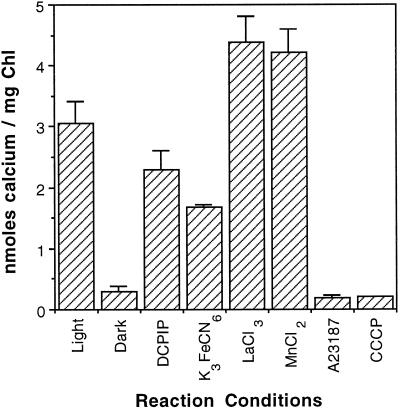
If the activity of the Ca2+/H+ antiporter is dependent on light-stimulated proton pumping, then the reaction rate may be enhanced by the addition of artificial electron acceptors to the isolated thylakoid membranes used in our reactions. However, as shown in Figure 5, attempts to optimize the activity of the Ca2+/H+ antiporter in intact isolated membranes by the addition of the electron acceptors DCPIP and K3FeCN6 proved unsuccessful. We also investigated the effect of several other ions that could be inhibitory. La3+ is a well-characterized inhibitor of Ca2+ channels and might also interact with this Ca2+ antiporter. Likewise, Mn2+ that is translocated across the thylakoid membrane by unknown mechanisms may utilize the Ca2+/H+ antiporter as a transport mechanism. Furthermore, the addition of either LaCl3 or MnCl2 at a concentration of 100 μm had no inhibitory effect on the Ca2+/H+ antiporter (Fig. 5). Incidentally, the presence or absence of 5 mm MgCl2 had no pronounced effect on the activity of the Ca2+/H+ antiporter in the light, but MgCl2 was required for optimal activity in the ATP-dependent reaction in the dark (data not shown). Finally, the Ca2+ ionophore A23187 and the uncoupler CCCP were shown to inhibit the transport reaction. To ensure that the transport of Ca2+ across the thylakoid membrane was applicable to other plant species, we performed sample reactions using thylakoid membranes from chloroplasts isolated from spinach. Spinach chloroplasts yielded results nearly identical to those of pea (data not shown).
Figure 5.
Characterization of 45Ca2+ transport across the thylakoid membrane. Light-dependent 45Ca2+ transport was assessed in the presence of DCPIP (10 μm), K3FeCN6 (1 mm), Ca2+ ionophore A23187 (5 μm), CCCP (5 μm), LaCl3 (100 μm), and MnCl2 (100 μm). One microliter of a concentrated stock of each effector was added to the thylakoid membranes before the reaction was initiated. Transport reactions were initiated by adding 45Ca2+ (1.5 μm final concentration) to thylakoid membranes suspended in import buffer at 25°C in the light or dark. Reactions were terminated after 15 min. Chl, Chlorophyll.
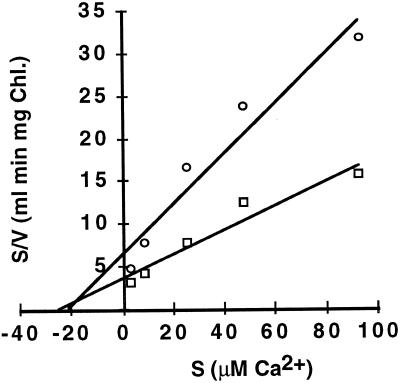
We investigated the kinetics of Ca2+ uptake by chloroplast thylakoid membranes and intact chloroplasts by measuring the initial rate of Ca2+ uptake as a function of increasing Ca2+ concentration (Fig. 6). Analysis of these data demonstrated that the thylakoid transport reaction has a Km for Ca2+ of 24 μm and a Vmax of 7 nmol min−1 mg−1 chlorophyll. The Km for Ca2+ of the thylakoid membrane Ca2+/H+ antiporter is comparable to reported Km values (10–70 μm) for the Ca2+/H+ antiporters isolated from higher-plant tonoplast membranes (Schumaker and Sze, 1986; Blackford et al., 1990). The transport of Ca2+ across thylakoid membranes and intact chloroplasts showed similar kinetics. Intact chloroplasts have a Km for Ca2+ of 22 μm and a Vmax of 3 nmol min−1 mg−1 chlorophyll (Fig. 7). However, the value we obtained for the Km for Ca2+ of the chloroplast Ca2+-transport reaction was significantly lower than the 180 and 188 μm values obtained by Muto et al. (1982) and Kreimer et al. (1985b), respectively. The difference in relative Ca2+ Km values between our data and previously reported values may reflect our differing methods but may also indicate that, like mitochondria, chloroplasts have multiple Ca2+-transport mechanisms (Gunter et al., 1994).
Figure 6.
Kinetic analysis of Ca2+ uptake by isolated thylakoids(□) and intact chloroplasts (○). A series of reaction solutions were prepared by combining CaCl2 and 3 μm of 45Ca2+ to a final Ca2+ concentration of 3.0, 8.6, 25.5, 48.0, or 93.0 μm. Light-dependent reactions were run in triplicate and initiated by the addition of thylakoid membranes. Reactions were terminated after 1.0, 2.5, and 4.0 min. Reaction rates for each Ca2+ concentration were determined by linear-regression analysis of the average Ca2+ uptake at the three different time points. Reactions with intact chloroplasts were run in an identical manner, except the reactions were terminated by centrifugation of the membranes through a layer of 100% AR-200 silicone oil. Results shown are the averages of two independent experiments. Chl, Chlorophyll.
Figure 7.
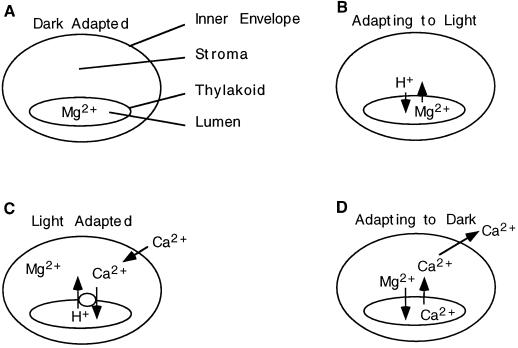
A model of Ca2+ flux in the intact chloroplast. Dark-adapted chloroplasts contain micromolar levels of free Ca2+ in the stroma, and Mg2+ ions are sequestered in the thylakoid lumen (A). The light-stimulated release of Mg2+ from the thylakoid lumen reduces the trans-thylakoid membrane potential (B). The Ca2+/H+ antiporter pumps Ca2+ into the thylakoid lumen and prevents Ca2+-mediated inhibition of CO2 fixation (C). Adaptation to the dark prompts the electroneutral exchange of Ca2+ and Mg2+ across the thylakoid membrane and results in Calvin-Benson-cycle inhibition by Ca2+ in the dark (D).
DISCUSSION
We initiated this study to elucidate the mechanism of Ca2+ transport across the thylakoid membrane and to assess its availability for assembly into the OEC. We utilized thylakoid membrane fractions prepared by osmotic lysis from freshly isolated Percoll-gradient-purified chloroplasts to ensure membrane purity and stability. Thylakoid membranes prepared the same way have been shown to be competent in the ΔpH- or ATP-dependent transport of the precursor forms of plastocyanin, OE33, OE23, and OE17 and in the integration of the precursor form of the light-harvesting chlorophyll a/b protein into the thylakoid membrane (Cline et al., 1992). Uptake of 45Ca2+ by the thylakoid membranes was monitored by silicone oil centrifugation and liquid-scintillation counting of the recovered membranes (Heldt, 1980). This method has enabled us to demonstrate that the import of a significant amount Ca2+ into the thylakoids is time and energy dependent. Our results demonstrate that the transthylakoid ΔpH is necessary and sufficient for Ca2+ import across the thylakoid membrane, and our results also strongly suggest that the mechanism for the import of Ca2+ is a Ca2+/H+ antiporter powered by a light- or ATP-induced proton gradient.
Early studies of Ca2+ transport across the membranes of isolated chloroplasts were initiated by Nobel and coworkers (Nobel and Packer, 1965; Nobel, 1967, 1969), who demonstrated that Ca2+ transport into chloroplast membranes is a light-dependent process that requires ATP and Mg2+ and is facilitated by the addition of thiol reagents and PMS. However, these studies did not suggest a mechanism of Ca2+ transport across the chloroplast membrane, nor did they separate out the role of the envelope or thylakoid membranes, light, ΔpH, or ATP in the transport process. Studies with intact chloroplasts have been carried out by Muto et al. (1982) and Kreimer et al. (1985a, 1985b, 1988). These studies demonstrated that Ca2+ transport from the cytosol into the chloroplast is a light-dependent process. According to their model, the total Ca2+ concentration in the chloroplast is 4 to 23 mm. However, much of the stromal Ca2+ is bound or sequestered, and the free Ca2+ concentration in the stroma is only 2 to 6 μm. Therefore, stroma-free Ca2+ is about 10-fold more concentrated than in the cytosol (100–300 nm), and transport must be energetically facilitated by an electrogenic Ca2+ pump in the inner envelope membrane (Kreimer et al., 1985a, 1985b, 1988). Other information about transthylakoid ion transport comes from more recent patch-clamp studies demonstrating the existence of thylakoid membrane cation channels that have conductance for K+, Mg2+, and Ca2+ (in order of their decreasing conductance) (Enz et al., 1993; Pottosin and Schönknecht, 1996). However, patch-clamp studies do not address the direction, energetics, or mechanism of Ca2+ transport across the thylakoid membrane.
A H+/Ca2+ antiporter in the thylakoid membrane would certainly supply the Ca2+ required for the assembly and maintenance of PSII and other thylakoid functions noted above. Additionally, the transport of Ca2+ across the thylakoid membrane may be associated with several important processes in chloroplasts. The transition from dark to light is a key regulatory period for stromal enzymes involved in carbon reduction. However, the role of Ca2+ in the regulation of Calvin-Benson-cycle enzymes is more complex. It appears that low levels of Ca2+ in the stroma are necessary for the activation of FBPase in the presence of reduced thioredoxin. Ca2+ apparently inhibits the catalytic activity of the enzyme (Charles and Halliwell, 1980; Hertig and Wolosiuk, 1980). Thus, there is an apparent discrepancy between the light-dependent transport of Ca2+ from the cytosol to the stroma and the activation of Calvin-Benson-cycle enzymes, because Ca2+ is known to inhibit the activity of FBPase, and elevated Ca2+ levels have a pronounced inhibitory effect on CO2 fixation. Ca2+ accumulated by chloroplasts in the light must be tightly bound in the stroma or sequestered in the thylakoid to prevent the inhibition of CO2 fixation by Ca2+ (Wolosiuk et al., 1993). The thylakoid membrane Ca2+/H+ antiporter would provide the means to sequester chloroplast Ca2+ in the thylakoid lumen in the light and thus prevent the Ca2+-mediated inhibition of Calvin-Benson-cycle enzymes.
Stores of Ca2+ in the thylakoid lumen may play an important role in the regulation of Calvin-Benson-cycle enzymes. Recently, Johnson et al. (1995) noted a sharp rise in stromal Ca2+ concentrations after the transition of plants from light to dark. This rise in stromal Ca2+ peaked 20 to 25 min after the transition from light to dark and may play a role in signaling the light-to-dark transition through the inhibition of Calvin-Benson-cycle enzymes. The peak is believed to represent a change in stromal free Ca2+ levels from a basal level of 150 nm to approximately 5 to 10 μm. Such a pronounced increase is unlikely to originate from the cytosol without the expenditure of a great deal of cellular energy in the dark. However, the rapid release of Ca2+ from luminal stores could result in dramatic changes in stromal Ca2+ concentrations, even if the Ca2+ was free to move from the stroma to the cytosol. We propose that the dramatic rise in stromal Ca2+ after the light-to-dark transition that was observed by Johnson et al. (1995) is coupled to the release of stores of Ca2+ from the thylakoid lumen.
On the basis of our results and the observations by Johnson et al. (1995), we propose that there are dramatic fluxes of Ca2+ across the thylakoid membrane that are induced by the transitions from dark to light and light to dark (Fig. 7). When dark-adapted chloroplasts are transferred to the light, there is an initial increase in trans-thylakoid membrane potential to about 60 to 80 mV (lumen positive), which subsequently decays within a few seconds to a steady state of 15 to 30 mV (Bulychev et al., 1972; Hangarter and Good, 1982; Remis et al., 1986). The collapse of the transient membrane potential is caused by the efflux of Mg2+ from the thylakoid lumen to the stroma (Fig. 7B). The magnitude of light-induced Mg2+ efflux is approximately 26 to 120 nmol Mg2+ mg−1 chlorophyll (Barber et al., 1974; Hind et al., 1974; Portis and Heldt, 1976; Krause, 1977; Enz et al., 1993).
Several Calvin-Benson-cycle enzymes, including Rubisco, FBPase, and sedoheptulose-1,7-bisphosphatase, are activated by Mg2+ ions (Portis and Heldt, 1976), which is in apparent agreement with the light-induced efflux of Mg2+ ions from the thylakoid lumen. However, the mechanism by which Mg2+ is returned to the thylakoid lumen in the dark has not yet been elucidated. We propose that such a mechanism may be the coupling of the exchange of luminal Ca2+ accumulated in the light (Fig. 7C) for stromal Mg2+ (Fig. 7D). The counterexchange of these ions would be electrically neutral. Any mechanism for exchanging Ca2+ and Mg2+ across the thylakoid membrane in the dark would need to be tightly regulated to prevent a futile cycle from forming. This exchange may be regulated by the state of oxidation or by the reduction of thioredoxin, as are several enzymes of the Calvin-Benson cycle (for review, see Wolosiuk, 1993). Cycling of Ca2+ and Mg2+ across the thylakoid membrane is correlated with the activation of Calvin-Benson-cycle enzymes by Mg2+ in the light and their inhibition by Ca2+ in the dark.
In conclusion, we have demonstrated the existence of a Ca2+/H+ antiporter in the thylakoid membrane. This an-tiporter provides the mechanism and energetics necessary to transport Ca2+ into the thylakoid lumen in the light and thereby supply Ca2+ for Ca2+-dependent processes in the thylakoid lumen, such as the assembly of the OEC. Additionally, our model of chloroplast Ca2+ homeostasis proposes that the uptake of cytosolic Ca2+ by chloroplasts in the light does not lead to a pronounced increase in stromal Ca2+ but, rather, results in an increase of Ca2+ stores in the thylakoid lumen. The Ca2+/H+ antiporter is critical for transporting stromal Ca2+ into the thylakoid lumen in the light, where it would have no inhibitory effect on Calvin-Benson-cycle enzymes. We propose that pools of Ca2+ sequestered in the lumen during a period of light are released from the lumen after a period of dark adaptation and complement the activity of thioredoxin in the inhibition of Calvin-Benson-cycle enzymes in the dark.
ACKNOWLEDGMENTS
We would like to thank Dr. Steven Theg for his valuable suggestions and Amanda B. Lehman and Hilary I. Van Hole for their excellent technical assistance. Silicone oils AR-200 and AR-20 were generously donated by Wacker Silicones.
Abbreviations:
- AMP-PNP
5‘-adenylyl imidodiphosphate
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- DCCD
N,N′-dicyclohexylcarbodiimide
- DCPIP
2,6-dichlorophenolindophenol
- FBPase
Fru-1,6-bisphosphatase
- OEC
oxygen-evolving complex of PSII
Footnotes
This research was funded by a grant to W.F.E. from the M.J. Murdock College Science Research Program.
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated chloroplasts. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J, Mills JB, Nicolson J. Studies with cation specific ionophores show that within the intact chloroplast Mg2+ acts as the main exchange cation for H+ pumping. FEBS Lett. 1974;49:106–110. doi: 10.1016/0014-5793(74)80643-5. [DOI] [PubMed] [Google Scholar]
- Becker DW, Callahan FE, Cheniae GM. Photoactivation of NH2OH-treated leaves: reassembly of released extrinsic PSII polypeptides and relegation of Mn into the polynuclear Mn catalyst of water oxidation. FEBS Lett. 1985;192:209–214. [Google Scholar]
- Blackford S, Rea PA, Sanders D. Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. J Biol Chem. 1990;265:9617–9620. [PubMed] [Google Scholar]
- Broussac A, Zimmermann J-L, Rutherford AW, Lavergne J. Histidine oxidation in the oxygen-evolving photosystem II enzyme. Nature. 1990;347:303–306. [Google Scholar]
- Bulychev AA, Andrianov VK, Kurella GA, Litvin FF. Micro-electrode measurements of the transmembrane potential of chloroplasts and its photoinduced changes. Nature. 1972;236:175–177. [Google Scholar]
- Bush DS. Regulation of cytosolic calcium in plants. Plant Physiol. 1993;103:7–13. doi: 10.1104/pp.103.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Charles SA, Halliwell B. Action of calcium ions on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase and other enzymes of the Calvin cycle. Biochem J. 1980;188:775–779. doi: 10.1042/bj1880775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Ettinger WF, Theg SM. Protein-specific energy requirements for protein transport across or into thylakoid membranes: two lumenal proteins are translocated in the absence of ATP. J Biol Chem. 1992;267:2688–2696. [PubMed] [Google Scholar]
- Cline K, Werner-Washburn M, Lubben TH, Keegstra K. Precursors to two nuclear encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985;260:3691–3696. [PubMed] [Google Scholar]
- Debus RJ. The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta. 1992;1102:269–352. doi: 10.1016/0005-2728(92)90133-m. [DOI] [PubMed] [Google Scholar]
- Dilley RA, Chiang GG. Chloroplast thylakoid membrane-bound Ca2+ acts in a gating mechanism to regulate energy-coupled proton fluxes. Ann NY Acad Sci. 1989;574:246–267. doi: 10.1111/j.1749-6632.1989.tb25163.x. [DOI] [PubMed] [Google Scholar]
- Enz C, Steinkanp T, Wagner R. Ion channels in the thylakoid membrane (a patch-clamp study) Biochim Biophys Acta. 1993;1143:67–76. [Google Scholar]
- Evans DE, Briars S-A, Williams LE. Active calcium transport by plant cell membranes. J Exp Biol. 1991;42:285–303. [Google Scholar]
- Ghanotakis DF, Babcock GT, Yocum CF. Calcium reconstitutes high levels of oxygen evolution in polypeptide-depleted photosystem II preparations. FEBS Lett. 1984;167:127–130. [Google Scholar]
- Gilroy S, Bethke PC, Jones RL. Calcium homeostasis in plants. J Cell Sci. 1993;106:453–462. doi: 10.1242/jcs.106.2.453. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu S-S, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Hangarter RP, Good NE. Energy thresholds for ATP synthesis in chloroplasts. Biochim Biophys Acta. 1982;681:397–404. [Google Scholar]
- Heldt HW. Measurement of metabolite movement across the envelope and of the pH in the stroma and the thylakoid space in intact chloroplasts. Methods Enzymol. 1980;69:604–613. [Google Scholar]
- Hertig C, Wolosiuk RA. A dual effect of Ca2+ on chloroplast fructose-1,6-bisphosphatase. Biochem Biophys Res Commun. 1980;97:325–333. doi: 10.1016/s0006-291x(80)80171-9. [DOI] [PubMed] [Google Scholar]
- Hind G, Nakatani HY, Izawa S. Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci USA. 1974;71:1484–1488. doi: 10.1073/pnas.71.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal T, Aro EM, Carlberg I, Andersson B. Restoration of light induced photosystem II inhibition without de novo protein synthesis. FEBS Lett. 1990a;267:203–206. doi: 10.1016/0014-5793(90)80925-9. [DOI] [PubMed] [Google Scholar]
- Hundal T, Virgin I, Styring S, Andersson B. Changes in the organization of photosystem II following light-induced D1 protein degradation. Biochim Biophys Acta. 1990b;1017:235–241. [Google Scholar]
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- Krause GH. Light-induced movement of magnesium ions in intact chloroplasts: spectroscopic studies with eriochrome blue SE. Biochim Biophys Acta. 1977;460:500–510. doi: 10.1016/0005-2728(77)90088-3. [DOI] [PubMed] [Google Scholar]
- Kreimer G, Melkonian M, Holtum JAM, Latzko E. Characterization of calcium fluxes across the envelope of intact spinach chloroplasts. Planta. 1985a;166:515–523. doi: 10.1007/BF00391276. [DOI] [PubMed] [Google Scholar]
- Kreimer G, Melkonian M, Holtum JAM, Latzko E. Stromal free calcium concentration and light-mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol. 1988;86:423–428. doi: 10.1104/pp.86.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer G, Melkonian M, Latzko E. An electrogenic uniport mediates light-dependent Ca2+-influx into intact spinach chloroplasts. FEBS Lett. 1985b;180:253–258. [Google Scholar]
- Mattoo AK, Marder JB, Edelman M. Dynamics of the photosystem II reaction center. Cell. 1989;56:241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- McNamera VP, Gounaris K. Granal photosystem II complexes contain only the high redox potential form of cytochrome b-559 which is stabilized by the ligation of calcium. Biochim Biophys Acta. 1995;1231:289–296. [Google Scholar]
- Miller A-F, Brudvig GW. Manganese and calcium requirements for reconstitution of oxygen-evolution in manganese-depleted photosystem II membranes. Biochemistry. 1989;28:8181–8190. doi: 10.1021/bi00446a033. [DOI] [PubMed] [Google Scholar]
- Muto S, Izawa S, Miyachi S. Light-induced Ca2+ uptake by intact chloroplasts. FEBS Lett. 1982;139:250–254. [Google Scholar]
- Nelson N, Eytan E, Notsani B-E, Sigrist H, Sigrist-Nelson K, Gitler C. Isolation of a chloroplast N,N′-dicyclohexylcarbodiimide-binding protein proteolipid, active in proton translocation. Proc Natl Acad Sci USA. 1977;74:2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS. Calcium uptake, ATPase and photophosphorylation by chloroplasts in vitro. Nature. 1967;214:875–877. doi: 10.1038/214875a0. [DOI] [PubMed] [Google Scholar]
- Nobel P. Light-induced changes in the ionic composition of chloroplasts in Pisum sativum. Biochim Biophys Acta. 1969;172:134–143. doi: 10.1016/0005-2728(69)90098-x. [DOI] [PubMed] [Google Scholar]
- Nobel PS, Packer L (1965) Light-dependent ion translocation in spinach chloroplasts. Plant Physiol 633–640 [DOI] [PMC free article] [PubMed]
- Portis AR, Heldt HW. Light dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976;449:434–446. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Pottosin, Schönknecht G. Ion channel permeable for divalent and monovalent cations in native spinach thylakoid membranes. J Membr Biol. 1996;152:223–233. doi: 10.1007/s002329900100. [DOI] [PubMed] [Google Scholar]
- Remis D, Bulychev AA, Kurella GA. The electrical and chemical components of the proton motive force in chloroplasts as measured with capillary and pH-sensitive electrodes. Biochim Biophys Acta. 1986;852:68–73. [Google Scholar]
- Robinson SP, Wiskich JT. Uptake of ATP analogs by isolated pea chloroplasts and their effect on CO2 fixation and electron transport. Biochim Biophys Acta. 1977;461:131–140. doi: 10.1016/0005-2728(77)90075-5. [DOI] [PubMed] [Google Scholar]
- Schumaker KS, Sze H. Calcium transport into the vacuole of oat roots: characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986;261:12172–12187. [PubMed] [Google Scholar]
- Shavit N, Dilley RA, San Peitro A. Ion translocation in isolated chloroplasts uncoupling of photophosphorylation and translocation of K+ and H+ by nigericin. Biochemistry. 1968;7:2356–2363. doi: 10.1021/bi00846a043. [DOI] [PubMed] [Google Scholar]
- Steele JA, Uchytil TF, Durbin RD, Bhatnagar P, Rich DH. Chloroplast coupling factor I: a species-specific receptor for tentoxin. Proc Natl Acad Sci USA. 1976;73:2245–2248. doi: 10.1073/pnas.73.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theg SM, Filar LJ, Dilley RA. Photoinactivation of chloroplasts already inhibited on the oxidizing side of photosystem II. Biochim Biophys Acta. 1986;849:104–111. [Google Scholar]
- Virgin I, Ghanotakis DF, Andersson B. Light induced D1-protein degradation in isolated photosystem II core complexes. FEBS Lett. 1990;269:45–48. doi: 10.1016/0014-5793(90)81115-5. [DOI] [PubMed] [Google Scholar]
- Wolosiuk RA, Ballicora MA, Hagelin K. The reductive pentose phosphate cycle for photosynthetic CO2 assimilation: enzyme modulation. FASEB J. 1993;7:622–737. doi: 10.1096/fasebj.7.8.8500687. [DOI] [PubMed] [Google Scholar]