Abstract
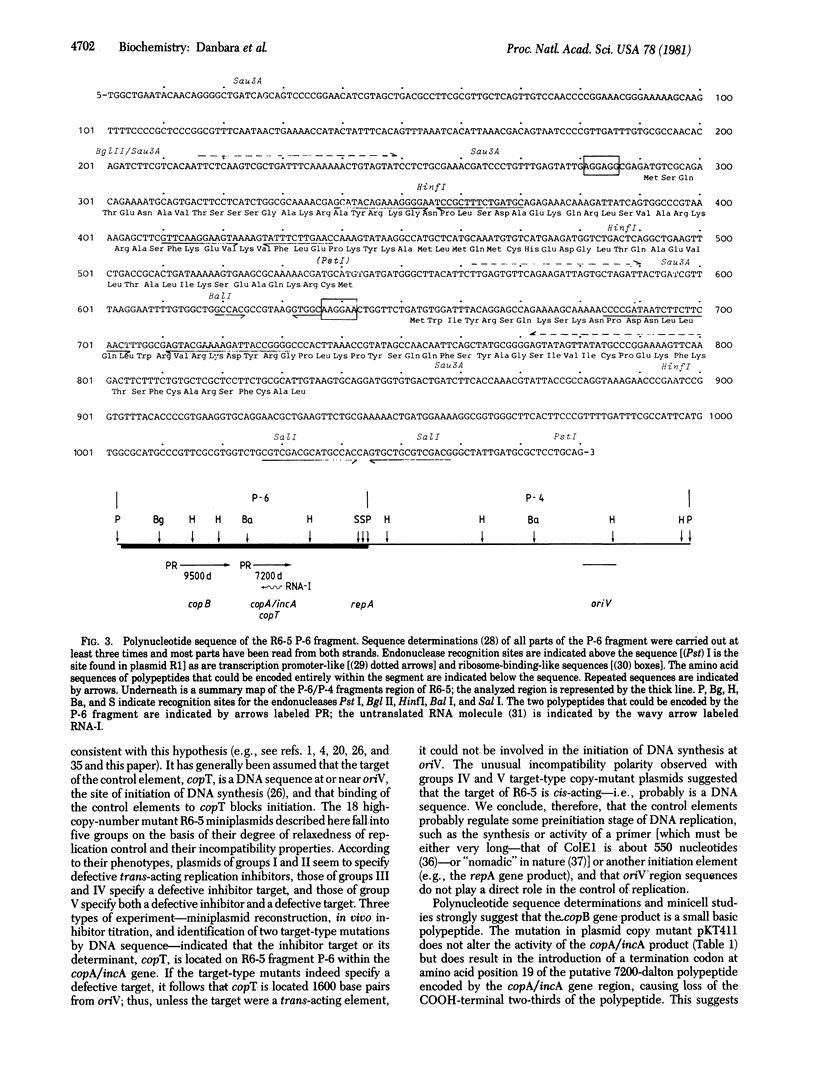
The replication control system of plasmid R6-5 has been investigated by characterization of high-copy-number mutant miniplasmids, development of an in vivo assay for the site of action or "target" of the replication control elements, and sequence analysis of the replication control regions of the wild-type plasmid and two copy-number mutant derivatives. These and other experiments have shown that three plasmid determinants--copA/incA, copB, and copT--are involved in DNA replication control. The products of the copB and copA/incA genes, a 9500-dalton basic polypeptide and either a 7200-dalton basic polypeptide or a short untranslated RNA molecule, respectively, are negative-acting elements that interact with the third element, their target, the copT DNA sequence, or its product to regulate the frequency of initiation of plasmid replication. The location of copT within the copA/incA gene and 1600 base pairs upstream from the origin of replication indicates that regulation is effected at a preinitiation stage of replication, such as the production of a primer or other initiation factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrés I., Slocombe P. M., Cabello F., Timmis J. K., Lurz R., Burkardt H. J., Timmis K. N. Plasmid replication functions. II. Cloning analysis of the repA replication region of antibiotic resistance plasmid R6-5. Mol Gen Genet. 1979 Jan 5;168(1):1–25. doi: 10.1007/BF00267929. [DOI] [PubMed] [Google Scholar]
- Backman K., Betlach M., Boyer H. W., Yanofsky S. Genetic and physical studies on the replication of ColE1-type plasmids. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):69–76. doi: 10.1101/sqb.1979.043.01.012. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbara H., Timmis J. K., Lurz R., Timmis K. N. Plasmid replication functions: two distinct segments of plasmid R1, RepA and RepD, express incompatibility and are capable of autonomous replication. J Bacteriol. 1980 Dec;144(3):1126–1138. doi: 10.1128/jb.144.3.1126-1138.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Goebel W., Bonewald R. Class of small multicopy plasmids originating from the mutant antibiotic resistance factor R1 drd-19B2. J Bacteriol. 1975 Aug;123(2):658–665. doi: 10.1128/jb.123.2.658-665.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Timmis K. N. Incompatibility properties of Col E1 and pMB1 derivative plasmids: random replication of multicopy replicons. Cell. 1981 Jan;23(1):229–238. doi: 10.1016/0092-8674(81)90287-7. [DOI] [PubMed] [Google Scholar]
- Hsiang M. W., Cole R. D., Takeda Y., Echols H. Amino acid sequence of Cro regulatory protein of bacteriophage lambda. Nature. 1977 Nov 17;270(5634):275–277. doi: 10.1038/270275a0. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci U S A. 1980 May;77(5):2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollek R., Oertel W., Goebel W. Isolation and characterization of the minimal fragment required for autonomous replication ("basic replicon") of a copy mutant (pKN102) of the antibiotic resistance factor R1. Mol Gen Genet. 1978 Jun 1;162(1):51–57. doi: 10.1007/BF00333850. [DOI] [PubMed] [Google Scholar]
- Matsubara K. Genetic structure and regulation of a replicon of plasmid lambdadv. J Mol Biol. 1976 Apr 15;102(3):427–439. doi: 10.1016/0022-2836(76)90325-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Bauer W. Isolation, by tetracycline selection, of small plasmids derived from R-factor R12 in Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):644–655. doi: 10.1128/jb.127.1.644-655.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Cloning of replication, incompatibility, and stability functions of R plasmid NR1. J Bacteriol. 1980 Jan;141(1):87–99. doi: 10.1128/jb.141.1.87-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Diaz R., Uhlin B. E., Nordström K. Runaway replication of plasmid R1 is not caused by loss of replication inhibitor activity of gene cop. J Bacteriol. 1980 Aug;143(2):1046–1048. doi: 10.1128/jb.143.2.1046-1048.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Nordström K. Control of plasmid R1 replication: functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol. 1980 Jan;141(1):111–120. doi: 10.1128/jb.141.1.111-120.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo E., Feingold J., Ohtsubo H., Mickel S., Bauer W. Unidirectional replication in Escherichia coli of three small plasmids derived from R factor R12. Plasmid. 1977 Nov;1(1):8–18. doi: 10.1016/0147-619x(77)90004-x. [DOI] [PubMed] [Google Scholar]
- Rosen J., Ryder T., Inokuchi H., Ohtsubo H., Ohtsubo E. Genes and sites involved in replication and incompatibility of an R100 plasmid derivative based on nucleotide sequence analysis. Mol Gen Genet. 1980;179(3):527–537. doi: 10.1007/BF00271742. [DOI] [PubMed] [Google Scholar]
- Rosen J., Ryder T., Ohtsubo H., Ohtsubo E. Role of RNA transcripts in replication incompatibility and copy number control in antibiotic resistance plasmid derivatives. Nature. 1981 Apr 30;290(5809):794–797. doi: 10.1038/290794a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Silver L., Chandler M., de la Tour E. B., Caro L. Origin and direction of replication of the drug resistance plasmid R100.1 and of a resistance transfer factor derivative in synchronized cultures. J Bacteriol. 1977 Sep;131(3):929–942. doi: 10.1128/jb.131.3.929-942.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Cohen S. N. Nonchromosomal antibiotic resistance in bacteria. V. Isolation and characterization of R factor mutants exhibiting temperature-sensitive repression of fertility. J Bacteriol. 1972 Jun;110(3):1082–1088. doi: 10.1128/jb.110.3.1082-1088.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synenki R. M., Nordheim A., Timmis K. N. Plasmid replication functions. III. Origin and direction of replication of a "mini" plasmid derived from R6-5. Mol Gen Genet. 1979 Jan 5;168(1):27–36. doi: 10.1007/BF00267930. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N. Structural and functional analysis of cloned DNA segments containing the replication and incompatibility regions of a miniplasmid derived from a copy number mutant of NR1. J Bacteriol. 1979 Jan;137(1):92–104. doi: 10.1128/jb.137.1.92-104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Slocombe P. M. Plasmid incompatibility: cloning analysis of an incFII determinant of R6-5. Nature. 1978 May 4;273(5657):27–32. doi: 10.1038/273027a0. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Danbara H., Brady G., Lurz R. Inheritance functions of group IncFII transmissible antibiotic resistance plasmids. Plasmid. 1981 Jan;5(1):53–75. doi: 10.1016/0147-619x(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. Plasmid incompatibility and control of replication: copy mutants of the R-factor R1 in Escherichia coli K-12. J Bacteriol. 1975 Nov;124(2):641–649. doi: 10.1128/jb.124.2.641-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M. Identification and mapping of the replication genes of an R factor, R100-1, integrated into the chromosome of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):1123–1131. doi: 10.1128/jb.118.3.1123-1131.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



