Abstract
Introduction
Tendons establish specific connections between muscles and the skeleton by transferring contraction forces from skeletal muscle to bone thereby allowing body movement. Tendon physiology and pathology are heavily dependent on mechanical stimuli. Tendon injuries clinically represent a serious and still unresolved problem since damaged tendon tissues heal very slowly and no surgical treatment can restore a damaged tendon to its normal structural integrity and mechanical strength. Understanding how mechanical stimuli regulate tendon tissue homeostasis and regeneration will improve the treatment of adult tendon injuries that still pose a great challenge in today's medicine.
Source of data
This review summarizes the current status of tendon treatment and discusses new directions from the point of view of cell-based therapy and regenerative medicine approach. We searched the available literature using PubMed for relevant original articles and reviews.
Growing points
Identification of tendon cell markers has enabled us to study precisely tendon healing and homeostasis. Clinically, tissue engineering for tendon injuries is an emerging technology comprising elements from the fields of cellular source, scaffold materials, growth factors/cytokines and gene delivering systems.
Areas timely for developing research
The clinical settings to establish appropriate microenvironment for injured tendons with the combination of these novel cellular- and molecular-based scaffolds will be critical for the treatment.
Keywords: tendon injury, tissue engineering, regenerative medicine, stem cells, scleraxis, mechanical force
Tendon physiology
Tendon, a fibrous connective tissue made of specialized fibroblasts called ‘tenocytes’ and an abundant collagenous extracellular matrix (ECM), is a tissue whose physiology and pathology is heavily dependent on mechanical stimuli.1 Tendons establish specific connections between muscles and the skeleton by transferring contraction forces from skeletal muscle to bone, thereby allowing body movement.2 Tendons exhibit high mechanical strength, good flexibility and an optimal level of elasticity to perform their unique role. The tensile strength of a tendon is related to its thickness and collagen content: for example, a tendon with an area of 1 cm2 is capable of bearing 500–1000 kg.3 Tendons have relatively few blood vessels and function at a low metabolic rate. Tendons receive oxygen and nutrients from three main sources: internally via the myotendinous junction and osteotendinous junctions, and externally through the paratenon or the synovial sheath.4
Tendon development and adult homeostasis
During embryonic development, tenocytes originate from mesodermal compartments, as do skeletal myoblasts, chondrocytes and osteoblasts.5 Some of the multipotent mesenchymal progenitor cells that arise from these compartments express the basic helix-loop-helix transcription factor scleraxis. However, once they are committed to become cells making up a specific tissue, only tendon progenitor cells and tenocytes retain the ability to express scleraxis. Therefore, scleraxis is a highly specific marker of tenogenic cells and mature differentiated tenocytes.6,7 The scleraxis gene is thus the first master gene found to be essential for establishing the tendon lineage during development.8 Tenomodulin is a type II transmembrane glycoprotein. Its robust expression is induced in mouse tendons in a late (embryonic day [E] 17.5) developmental phase and is also observed in adult tendons showing that tenomodulin is a marker of mature (differentiated) tenocytes.9 In vitro experimental evidence shows that the genes composed of tendon-specific ECM are tightly regulated in tenocytes by mechanical forces.2 Very recently, tendon stem/progenitor cells have been discovered in human and mouse tendon, and the proteoglycans biglycan and fibromodulin have been identified as essential elements in a microenvironment to keep phenotypes and differentiation of stem/progenitor cells.10 However, the roles of these stem/progenitor cells in adult tendon homeostasis and/or wound healing remains unknown.
Mechanical force and tenocytes
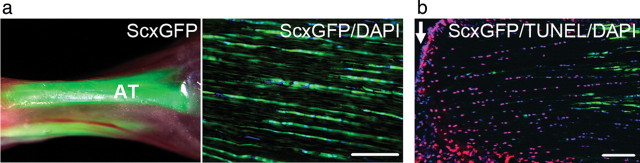
Since tendon tissues are constantly exposed to variable transmittal forces from skeletal muscles, our laboratory examined the functional links between mechanical forces and tenocyte phenotypes using scleraxis as a tenocyte marker. We utilized a transgenic mouse strain, which expresses the green fluorescent protein (GFP) marker driven by the scleraxis gene in a way that GFP is produced in a pattern that mimics its expression in the body6 (Scleraxis-GFP transgenic mice were kindly provided by Dr Ronen Schweitzer, Research Division, Shriners Hospital for Children, Portland, OR, USA). The vast majority of tenocytes show robust expression of scleraxis-GFP in adult tendon tissues under the fluorescence microscope (Fig. 1a). Strikingly, the sudden interruption of continuous tensile loading, such as by complete transection tendon injury, leads to a decreased expression of scleraxis and tenocyte death (Fig. 1b). Thus, these findings indicate a critical role for mechanical forces in adult tendon homeostasis and strongly suggest new directions for therapy following tendon injuries.11
Fig. 1.
Tensile loading plays a crucial role in tenocytes. (a) Achilles tendons in adult Scleraxis-GFP transgenic mice. Left panel: under fluorescence stereomicroscope; right panel: under microscope with GFP/ultraviolet (UV) filters to show scleraxis-GFP (green) and nucleus [4′6-diamidino-2-phenylinodole (DAPI) blue]. AT, Achilles tendon. Bar = 100 µm. (b) Analysis of cell death at 2 h after complete transection of adult Achilles tendon in Scleraxis-GFP transgenic mice. Arrows indicate the transection edge of tendons. Note that the expression of scleraxis-GFP (green) is diminished and cells positive by terminal deoxynucleotidyl transferase dUTP nick-end labeling assay (TUNEL assay: a marker for cell death, red) are evident in the transected Achilles tendon. Bar = 100 µm.
Tendon injury
Incidence of tendon injury
Soft-tissue injuries, including injury to tendon, ligament or meniscus, can induce abnormal joint motions and altered loading in the short term and they could contribute to degenerative joint disease and osteoarthritis in the long term.12. These injuries can be acute or chronic and are caused by intrinsic or extrinsic factors, either alone or in combination.4 Acute tendon injury interrupts tendon continuity with consequent disruption of ECM architecture and dramatic loss of transmittal forces from skeletal muscle.4 Tendon injuries represent a serious and still unresolved problem. More than 130 000 patients per year undergo tendon-related surgery in the USA.13 The tendons most frequently affected are shoulder rotator cuff (51 000 cases), Achilles tendon (44 000 cases) and patellar tendon (42 000 cases).13 Injuries to Achilles tendon, patellar tendon, hand flexor tendon and shoulder rotator cuff have clinical importance since they can lead to loss of muscle function, significant disability, joint instability and secondary osteoarthritis, adversely affecting a patient's activities of daily living and quality of life. The incidence of tendon injury has increased in recent years as the number of aging adults continues to grow.14 The altered activity of mechanical loading, and vasculature and angiogenesis are suggested to play a significant role in degenerative tendon diseases.15,16
Tendon healing
Tendon wound healing involves regeneration of tenocytes and reconstruction of dense collagen fibrils, and the tendon repair process in transected experimental animal tendons is known to involve three overlapping phases, as for other organs/tissues.4,13. An initial, inflammatory phase occurs until Day 2 after injury. It involves extensive cell death in the injured area and subsequent inflammatory cell infiltration. A second, proliferative phase starts at Day 3. It involves cell migration into the injured area, extensive proliferation and production of collagen fibrils. A third, remodeling phase occurs from 6 weeks on. This phase can be divided into a consolidation stage, from 6 to 10 weeks after injury, and a maturation stage, after 10 weeks. It is characterized by decreased cellularity and collagen synthesis, and the alignment of tenocytes and collagen fibrils in the direction of stress. ECM-remodeling during tendon wound healing follows in general the same processes as in other tissues, i.e. in an early stage, provisional matrix formation by the plasma proteins fibrinogen and fibronectin, followed by replacement of the provisional matrix by collagen fibrils.2,4
In the inflammatory phase, vasoactive and chemotactic factors such as cytokines and growth factors are released and lead to an increased vascular permeability, initiation of angiogenesis and stimulation of tenocyte proliferation. In particular, various growth factors/cytokines play a number of important roles, including stimulation of tenocyte proliferation, cell migration to the wound and synthesis of the new ECM during tendon healing.17,18 In the proliferation phase, two mechanisms, intrinsic and extrinsic mechanisms, are likely to contribute to the healing process. The intrinsic mechanism involves the proliferation of tenocytes from the tendon and epitenon. These tenocytes contribute to synthesize the new ECM, which consists largely of collagens and glycosaminoglycans. Such intrinsic healing results in improved biomechanics and fewer post-injury complications. In contrast, the extrinsic mechanism involves the migration of inflammatory cells and fibroblasts from the overlying sheath and synovium into the wounded site.4,19 This often causes scar tissue and results in adhesion formation, which disrupts tendon gliding. Although tendon healing follows the same process as that in various connective tissues, including skin and muscle, tendon tissues heal more slowly than other connective tissues, probably because of the low metabolic rate of tendons and their dense and hypocellular composition (only 5% of the volume is occupied by cells).4,5 Furthermore, ECM remodeling such as increasing diameters and cross-linking of collagen fibrils occurs over a period of up to 2 years following tendon injury.3 Despite such remodeling, the biochemical and mechanical properties of healed tendons never match those of intact ones.3 The final tensile strength in the healed tendons is eventually reduced to ∼30% the strength of intact tendons.20
Therapies in tendon in injury
General concepts in therapies in tendon injury
Clinically, two main strategies for treating injured tendons are followed:21 (i) leave them untreated to heal naturally or (ii) perform surgery for primary repair of injured tendons using various techniques (Table 1). These treatments are determined in general according to clinical factors, such as age, tendon location, mode of injury and size of defect. Surgical treatment of an acute Achilles tendon rupture has been considered superior to non-operative treatment to prevent the risk of re-rupture.22,23 However, investigators in a recent multicenter randomized trial (NCT00284648 in ClinicalTrials.gov) reported that similar clinical outcomes are observed between (i) non-operative groups with accelerated functional rehabilitation and (ii) operative groups.24 Thus, the treatment for acute Achilles tendon rupture remains inconclusive.22,24 Furthermore, for injuries to the hand flexor tendon or shoulder rotator cuff, long-term clinical outcomes of primary repair remain unsatisfactory.13,25 Despite improved surgical techniques and advances in postoperative therapy regimens, these treatments still have potential complications such as re-rupture of the repair site or the formation of restrictive adhesions.25,26 Recently, the biological grafts, such as autologous fascia, porcine small intestinal submucosa or synthetic materials, have been developed and used in tendon graft procedures to fill large tissue defects (Table 2). However, no graft method exists to restore a damaged tendon to its normal function. Grafting poses several potential complications, including increased inflammatory responses, antigenic reactions, failure at the fixation sites and deficiencies in long-term biocompatibility.25,26 Thus, current knowledge of the biology in tendon healing has yet to lead to clinically successful strategies for treatment.19 New treatment modalities are required to promote the regeneration of tendon tissues.
Table 1.
Characteristics for treatment in tendon injury.
| Treatment type | Indication | Advantages | Disadvantages |
|---|---|---|---|
| Immobilization | Acute Achilles tendon rupture | No surgical complication | Failure to heal |
| Plaster | Tendinopathy | Rerupture | |
| Brace | Muscle atrophy | ||
| Tendon atrophy | |||
| Limitation of range of motion | |||
| Decreasing of tensile strength | |||
| Primary repair | Most acute tendon injury | First clinical choice | Suture failure |
| Chronic tear ofrotator cuff | Recovery of muscle contraction | Rerupture | |
| Inability to achieve functional results | |||
| Postoperative adhesion | |||
| Infection | |||
| Tendon transfer | Large tendon defect | Filling of tissue defect | Suture failure |
| Primary repair failure | Recovery of muscle contraction | Rerupture | |
| Chronic tendon injury | Inability to achieve functional results | ||
| Postoperative adhesion | |||
| Infection | |||
| Altered biomechanics of joint | |||
| Tendon graft | Large tendon defect | Filling of tissue defect | Suture failure |
| Autograft | Primary repair failure | Recovery of muscle contraction | Rerupture |
| Allograft | Chronic tendon injury | Inability to achieve functional results | |
| Xenograft | Destructive disease | Postoperative adhesion | |
| Synthetic material | Congenital disorder | Infection | |
| Limited availability of material | |||
| Altered biomechanics of joint | |||
| Deficiency of biocompatibility | |||
| Immunologic rejection | |||
| Antigenic reaction | |||
| Disease transmission |
Table 2.
Scaffold materials for tendon injury.
| Biologic (naturally occurring) | |
| Human tissue | Dermis |
| Dura mater | |
| Animal tissue | Porcine small intestinal submucosa |
| Porcine dermis | |
| Biopolymers | Type I collagen |
| Synthetic (manufactured) | |
| Resorbable | Polyethylene |
| Polyglycolic acid | |
| Polylactic acid | |
| Poly-N-acetyl-d-glucosamine | |
| Non-resorbable | Carbon fibers |
| Dacron® | |
| Nylon | |
| Polyacrylamide | |
| Silicone | |
| Teflon® | |
| Silk | |
New treatment strategies for tendon healing
To design efficient strategies to enhance tendon repair following injury, it is essential that scientists and clinicians understand the cellular and molecular mechanisms responsible for the development, homeostasis, regeneration and repair of tendons. To date, however, the molecular mechanisms underlying tendon repair remain largely unknown. The target molecules for tendon repair are based on knowledge from general wound healing, not on results generated specifically from analysis of tendon healing.27
Tissue engineering is an emerging technology that offers a novel approach for treating tendon injuries and restoring tissue and joint function.28 The most common tissue-engineering principles are (i) the use of healthy multipotent cells that are non-immunogenic, easy to isolate and highly responsive to distinct environmental cues; (ii) the development of carrier scaffolds that provide short-term mechanical stability of the transplant as well as a template for spatial growth of the regenerating tissue; and (iii) the delivery of growth factors that drive the process of cell differentiation and maturation.25
Orthopedic tissue engineering comprises elements from the fields of cell biology, materials science, mechanical engineering and surgery.12 Various types of scaffolds, such as naturally occurring ECMs as well as cell-based strategies have been developed12,25,26,29–33 (Table 2). Mesenchymal stem cells (MSCs) are becoming a subject of increasing interest because of their potential utility in tissue-engineering applications34,35 (Table 3). MSCs exist in adult bone marrow and can be induced to form different mesenchymal tissue lineage cells such as chondrocytes, adipocytes or osteoblasts. In fact, MSCs-based scaffolds have been tried in animal models of tendon wound healing.
Table 3.
New modalities for treatment in tendon injury.
| Treatment type | Methods of delivery | Advantages | Disadvantages |
|---|---|---|---|
| Scaffolds | |||
| Biologic material | Direct implantation | Abundant supply (type I collagen) | Limited clinical applications (autograft) |
| Limited recovery of strength | |||
| Relatively low complications (type I collagen) | Potential complications as in Table 1 | ||
| Synthetic material | Direct implantation | Abundant supply | Limited recovery of strength |
| Limited regeneration | |||
| Potential complications as in Table 1 | |||
| Cell therapy | |||
| Tenocyte | Direct implantationwith collagen materials | Differentiated cell | Limited cellular source |
| Low proliferative ability | |||
| Low morbidity | |||
| Mesenchymal stem cell | Direct implantationwith collagen materials | Excellent regenerative capacity | Unclearness of mechanisms in differentiation |
| High proliferative ability | |||
| Low morbidity | |||
| Invasive procurement procedure | |||
| Low yielding | |||
| Growth factors | Direct administration | Easy administration | Unclearness of growth factor stability |
| Unclearness of effective concentration | |||
| Gene therapy | |||
| Viral method | Direct viral infection | High transduction efficiency | Inflammatory response |
| Transient expression | Non-specific infection | ||
| Non-viralmethod | Direct administration with liposomes | Low pathogenic response | Relatively low transduction efficiency |
Cellular scaffold-based therapy
Scaffolds
The underlying concept for tissue engineering technologies has been changing. Traditionally, a graft was composed of some material (such as nylon or silk) meant solely to fill the tissue defect. Nowadays, implants are expected to serve as ‘biocompatible scaffolds’ (natural or synthetic materials that can be replaced by host tissues without undesirable responses). These biocompatible scaffolds are suitable as vehicles for implanted cells, the delivery of growth factors, or the transfer of genes25,26. Both biologic and synthetic materials are used to create scaffolds for tendon reconstruction with a three-dimensional biocompatible construct that serves as a temporary or permanent implant26. As described, injured tendons have very limited spontaneous healing capabilities. Thus, ideal scaffold materials need to play at least two essential roles: to stimulate regeneration (including proliferation and differentiation of cells) at implanted sites and to establish the specific composition and structure of an ECM that can then provide an appropriate microenvironment for regenerating cells. The major ECM component in tendons is type I collagen. The advantages of using type I collagen for tendon reconstruction include its strength, capacity to resorb and ability to induce the alignment of host connective tissues.26. Scaffolds of type I collagen cross-linked with glutaraldehyde or carbodiimide are used in research to regenerate tendon tissue because of the low antigenicity and strength.26,36 Indeed, they have improved graft strength in a rabbit Achilles tendon model.36 Synthetic non-resorbable materials, including nylon, silk and carbon, are not biocompatible because of host foreign body responses and late mechanical failure.26 To circumvent these problems, synthetic resorbable materials have been developed using polyglycolic acid or polylactic acid.25 They can be fabricated into three-dimensional scaffolds of variable structure and porosity with a correspondingly wide range of mechanical and degradation properties.25 Unfortunately, some synthetic resorbable scaffolds alter the mechanical properties of the repaired tendon, lose strength and integrity over time, limit tendon ingrowth, cause abrasions of surrounding tissues, enhance the inflammatory response and cause undesirable scar formation around the repair site.26 A study in a goat shoulder rotator-cuff repair model indicates that the polylactic acid-scaffold does not show significant increase in the load-to-failure strength, even though the polylactic acid patch is occupied by cellular fibrous tissues.37 Therefore, despite their potential roles in tendon reconstruction, further investigation will be necessary to find an alternative to natural materials.
Cell-based therapy
Cell-based therapy is also a novel technique to improve the composition, structure and biomechanical properties of new tendon tissue: cells are initially seeded onto scaffolds, and then they are delivered to the injured sites as cell- and scaffold-combined materials.26 To date, several different combinations of cell types and biomaterial scaffolds have been used in experimental animal models (including MSCs-type I collagen gel, MSCs-knitted polylactide-co-glycolide matrix, tenocytes-non-woven polyglycolic acid fibers), and they have the capacity to enhance tendon formations.30–33,38 In these biomaterial scaffolds, a plenty of materials such as collagen gel or synthetic biodegradable polymers are commercially available. On the other hand, cells seeded on such a scaffold need to proliferate rapidly in vitro to provide adequate numbers for in vivo implantation.25 An important prerequisite for cell-based therapy is the successful isolation and selection of appropriate cells.25 A tenocyte-based method is one of the potential cell-based therapies, but a number of concerns still limit the practicality of its use: (i) a limited availability of donor sites tenocytes from which tenocytes may be obtained for implantation, (ii) the time requirements for lengthy in vitro culture to expand the number of cells and (iii) the morbidity of tenocytes themselves.39 To circumvent the negative impact of this tenocyte-based method, MSCs have been investigated as an alternative source for tendon engineering. MSCs, which show an excellent capability for regeneration and rapid proliferation, have the potential to differentiate into a spectrum of specialized mesenchymal tissues, tendon, ligament, bone, cartilage, muscle, fat and marrow stroma.25 In addition, MSCs can be relatively easily isolated from bone marrow, but they are also found in muscle, adipose tissue, skin and around blood vessels.40 The ability of MSCs for tendinogenic differentiation has been documented in several studies.31–33 In fact, recruitment of MSCs to accelerate repair and tissue regeneration was shown in vivo in a rabbit tendon tissue model.32 However, no significant differences were observed in mechanical properties between MSC-transplanted and non-transplanted repaired tissues. Moreover, 28% of MSC-treated tendons developed foci of ectopic bone, whereas no bone formed in naturally healing contralateral controls.29,41 These studies clearly indicate that the determination of an appropriate MSC microenvironment for tenocyte differentiation is a critical issue that needs further investigation. We also need to take into consideration several more issues relating to the clinical application of MSC-based therapy: long-term safety of the patient, large-scale culture and storage of cells, ideal scaffold materials, optimal cell seeding conditions and an alternative mode of applying MSC-material composite to the injured site.4,25
Molecular-based therapy
Growth factors and cytokines
Growth factors/cytokines represent one of the largest molecular families involved in the wound healing process, and a considerable number of studies have been undertaken in an effort to elucidate their many functions and behaviours during healing progression.17 Several molecules have been identified as key factors during the repair process of tendons, including transforming growth factor-β (TGF-β), insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and growth and differentiation factor (GDF)-5 through 7.26 Since TGF-β regulates a wide variety of cellular processes, including the expression of scleraxis during tendon formation in embryonic development,42 such multifunctional aspects of TGF-β have been extensively studied in relation to adult tendon injury and homeostasis. The expression levels of TGF-β in adult tendons are dramatically upregulated in a short time after injury, and TGF-β initiates an inflammatory response to tissue damage.17 In contrast, TGF-β upregulates the production of ECMs, which results in excessive scar formation. Indeed, the local administration of a neutralizing antibody of TGF-β can diminish excessive production of ECM and improve the postoperative range of motion in a rabbit model of complete transection of the hand flexor tendon.43 Thus, such contradictory functional aspects of TGF-β make it difficult to rely on TGF-β for clinical use in tendon healing.3 IGF-1 stimulates synthesis of DNA, collagen and proteoglycans, as well as tenocyte proliferation and migration in vitro.44 IGF-1 also acts synergistically with PDGF to stimulate tenocyte migration.44 A study in a rat Achilles tendon transection model indicates that the injection of IGF-1 at injured sites accelerates functional recovery of Achilles tendon.45 GDF-5, -6 and -7 (members of the TGF-β superfamily that are related to bone morphogenetic proteins) can induce neotendon formation, as assessed by histochemical analysis when injected at subcutaneous sites in rats.18 Another study shows that the injection of GDF-5, -6 or -7 into injured Achilles tendons in rats results in a significant dose-related increase of mechanical properties in rat Achilles tendon.46
Some success has been achieved utilizing single growth factors as therapeutics.17 Direct injection of a growth factor at the injured site may give a temporary boost of a single healing signal but has only limited effect on the final outcome.17 The combination of patients’ own growth factors to promote healing in injured tissues is a potentially very fruitful area of research.17 Platelet-rich plasma (PRP), easily harvested from whole blood by a few centrifugation steps, contains autologous growth factors such as PDGF, TGF-β, IGF-1 and -2 and bFGF.47 Postoperative direct injection of PRP significantly improves mechanical strength and stiffness in a rat Achilles tendon repair model.48 Recently, there has been increasing interest in the field of sports medicine to facilitate healing and earlier return to activity after tendon and ligament injury.49 Several clinical trials investigating the efficacy of PRP treatment have been performed for Achilles tendon rupture (NCT00731068 in ClinicalTrials. gov) and rotator cuff injury (NCT01000935; NCT01152658; NCT01170312 in ClinicalTrials.gov). However, recent randomized clinical trials indicate that PRP treatment has no significant effect on the healing of tendon and ligament injuries.49,50
Gene therapy
A variety of gene transfer methods have been used both in vitro and in vivo to induce local production of growth factors such as GDF-7 or PDGF by means of viral (e.g. adenovirus, retrovirus) or non-viral vectors (e.g. liposomes) to accelerate the tendon repair process.51,52 The use of a non-viral vector-mediated delivery is less pathogenic because of the absence of viral proteins but is also less efficient than the use of viral vectors.25 The expression of a transgene is transient but generally manipulated for up to 8–10 weeks: this method is more beneficial than repeating local injection of growth factors/cytokines.3,25 To date, the recombinant viral system is well suited for a study of its potential as a therapy of tendon injuries using experimental animal models. Two main strategies for gene transfer using vectors can be envisioned: (i) in vivo transfer with a vector that is applied directly to the relevant tissue (in vivo direct gene transfer method) and (ii) the removal of cells from the body, transfer of the gene in vitro, and then reintroduction into the target site in the body (ex vivo indirect gene transfer method).25 The in vivo direct gene transfer method is less invasive and technically easier than transfer of cells in vitro. However, the disadvantages of in vivo transfer are potential inflammatory responses and non-specific infections at the injury site. The ex vivo indirect gene transfer method can ensure the collection of only selected/targeted cells in vitro that express the transgene at high concentrations to the injury site with less chance of contamination of viral DNA and proteins. Therefore, the ex vivo indirect gene transfer method could be preferable for the treatment of a large and degenerative tendon injury such as that to the rotator cuff, rather than an acute tendon injury.
Future directions for tendon injury treatment
Tendon injuries clinically represent the serious and still unresolved problem of how best to restore a damaged tendon to more nearly normal structural integrity and mechanical strength. Considering the unique feature of tendons with their constant and high mechanical loading, new treatment modalities are required to promote the regeneration of tendon tissues. Tissue engineering offers many approaches for treating tendon injuries and restoring tissues and joint functions. Indeed, a variety of synthetic and biologic scaffolds have been developed. The combination of several scaffolds, including synthetic materials, cells such as MSCs or tendon progenitor cells and growth factors/cytokines, could be an attractive way to generate an appropriate microenvironment for both transplanted and tendon cells at the injured site. We also foresee many other novel cellular sources and technologies, induced pluripotent stem (iPS) cells or tendon stem/progenitor cells and nanomedicine scaffolds. The emerging interventions using those orthopaedic tissue engineering technologies have shown a therapeutic effect in experimental animal models of tendon injury. However, their efficacy and safety in humans remains to be elucidated in the clinical setting. Unfortunately, to date, the development of new treatment strategies for injured tendons has been hindered because of our limited understanding of basic tendon biology. Nevertheless, the translation of basic research into improved therapeutic approaches is an essential step in the management of patients with tendon injuries, having the potential for significant benefit to public health.
Funding
This work was supported in part by National Institutes of Health, grant no. R01 DK074538, The Cleveland Clinic and the Sumitomo Foundation, Japan (to T. Sakai).
Acknowledgements
We wish to acknowledge many outstanding contributions of investigators in the field whose work could not be cited because of space constraints.
References
- 1.Zhang G, Young BB, Ezura Y, et al. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- 2.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. doi:10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Maffulli N. The future: rehabilitation, gene therapy, optimization of healing. Foot Ankle Clin. 2005;10:383–97. doi: 10.1016/j.fcl.2005.01.005. doi:10.1016/j.fcl.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. doi:10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 5.Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–36. doi: 10.1002/bdrc.20049. doi:10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- 6.Pryce BA, Brent AE, Murchison ND, et al. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–82. doi: 10.1002/dvdy.21179. doi:10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 7.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 8.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development. 2010;137:2807–17. doi: 10.1242/dev.047498. doi:10.1242/dev.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docheva D, Hunziker EB, Fassler R, et al. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. doi:10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. doi:10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Sakabe T, Sunaga A, et al. Curr Biol. 2011. Conversion of mechanical force into TGF-β-mediated biochemical signals. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler DL, Shearn JT, Juncosa N, et al. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin Orthop Relat Res. 2004;427S:S190–9. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- 13.Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–29. doi: 10.1146/annurev.bioeng.6.040803.140240. doi:10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- 14.Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports. 1997;7:113–8. doi: 10.1111/j.1600-0838.1997.tb00127.x. doi:10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 15.Pufe T, Petersen WJ, Mentlein R, et al. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–22. doi: 10.1111/j.1600-0838.2005.00465.x. doi:10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 16.Boesen MI, Koenig MJ, Torp-Pedersen S, et al. Tendinopathy and Doppler activity: the vascular response of the Achilles tendon to exercise. Scand J Med Sci Sports. 2006;16:463–9. doi: 10.1111/j.1600-0838.2005.00512.x. doi:10.1111/j.1600-0838.2005.00512.x. [DOI] [PubMed] [Google Scholar]
- 17.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–94. doi: 10.2165/00007256-200333050-00004. doi:10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 18.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–30. doi: 10.1172/JCI119537. doi:10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am. 2004;29:551–63. doi: 10.1016/j.jhsa.2004.04.020. doi:10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11:533–78. [PubMed] [Google Scholar]
- 21.Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. J Bone Joint Surg Am. 2010;92:2604–13. doi: 10.2106/JBJS.I.01744. doi:10.2106/JBJS.I.01744. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari M, Guyatt GH, Siddiqui F, et al. Treatment of acute Achilles tendon ruptures: a systematic overview and metaanalysis. Clin Orthop Relat Res. 2002;400:190–200. doi: 10.1097/00003086-200207000-00024. doi:10.1097/00003086-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Metz R, Verleisdonk EJ, van der Heijden GJ, et al. Acute Achilles tendon rupture: minimally invasive surgery versus nonoperative treatment with immediate full weightbearing—a randomized controlled trial. Am J Sports Med. 2008;36:1688–94. doi: 10.1177/0363546508319312. doi:10.1177/0363546508319312. [DOI] [PubMed] [Google Scholar]
- 24.Willits K, Amendola A, Bryant D, et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am. 2010;92:2767–75. doi: 10.2106/JBJS.I.01401. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Balian G, Chhabra AB. Tendon tissue engineering and gene transfer: the future of surgical treatment. J Hand Surg Am. 2006;31:693–704. doi: 10.1016/j.jhsa.2005.10.022. doi:10.1016/j.jhsa.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 26.DeFranco MJ, Derwin K, Iannotti JP. New therapies in tendon reconstruction. J Am Acad Orthop Surg. 2004;12:298–304. doi: 10.5435/00124635-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand KA, Frank CB, Hart DA. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther. 2004;11:368–78. doi: 10.1038/sj.gt.3302198. doi:10.1038/sj.gt.3302198. [DOI] [PubMed] [Google Scholar]
- 28.Rodeo SA, Delos D, Weber A, et al. What's new in orthopaedic research. J Bone Joint Surg Am. 2010;92:2491–501. doi: 10.2106/JBJS.J.01174. doi:10.2106/JBJS.J.01174. [DOI] [PubMed] [Google Scholar]
- 29.Awad HA, Boivin GP, Dressler MR, et al. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–31. doi: 10.1016/S0736-0266(02)00163-8. doi:10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Vacanti JP, Ma X, et al. Generation of neo-tendon using synthetic polymers seeded with tenocytes. Transplant Proc. 1994;26:3390–2. [PubMed] [Google Scholar]
- 31.Butler DL, Awad HA. Perspectives on cell and collagen composites for tendon repair. Clin Orthop Relat Res. 1999;367S:S324–32. doi: 10.1097/00003086-199910001-00031. doi:10.1097/00003086-199910001-00031. [DOI] [PubMed] [Google Scholar]
- 32.Young RG, Butler DL, Weber W, et al. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–13. doi: 10.1002/jor.1100160403. doi:10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 33.Awad HA, Butler DL, Boivin GP, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–77. doi: 10.1089/ten.1999.5.267. doi:10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 34.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–211. doi: 10.1089/ten.2005.11.1198. doi:10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 35.Shenaq DS, Rastegar F, Petkovic D, et al. Mesenchymal progenitor cells and their orthopedic applications: forging a path towards clinical trials. Stem Cells Int. 2010;2010:519028. doi: 10.4061/2010/519028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato YP, Dunn MG, Zawadsky JP, et al. Regeneration of Achilles tendon with a collagen tendon prosthesis. Results of a one-year implantation study. J Bone Joint Surg Am. 1991;73:561–74. [PubMed] [Google Scholar]
- 37.MacGillivray JD, Fealy S, Terry MA, et al. Biomechanical evaluation of a rotator cuff defect model augmented with a bioresorbable scaffold in goats. J Shoulder Elbow Surg. 2006;15:639–44. doi: 10.1016/j.jse.2005.11.009. doi:10.1016/j.jse.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang HW, Goh JC, Thambyah A, et al. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003;9:431–9. doi: 10.1089/107632703322066615. doi:10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- 39.Hankemeier S, Keus M, Zeichen J, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11:41–9. doi: 10.1089/ten.2005.11.41. doi:10.1089/ten.2005.11.41. [DOI] [PubMed] [Google Scholar]
- 40.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–64. doi: 10.1016/s1471-4914(01)02016-0. doi:10.1016/S1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 41.Harris MT, Butler DL, Boivin GP, et al. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. doi:10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Pryce BA, Watson SS, Murchison ND, et al. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–61. doi: 10.1242/dev.027342. doi:10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang J, Thunder R, Most D, et al. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105:148–55. doi: 10.1097/00006534-200001000-00025. doi:10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 44.Abrahamsson SO, Lundborg G, Lohmander LS. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J Orthop Res. 1991;9:495–502. doi: 10.1002/jor.1100090405. doi:10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- 45.Kurtz CA, Loebig TG, Anderson DD, et al. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med. 1999;27:363–9. doi: 10.1177/03635465990270031701. [DOI] [PubMed] [Google Scholar]
- 46.Forslund C, Rueger D, Aspenberg P. A comparative dose-response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res. 2003;21:617–21. doi: 10.1016/S0736-0266(03)00010-X. doi:10.1016/S0736-0266(03)00010-X. [DOI] [PubMed] [Google Scholar]
- 47.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. doi:10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 48.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75:93–9. doi: 10.1080/00016470410001708190. doi:10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 49.Paoloni J, De Vos RJ, Hamilton B, et al. Platelet-rich plasma treatment for ligament and tendon injuries. Clin J Sport Med. 2011;21:37–45. doi: 10.1097/JSM.0b013e31820758c7. doi:10.1097/JSM.0b013e31820758c7. [DOI] [PubMed] [Google Scholar]
- 50.Schepull T, Kvist J, Norrman H, et al. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39:38–47. doi: 10.1177/0363546510383515. doi:10.1177/0363546510383515. [DOI] [PubMed] [Google Scholar]
- 51.Lou J, Tu Y, Burns M, et al. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–202. doi: 10.1016/S0736-0266(01)00042-0. doi:10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura N, Shino K, Natsuume T, et al. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998;5:1165–70. doi: 10.1038/sj.gt.3300712. doi:10.1038/sj.gt.3300712. [DOI] [PubMed] [Google Scholar]