Our age-specific analysis of the mortality patterns of the influenza A/H1N1 pandemic in Mexico suggests a high excess of mortality burden relative to other countries, especially among individuals aged 5-59 years.
Abstract
Background. The mortality burden of the 2009 A/H1N1 influenza pandemic remains controversial, in part because of delays in reporting of vital statistics that are traditionally used to measure influenza-related excess mortality. Here, we compare excess mortality rates and years of life lost (YLL) for pandemic and seasonal influenza in Mexico and evaluate laboratory-confirmed death reports.
Methods. Monthly age- and cause-specific death rates from January 2000 through April 2010 and population-based surveillance of influenza virus activity were used to estimate excess mortality and YLL in Mexico. Age-stratified laboratory-confirmed A/H1N1 death reports were obtained from an active surveillance system covering 40% of the population.
Results. The A/H1N1 pandemic was associated with 11.1 excess all-cause deaths per 100 000 population and 445 000 YLL during the 3 waves of virus activity in Mexico, April–December 2009. The pandemic mortality burden was 0.6–2.6 times that of a typical influenza season and lower than that of the severe 2003–2004 influenza epidemic. Individuals aged 5–19 and 20–59 years were disproportionately affected relative to their experience with seasonal influenza. Laboratory-confirmed deaths captured 1 of 7 pandemic excess deaths overall but only 1 of 41 deaths in persons >60 years of age in 2009. A recrudescence of excess mortality was observed in older persons during winter 2010, in a period when influenza and respiratory syncytial virus cocirculated.
Conclusions. Mexico experienced higher 2009 A/H1N1 pandemic mortality burden than other countries for which estimates are available. Further analyses of detailed vital statistics are required to assess geographical variation in the mortality patterns of this pandemic.
More than 2 years after the identification and rapid global spread of a novel pandemic A/H1N1 influenza virus in April 2009 [1], there is still debate about the morbidity and mortality burden of this pandemic relative to that of past influenza seasons [2–5]. Quantifying the health burden of influenza is notoriously difficult because diagnostic tests are not conducted routinely, influenza rarely appears on medical records, and death can occur after secondary bacterial infection or exacerbation of comorbidities several weeks after the primary viral infection has subsided [6]. Despite a remarkable increase in laboratory testing during the pandemic, laboratory-confirmed influenza hospitalizations and deaths crudely underestimate pandemic burden [2, 7, 8].
Because of limitations in the identification of influenza as a direct cause of death, influenza-related mortality is traditionally estimated by applying statistical time series methods to broad death outcomes [9]. Age- and cause-specific excess mortality estimates generated by this approach are essential to capture the full burden of the A/H1N1 pandemic and compare its impact with that of previous seasons. Preliminary estimates suggest that this pandemic was relatively mild, especially in Europe [2–5, 10–12]. Available mortality estimates lack precision, however, because they rely on laboratory-confirmed deaths [2, 5, 13] or on nonspecific mortality indicators [4, 10, 12] or concentrate on geographically limited areas [11, 12] that may not be representative of larger populations because of geographic heterogeneity in pandemic transmission dynamics [13].
To compare the mortality burden of the pandemic with interpandemic influenza seasons, it is important to consider the different texture of mortality resulting from the shift of pandemic-related deaths toward younger age groups [14–16]. This can be captured by applying the years of life lost (YLL) approach [3, 17–20]. Here, we use detailed cause- and age-specific vital statistics and viral surveillance data from Mexico to compare the mortality and YLL burdens of the A/H1N1 pandemic with those during seasonal epidemics during 2000–2008. Furthermore, we compare pandemic excess mortality estimates with those from laboratory-confirmed influenza death reports and discuss the implications of our findings for pandemic surveillance and control.
METHODS
Mortality and Population Data
Monthly deaths from pneumonia and influenza (P&I), respiratory, cardiac, and all causes were compiled from Mexican vital statistics, 2000–2010 ([21] and Table 1; online only for disease codes). We used mortality data available until April 2010 to capture the first year of pandemic A/H1N1 virus circulation in Mexico [22]. Sensitivity analyses suggest that mortality reports were >99% complete during this period (supplementary data). Mortality data were stratified by 19 age groups (<1, 1–4, 5–9, … 80–84, >85 years) to quantify refined age variation in influenza-related mortality and YLL.
We derived monthly age-specific population estimates by linear interpolation of semiannual estimates published by the Mexican population bureau [23] and calculated monthly age-specific death rates.
Virus Activity and Laboratory-Confirmed Deaths
To augment our mortality datasets with information on influenza virus activity, we obtained weekly numbers and percentages of isolates testing positive for influenza during 2000–2010 from a network of 38 laboratories located throughout Mexico [22] (Figure 1; online only). We identified the dominant influenza subtype each season as the subtype(s) comprising >75% of all influenza viruses isolated during that season (Table 2; online only).
To compare estimates of influenza-related mortality derived from vital statistics and traditional influenza surveillance, we obtained age-specific death rates from laboratory-confirmed pandemic A/H1N1 cases reported to the Mexican Institute for Social Security (IMSS) during April–December 2009 [13, 24] (supplementary data). IMSS is a health system for private sector workers and their families that covers approximately 40% of the Mexican population (107 million individuals). Influenza A/H1N1 deaths were identified from patients admitted to primary care clinics or hospitals presenting with influenza-like illness [24]; influenza testing rates remained stable across age groups and throughout 2009 [13].
Excess Mortality Rates Estimation
To estimate influenza-related mortality rates for interpandemic and pandemic seasons during 2000–2010, we applied Serfling cyclical regression models to monthly death rates for 4 outcomes (P&I, respiratory, respiratory and cardiac, and all cause) and 19 age groups separately ([25, 26] and supplementary data). In brief, Serfling models use seasonal and temporal trends to provide baselines of expected mortality in the absence of influenza virus circulation. We defined influenza-epidemic periods as the months in which observed P&I mortality was greater than the upper 95% confidence limit of baseline mortality. Excess mortality, defined as deaths occurring above baseline during epidemic months, is a standard measure of deaths attributable to influenza [6, 9, 10, 25, 26]. Monthly excess mortality was summed for all epidemic months of a given season to provide cumulative seasonal mortality burden estimates. Ninety-five percent confidence intervals (CIs) were generated on the basis of the variance of model baseline estimates.
To check the robustness of our excess mortality estimates, we also used negative binomial models that explicitly incorporated influenza viral activity and proxies for the circulation of respiratory syncytial virus (RSV; Supplementary Figure 2; online only).
Years of Life Lost Estimation
We used the YLL approach, as previously applied to influenza [3, 17–20], and multiplied the number of excess deaths in each age group, death outcome, and season by the life expectancy of that age group, using 2008 estimates for Mexico [27]. Age-specific estimates of YLL were summed across all age groups to give national seasonal estimates.
RESULTS
Influenza Virus Activity and Seasonal Mortality
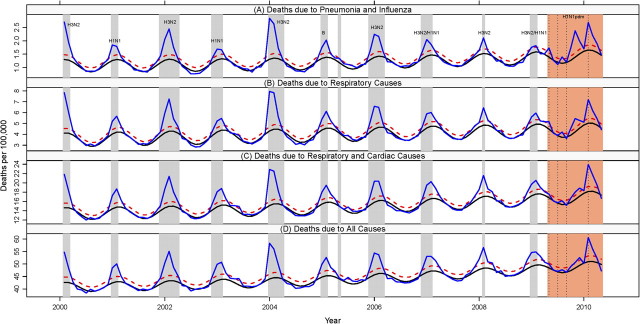
Monthly mortality rates due to P&I, respiratory diseases, respiratory and cardiac diseases, and all causes during 2000–2010 reveal a series of synchronous peaks during November–April, broadly coinciding with viral surveillance data (Figures 1–3, Figures 2–4; online only). The timing of peak viral activity occurred within a month of peak P&I mortality in 6 of the 9 interpandemic seasons studied and during the pandemic (Supplementary Table 2; online only). The A/H3N2 subtype was dominant in 4 of the 9 interpandemic seasons. The A/H1N1 pandemic virus became the predominant influenza subtype circulating in Mexico the week of 29 March 2009 and represented 96% of influenza-positive samples collected through 30 April 2010 (Supplementary Table 2; online only). During this period, we identified 4 distinct peaks of P&I mortality and divided the pandemic period into 4 successive waves: spring (April–May 2009), summer (June–July 2009), fall (August–December 2009), and winter (January–April 2010). Because of substantially lower A/H1N1 viral activity in winter 2010 than in 2009 and RSV cocirculation (Supplementary Figure 2; online only), we restricted our main excess mortality estimates to the 3 pandemic waves in 2009 and provide estimates including the fourth wave in winter 2010 as a sensitivity analysis.
Figure 1.
Time series of monthly mortality rates coded as pneumonia and influenza (A), respiratory causes (B), respiratory and cardiac causes (C), and all causes (D), from January 2000 through April 2010, Mexico. Blue line: observed mortality rates; black line: baseline rates predicted by a seasonal regression model; red dashed line: upper 95% confidence interval of the baseline. Periods highlighted in gray mark seasonal influenza epidemic periods, while periods highlighted in orange highlight the 4 different waves of the A/H1N1 pandemic (spring 2009, summer 2009, fall 2009, winter 2010). Influenza dominant subtypes are indicated each season. See Supplementary Table 2 and Supplementary Figure 1 (online only) for additional virus surveillance data.
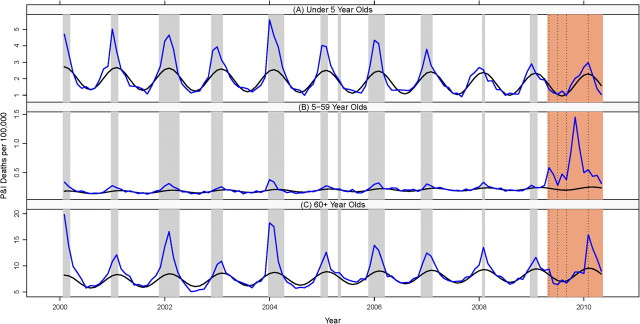
Figure 2.
Monthly pneumonia and influenza (P&I) mortality rates in persons aged <5 (A), 5–59 (B), and 60–99 (C) years, Mexico, January 2000–April 2010. Blue line: observed rate; black line: baseline rates predicted by a seasonal regression model. Gray bars: influenza epidemic periods; orange bar: influenza A/H1N1 pandemic period.
Figure 3.
Monthly respiratory mortality rates in persons aged <5 (A), 5–59 (B), and 60–99 (C) years, Mexico, January 2000–April 2010. Blue line: observed rate; black line: baseline rates predicted by a seasonal regression model. Gray bars: influenza epidemic periods; orange bar: influenza A/H1N1 pandemic period. See Supplementary Figures 2 and 3 (online only) for time series of cardiorespiratory and all-cause deaths by age.
Excess Mortality and Years of Life Lost Estimates
Overall, our Serfling approach attributes 4200 excess P&I deaths and 12 000 excess all-cause deaths to the 3 waves of pandemic A/H1N1 virus activity in Mexico, April–December 2009, corresponding to mortality rates of 3.9 and 11.1 deaths per 100 000 population, respectively. Excess mortality rates due to respiratory and cardiorespiratory diseases were intermediate (Table 1). The impact of each pandemic wave differed greatly, with 14%, 11%, and 75% of the total excess all-cause deaths occurring in spring, summer, and fall, respectively (Table 2). Sensitivity analyses using negative binomial models gave consistent estimates with the Serfling approach, yielding an all-cause excess death rate of 14.2 deaths per 100 000 population (Table 3; online only).
Table 1.
Excess Mortality Rates Associated With the 2009–2010 A/H1N1 Influenza Pandemic and Past Epidemic Seasons, by Age and Death Outcome, Mexico
| Excess death rate per 100 000 population, by age group (95% CI) |
|||||
| Season and mortality outcome | All ages | <5 Years | 5–19 Years | 20–59 Years | ≥60 Years |
| A/H1N1 pandemic (April 2009–December 2009a) | |||||
| Pneumonia and influenza | 3.9 (3.5–4.3) | 2.1 (0.2–4.0) | 0.9 (0.7–1.0) | 5.7 (5.5–5.9) | 4.8 (0.7–9.0) |
| Respiratory diseases | 4.9 (4.0–5.8) | 2.6 (−0.7 to 5.8) | 1.1 (0.9–1.3) | 6.6 (6.1–7.0) | 10.5 (0.9–20.0) |
| Cardiorespiratory diseases | 7.2 (5.2–9.1) | 1.8 (−2.2 to 5.7) | 1.4 (1.1–1.6) | 7.6 (6.5–8.6) | 30.6 (8.7–52.6) |
| All causes | 11.1 (7.4–14.9) | 5.5 (−4.4 to 15.4) | 2.4 (0.9–3.8) | 12.6 (9.8–15.4) | 38.8 (−0.4 to 78.0) |
| A/H1N1 pandemic (April 2009–April 2010) | |||||
| Pneumonia and influenza | 5.8 (5.3–6.3) | 2.8 (0.2–5.4) | 1.0 (0.8–1.2) | 7.0 (6.7–7.3) | 18.5 (12.0–24.0) |
| Respiratory diseases | 8.0 (6.8–9.2) | 2.6 (−1.8 to 7.0) | 1.3 (1.0–1.6) | 8.3 (7.8–8.8) | 35.7 (22.8–48.6) |
| Cardiorespiratory diseases | 15.4 (12.7–18.1) | 1.8 (−3.6 to 7.2) | 1.5 (1.1–1.9) | 10.0 (8.5–11.4) | 112.8 (82.9–142.7) |
| All causes | 24.6 (19.5–29.6) | 6.1 (−6.9 to 19.1) | 2.6 (0.7–4.4) | 15.8 (12.2–19.5) | 176.2 (123.3–229.2) |
| 2003–2004 A/H3N2 epidemic | |||||
| Pneumonia and influenza | 2.7 (2.4–3.0) | 6.7 (5.2–8.2) | 0.2 (0.1–0.3) | 0.5 (0.4–0.7) | 20.6 (17.4–23.8) |
| Respiratory diseases | 7.1 (6.4–7.8) | 10.2 (7.6–12.8) | 0.4 (0.2–0.5) | 1.4 (1.1–1.7) | 62.9 (55.4–70.5) |
| Cardiorespiratory diseases | 15.4 (13.9–16.9) | 12.0 (9.1–14.9) | 0.4 (0.2–0.6) | 3.3 (2.5–4.0) | 147.3 (130.6–163.9) |
| All causes | 28.9 (26.2–31.6) | 27.1 (20.6–33.5) | 1.0 (0.0–1.9) | 10.3 (8.5–12.1) | 245.8 (216.4–275.2) |
| Seasonal epidemicsb | |||||
| Pneumonia and influenza | 1.5 (1.1–1.8) | 3.8 (2.3–5.2) | 0.1 (0.0–0.2) | 0.4 (0.2–0.5) | 10.4 (7.3–13.6) |
| Respiratory diseases | 3.7 (3.0–4.4) | 6.0 (3.4–8.5) | 0.2 (0.0–0.3) | 0.9 (0.6–1.2) | 31.2 (23.8–38.5) |
| Cardiorespiratory diseases | 9.0 (7.5–10.4) | 6.6 (3.8–9.4) | 0.2 (0.0–0.4) | 2.2 (1.5–3.0) | 84.4 (68.2–100.7) |
| All causes | 17.9 (15.2–20.6) | 15.7 (9.2–22.2) | 0.8 (–0.2 to 1.7) | 7.1 (5.4–9.0) | 147.4 (118.6–176.2) |
Abbreviation: CI, confidence interval. As a sensitivity analysis, we considered 2 pandemic periods: April–December 2009, a period when A/H1N1 viral activity was most intense, providing conservative estimates of pandemic burden, and April 2009–April 2010, a period when the pandemic A/H1N1 virus was predominant in more than 99% of all influenza-positive respiratory specimens but respiratory syncytial virus was cocirculating (see also Supplementary Table 2; online only, Supplementary Figure 2; online only).
Estimates restricted to the period when pandemic A/H1N1 virus activity was most intense (see Supplementary Figure 1; online only).
Average of 8 interpandemic seasons from 2000–2001 through 2007–2008.
Table 2.
Number and Age Distribution of Excess Deaths and Years of Life Lost Associated With Each Wave of the A/H1N1 Influenza Pandemic, 2009–2010, and Past Epidemic Seasons, 2000–2008, Mexico
| Excess all-cause excess deaths |
Years of life lost |
|||
| No. (95% CI) | Percentage in persons <60 years | No. in thousands (95% CI) | Percentage in persons <60 years | |
| A/H1N1 pandemic | ||||
| Spring 2009a | 2300 (700–3900) | 78 | 95.1 (53.3–199.7) | 91 |
| Summer 2009a | 1800 (200–3300) | 71 | 71.4 (29.0–128.2) | 93 |
| Fall 2009a | 7900 (4600–11 300) | 68 | 278.1 (190.0–366.2) | 87 |
| Winter 2010a,b | 14 300 (10 700–18 000) | 14 | 203.3 (112.4–294.2) | 36 |
| Total April 2009–April 2010 | 26 500 (21 000–31 900) | 40 | 649.5 (509.7–789.4) | 72 |
| 2003–2004 A/H3N2 epidemic | 31 100 (28 200–34 000) | 28 | 668.5 (598.1–739.0) | 62 |
| Seasonal epidemicsc | 19 200 (16 300–22 100) | 30 | 426.4 (356.1–496.8) | 63 |
Abbreviation: CI, confidence interval. Years of life lost are derived from all-cause excess mortality rates. Excess deaths and years of life lost have been rounded to the nearest hundredth.
Spring 2009: April–May 2009; summer 2009: June–July 2009; fall 2009: August–December 2009; winter 2010: January–April 2010.
Period when pandemic A/H1N1 influenza virus and respiratory syncytial virus cocirculated.
Average of 8 interpandemic seasons 2000–2001 through 2007–2008.
Excess mortality rates during the pandemic period were 0.6–2.6 times those in an average epidemic season from 2000–2001 through 2007–2008, depending on the mortality outcome, and were 0.4–1.4 times those in the severe 2003–2004 influenza season. The more specific the mortality outcome, the higher the excess mortality burden of the pandemic was relative to seasonal influenza.
On the basis of all-cause mortality data, we estimate that 445 000 YLL (95% CI, 339 000–551 000 YLL) were associated with the A/H1N1 pandemic in Mexico in 2009, which is approximately 4% higher than the YLL burden of an average epidemic season and is 67% of the YLL burden of the severe 2003–2004 influenza epidemic (Table 2). YLL estimates based on more specific causes of deaths exacerbated the impact of the A/H1N1 pandemic relative to previous seasons, yielding a 3.7-fold increase in P&I-derived YLL impact over an average epidemic season.
Sensitivity analyses based on the longer period April 2009–April 2010 provided substantially higher burden estimates, with 6200 excess P&I deaths and 26 500 excess all-cause deaths, corresponding to mortality rates of 5.8 and 24.6 deaths per 100 000 population, respectively. The pandemic YLL burden was comparable to that during the severe 2003–2004 influenza epidemic in this analysis.
Age Patterns of Excess Mortality and Years of Life Lost
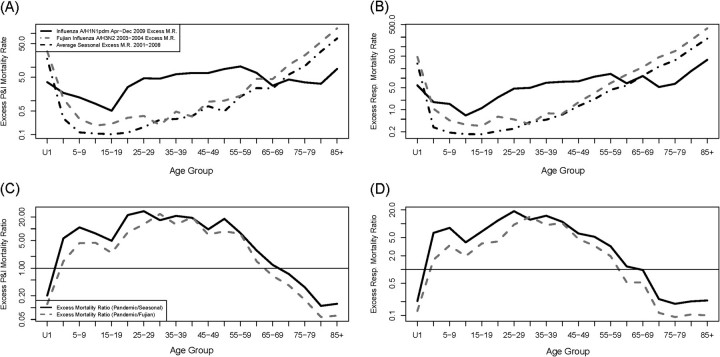
Next, we compared influenza age mortality patterns in pandemic and epidemic seasons. Excess mortality rates among persons aged 5–19 and 20–59 years were markedly elevated during the April–December 2009 pandemic period, relative to epidemic seasons, in all death outcomes studied (Table 1 and Figure 4). Among persons aged 20–59 years, pandemic excess mortality rates were 1.7–14.3-fold higher than in an average influenza season, depending on the outcome, and 1.2–11.4-fold higher than in the 2003–2004 season, with the most pronounced ratio in P&I data. Ratios comparing pandemic with epidemic seasons were slightly lower among persons aged 5–19 years (Table 1). Estimates for more detailed age groups suggest that the mortality risk culminated in adults aged 20–30 years during the pandemic, relative to seasonal influenza (Figure 4; also Supplementary Figures 5–7; online only).
Figure 4.
Age-specific comparison of the impact of pandemic (April–December 2009) and epidemic influenza (top, A and B). Age-specific rates of excess pneumonia and influenza (P&I) and respiratory deaths. Black solid line: 2009 A/H1N1; gray dashed line: 2003–2004 A/H3N2 season; black dashed line: average seasonal influenza, 2000–2008 (bottom, C and D) Relative risk of excess pneumonia and influenza (P&I) and respiratory mortality rates during the pandemic, compared with an average seasonal influenza epidemic (black solid line) and the particularly severe 2003–2004 epidemic (grey dashed line). See Supplementary Figure 5 (online only) for additional causes of death and Supplementary Figures 6 and 7 (online only) for comparisons involving the April 2009–April 2010 period.
In contrast to middle age groups, pandemic excess mortality rates were unusually low among children <5 years of age, and some of the estimates were not significantly different from zero, with ratios of pandemic to epidemic mortality of 0.4–0.6 (Table 1). Similarly, the pandemic impact among older persons >60 years of age was 0.3–0.5-fold lower than for average seasonal epidemics.
Sensitivity analyses based on the longer period April 2009–April 2010 suggest an age shift in excess mortality burden over time. Although the majority of excess deaths was among individuals <60 years of age in 2009 (71%), this changed substantially in winter 2010, when 87% of excess deaths occurred among seniors (Table 2). A more extreme age shift was seen in YLL estimates. Consequently, pandemic estimates limited to April–December 2009 were markedly lower than those including the winter 2010 period among older persons, whereas there was little difference in younger age groups.
Effectiveness of Laboratory-Confirmed Death Reporting
We compared excess mortality estimates and laboratory-confirmed A/H1N1 deaths for the main pandemic period April–December 2009. We estimate that the laboratory-confirmed deaths captured 39.7% of excess P&I mortality and 13.8% of excess all-cause mortality during this period in Mexico (Table 3). Age-specific patterns in laboratory-confirmed death and excess P&I mortality matched relatively well, except in individuals >60 years of age (Figure 8; online only). In this age group, laboratory-confirmed deaths captured only 19.0% of excess P&I mortality and 2.4% of excess all-cause mortality. Overall, the proportion of influenza-related excess deaths captured by laboratory-confirmed deaths decreased with less specific outcomes and with older age.
Table 3.
Comparisons of Pandemic-Related Mortality Rates Derived From Excess Mortality Models and Mexican Institute for Social Security Reports of Laboratory-Confirmed Deaths by Age, April–December 2009, Mexico
| Death rate per 100 000 population |
|||||
| Age group | Excess P&I | Excess respiratory | Excess R&C | Excess all-cause | IMSS laboratory-confirmeda |
| <5 years | 2.09 | 2.57 | 1.75 | 5.52 | 1.53 |
| 5–19 years | 0.86 | 1.10 | 1.35 | 2.36 | 0.61 |
| 20–59 years | 5.71 | 6.55 | 7.56 | 12.59 | 2.13 |
| >60 years | 4.84 | 10.45 | 30.63 | 38.79 | 0.92 |
| All ages | 3.88 | 4.91 | 7.15 | 11.15 | 1.54 |
Abbreviations: P&I, pneumonia and influenza; R&C, respiratory and cardiac; IMSS, Mexican Institute for Social Security.
A total of 585 deaths were reported to Mexican Institute for Social Security during April–December 2009, corresponding to a population of 38 million.
DISCUSSION
To our knowledge, this is the first national study to estimate age- and cause-specific excess mortality and YLL associated with pandemic influenza A/H1N1 in its first year of circulation with use of traditional statistical approaches, because of the availability of relatively timely and detailed vital statistics from Mexico. We estimate that approximately 12 000 excess all-cause deaths (rate of 11.1 deaths per 100 000 population) and approximately 445 000 YLL were attributable to the pandemic in 2009. Adults aged 20–59 years were most severely affected by the pandemic relative to their experience with seasonal influenza, followed by persons aged 5–19 years. By contrast, persons >60 years of age and children <5 years of age were less affected by pandemic than seasonal influenza. We observed substantial excess mortality among persons >60 years of age in winter 2010, a period when A/H1N1 was circulating at low levels. Finally, efforts to capture pandemic mortality burden based on laboratory-confirmed deaths substantially underestimated burden among older persons in 2009. Overall, 1 of 7 pandemic-related excess all-cause deaths and only 1 of 41 deaths in older persons were captured by this system.
Large variation in the mortality burden of historical pandemics has been reported among regions, countries, and cities [28, 29]. The early response to the 2009 pandemic was hampered by confusion about its severity [8]. Our study suggests that Mexico experienced higher pandemic-related mortality rates than did the United States, Europe, or Australia [2, 4, 5, 10, 12, 19], in line with the high case-fatality rate reported for Mexico [13]. In particular, a US study estimated a death rate of 4 deaths per 100 000 population during April 2009–April 2010 with use of laboratory-confirmed influenza deaths corrected for underreporting [2]. Our conservative estimate for Mexico, based on national vital statistics and limited to April–December 2009, suggests a much higher pandemic burden at 11.1 deaths per 100 000 population. The US study relied on age-specific correction factors calculated during spring 2009 [7], which could underestimate burden in subsequent months. Surprisingly, excess mortality rates for seasonal influenza were similar, if not lower, in Mexico than in the United States (this study and [9]), further suggesting an unusual severity of the A/H1N1 pandemic in Mexico. In addition, pandemic estimates for pediatric populations were higher in Mexico than elsewhere [2, 4, 5, 12, 19, 30, 31], although CIs are broad and methodological differences could partly account for the reported variation.
The fall 2009 pandemic wave was responsible for an estimated 16.1 excess all-cause deaths per 100 000 population in the central Mexican state of San Luis Potosi, with 74% of excess deaths occurring in persons >60 years of age [11]. These are higher than our national estimates, at 7.3 deaths per 100 000 population for the fall wave and with only 32% of excess all-cause deaths occurring among persons >60 years of age. These differences might be explained by heterogeneity in pandemic influenza transmission rates and socioeconomic status across Mexico [13].
Previous studies based on pneumonia or laboratory-confirmed influenza deaths have reported elevated pandemic mortality among persons <60 years of age and relative protection among older persons in 2009 [2, 5, 13, 14, 19], in line with our findings from Mexico. Surprisingly, we identified a recrudescent wave of excess mortality in winter 2010 that focused on older Mexican persons, and may be attributed in part to pandemic A/H1N1 activity. Although influenza activity decreased in winter 2010 in Mexico, pandemic A/H1N1 represented >99% of influenza-positive specimens collected during this period and was identified in >18% of respiratory specimens tested. This is a higher prevalence than for seasonal epidemics, including the severe 2003–2004 season [22]. Furthermore, the sharp peaks of mortality observed among older persons in January 2010 in various death outcomes traditionally linked to influenza are suggestive of an influenza etiology.
Despite evidence of A/H1N1 virus circulation in winter 2010, we cannot rule out the contributions of other respiratory pathogens, such as RSV, and of environmental factors, to excess mortality among older persons. Although there is no national RSV surveillance in Mexico, our mortality proxy of RSV activity suggests increased activity in winter 2010 (supplementary data). A laboratory-based hospital study in San Luis Potosi confirms that RSV circulated in winter 2010, although only 12.5% of adult patients with respiratory symptoms tested positive for RSV during peak RSV activity [32]. Alternatively, temperature effects could have contributed to elevated mortality among older persons in winter 2010, as they did in the United Kingdom [10]. However, we did not find any association between temperature and excess mortality in winter 2010 in Mexico (supplementary data). Overall, although the exact etiology of the winter 2010 period of excess mortality remains inconclusive, the Mexican mortality patterns are reminiscent of the 1918–1920 and 1957–1958 influenza pandemics in the United States and Europe [15, 33, 34].
If the excess mortality observed in winter 2010 in Mexico was attributable to influenza, this would suggest a rapid change in mortality risk age profile over the first year of A/H1N1 circulation. In the absence of clear genetic changes in A/H1N1 viruses during 2009–2010 [35], age differences in contact rates could potentially explain the reported mortality age shift, as suggested by empirical data and theoretical models [13, 36]. Alternatively, increased cocirculation of bacterial respiratory pathogens may have increased the severity of influenza infections in older persons in winter 2010 [37].
A strengthened influenza surveillance system established by a large social security health network [13] provided a unique opportunity to compare age patterns in excess mortality and laboratory-confirmed deaths in 2009 in Mexico. Although laboratory surveillance was relatively robust among pediatric and young adult populations, it captured only approximately 2% of excess deaths in individuals >60 years of age. This is intriguing because one-third of influenza-like illnesses were systematically tested for influenza in all age groups [13]. Prospective studies conducted during the 2009 pandemic suggest that an influenza code was present on the death certificates of approximately 80% of British children who died of laboratory-confirmed A/H1N1 [38], whereas a respiratory code was present in only approximately 60% of laboratory-confirmed A/H1N1 deaths in the general US population [39]. Overall, our study and others highlight the difficulties in identifying and coding influenza-related deaths among older patients, even with increased diagnostic rates. These observations could be useful to improve laboratory surveillance in future pandemics.
Several limitations should be mentioned. Our study was ecological in design, and therefore, causes other than influenza activity may contribute to the estimated excess mortality. However, synchrony between high excess mortality seasons and elevated A/H3N2 virus activity in the interpandemic period (Figure 1) and similarities in the age distributions of excess mortality across different mortality outcomes lend strength to our approach. Also, our burden estimates were derived from 2 independent excess mortality approaches. A second limitation relates to the lack of individual patient information in Mexican data, preventing us from incorporating comorbidities into our analysis. Because 73%–78% of laboratory-confirmed pandemic deaths were in patients with chronic conditions [38, 39], we have likely overestimated the absolute YLL pandemic burden [19]. However, by applying the same approach to all influenza seasons during 2000–2010, we provide a fair comparison of the relative burden of pandemic and seasonal influenza [3].
Robust estimation of pandemic age mortality patterns is crucial to guide intervention strategies and to define high-risk priority groups for vaccination in resource-limited settings. Here, we show that the A/H1N1 virus had an atypical impact on deaths and YLL in Mexico in 2009 in individuals 5–59 years of age, corroborating the international policy targeting vaccines to younger populations. Vaccinating school-age children may provide not only direct mortality benefits but also indirect benefits through herd immunity, especially during pandemic seasons [13, 40]. Vaccination of middle-aged adults (20–59 years) may also be important in light of this pandemic and previous pandemics [18]. Additional studies should concentrate on elucidating the etiology of the 2010 winter wave of excess mortality concentrated among older Mexican persons. Finally, multinational comparisons will be particularly important to assess geographical variation in A/H1N1 pandemic mortality burden globally and to confirm whether Mexico fared particularly poorly during this pandemic.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the authors that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We thank James Tamerius for providing daily temperature data for Mexico City, 2000–2010. This research was conducted in the context of the Multinational Influenza Seasonal Mortality Study (MISMS), an ongoing international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health (http://www.origem.info/misms/index.php).
Financial support.
This work was supported by the in-house Influenza Research Program of the Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, which is funded by the International Influenza Unit, Office of Global Affairs, Department of Health and Human Services, and by the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security (to L. S.).
Potential conflicts of interest.
All authors:No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ginsberg M, Hopkins J, Maroufi A, et al. Swine influenza A (H1N1) infection in two children–Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:400–2. [PubMed] [Google Scholar]
- 2.Shrestha SS, Swerdlow DL, Borse RH, Prabhu VS, Finelli L, et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010) Clin Infect Dis. 2011;52(Suppl 1):S75–82. doi: 10.1093/cid/ciq012. [DOI] [PubMed] [Google Scholar]
- 3.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010:RRN1153. doi: 10.1371/currents.RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazick A, Gergonne B, Wuillaume F, Danis K, Vantarakis A, et al. Higher all-cause mortality in children during autumn 2009 compared with the three previous years: pooled results from eight European countries. Euro Surveill. 2010;15:pii19480. [PubMed] [Google Scholar]
- 5.Donaldson LJ, Rutter PD, Ellis BM, Greaves FEC, Mytton OT, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:b5213–b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17(Suppl 1):S3–10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 7.Reed C, Angulo FJ, Swerdlow DL, Lipsitch M, Meltzer MI, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April–July 2009. Emerging Infect Dis. 2009;15:2004–7. doi: 10.3201/eid1512.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Presanis AM, De Angelis D, Hagy A, Reed C, Riley S, et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6:e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Hardelid P, Andrews N, Pebody R. Excess mortality monitoring in England and Wales during the influenza A (H1N1) 2009 pandemic. Epidemiol Infect. 2011;139:1431–9. doi: 10.1017/S0950268811000410. [DOI] [PubMed] [Google Scholar]
- 11.Comas-García A, García-Sepúlveda CA, Méndez-de Lira JJ, Aranda-Romo S, Hernández-Salinas AE, et al. Mortality attributable to pandemic influenza A (H1N1) 2009 in San Luis Potosí, Mexico. Influenza Other Respi Viruses. 2011;5:76–82. doi: 10.1111/j.1750-2659.2010.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muscatello DJ, Cretikos MA, Macintyre CR. All-cause mortality during first wave of pandemic (H1N1) 2009, New South Wales, Australia, 2009. Emerging Infect Dis. 2010;16:1396–402. doi: 10.3201/eid1609.091723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowell G, Echevarría-Zuno S, Viboud C, Simonsen L, Tamerius J, et al. Characterizing the epidemiology of the 2009 influenza A/H1N1 pandemic in Mexico. PLoS Med. 2011;8:e1000436. doi: 10.1371/journal.pmed.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–9. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 15.Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med. 2009;360:2595–8. doi: 10.1056/NEJMp0903906. [DOI] [PubMed] [Google Scholar]
- 16.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, et al. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178:53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 17.Butler D. Portrait of a year-old pandemic. Nature. 2010;464:1112–3. doi: 10.1038/4641112a. [DOI] [PubMed] [Google Scholar]
- 18.Miller MA, Viboud C, Olson DR, Grais RF, Rabaa MA, et al. Prioritization of influenza pandemic vaccination to minimize years of life lost. J Infect Dis. 2008;198:305–11. doi: 10.1086/589716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wielders CCH, van Lier EA, van 't Klooster TM, van Gageldonk-Lafeber AB, van den Wijngaard CC, et al. The burden of 2009 pandemic influenza A (H1N1) in the Netherlands. Eur J Public Health. 2010 doi: 10.1093/eurpub/ckq187. Dec 22. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Molinari NM, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Sistema Nacional de Informacion en Salud (SINAIS) Introducción: conoce al SINAIS: SINAIS | SALUD MÉXICO. Available at: http://www.sinais.salud.gob.mx/acercade/index.html. Accessed 17 May 2011. [Google Scholar]
- 22.World Health Organization. Global Health Atlas. Available at: http://apps.who.int/globalatlas/dataQuery/default.asp. Accessed 3 May 2011. [Google Scholar]
- 23.Consejo Nacional de Población. Available at: http://www.conapo.gob.mx/. Accessed 3 May 2011. [Google Scholar]
- 24.Echevarría-Zuno S, Mejía-Aranguré JM, Mar-Obeso AJ, Grajales-Muñiz C, Robles-Pérez E, et al. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet. 2009;374:2072–9. doi: 10.1016/S0140-6736(09)61638-X. [DOI] [PubMed] [Google Scholar]
- 25.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78:494–506. [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen C, Simonsen L, Kang J, Miller M, McAnerney J, et al. Elevated influenza-related excess mortality in South African elderly individuals, 1998–2005. Clin Infect Dis. 2010;51:1362–9. doi: 10.1086/657314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Global health observatory database. Available at: http://apps.who.int/ghodata/?vid=720. Accessed 2 May 2011. [Google Scholar]
- 28.Murray CJL, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918-20 pandemic: a quantitative analysis. Lancet. 2006;368:2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 29.Viboud C, Grais RF, Lafont BAP, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192:233–48. doi: 10.1086/431150. [DOI] [PubMed] [Google Scholar]
- 30.Libster R, Coviello S, Cavalieri ML, Morosi A, Alabart N, et al. Pediatric hospitalizations due to influenza in 2010 in Argentina. N Engl J Med. 2010;363:2472–3. doi: 10.1056/NEJMc1008806. [DOI] [PubMed] [Google Scholar]
- 31.Wilking H, Buda S, von der Lippe E, Altmann D, Krause G, et al. Mortality of 2009 pandemic influenza A (H1N1) in Germany. Euro Surveill. 2010;15:pii19741. doi: 10.2807/ese.15.49.19741-en. [DOI] [PubMed] [Google Scholar]
- 32.Lovato-Salas F, Matienzo-Serment L, Monjarás-Ávila C, Godoy-Lozano EE, Comas-García A, et al. Pandemic influenza A(H1N1) 2009 and respiratory syncytial virus associated hospitalizations. J Infect. 2010;61:382–90. doi: 10.1016/j.jinf.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Dauer CC. Serfling RE: Mortality from influenza, 1957–58 and 1959–60. Am. Rev. Resp. Dis. 1961;83:15–28. [Google Scholar]
- 34.Saglanmak N, Andreasen V, Simonsen L, Molbak K, Miller MA, et al. Gradual changes in the age distribution of excess deaths in the years following the 1918 influenza pandemic in Copenhagen: using epidemiological evidence to detect antigenic drift. Vaccine. 2011;29(S2):B42–8. doi: 10.1016/j.vaccine.2011.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Recommended viruses for influenza vaccines for use in the 2011 southern hemisphere influenza season. 2010 http://www.who.int/csr/disease/influenza/201009_Recommendation.pdf. Accessed 30 Aug 2011. [Google Scholar]
- 36.Cauchemez S, Donnelly CA, Reed C, Ghani AC, Fraser C, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361:2619–27. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–12. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet. 2010;376:1846–52. doi: 10.1016/S0140-6736(10)61195-6. [DOI] [PubMed] [Google Scholar]
- 39.Fowlkes AL, Arguin P, Biggerstaff MS, et al. Epidemiology of 2009 pandemic influenza A (H1N1) deaths in the United States, April–July 2009. Clin Infect Dis. 2011;52(Suppl 1):S60–68. doi: 10.1093/cid/ciq022. [DOI] [PubMed] [Google Scholar]
- 40.Chowell G, Viboud C, Wang X, Bertozzi SM, Miller MA. Adaptive vaccination strategies to mitigate pandemic influenza: Mexico as a case study. PLoS One. 2009;4:e8164. doi: 10.1371/journal.pone.0008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.