We showed a dramatic decrease in the human immunodeficiency virus type 1 (HIV-1) ribonucleic acid (RNA) level in a community of patients who receive care in an urban region in the United States, suggesting a decreased risk of HIV transmission as treatment has improved in those under care.
Abstract
(See the Editorial Commentary by Sax, on pages 605–608.)
Background. We have previously showed that as antiretroviral therapy has improved over time since 1995–1996, the likelihood of achieving virologic suppression has also improved. Antiretroviral therapy and antiretroviral therapy guidelines have continued to evolve, and we wished to determine the trend in human immunodeficiency virus (HIV-1) RNA levels over time in HIV-infected persons receiving care in our large urban HIV clinical practice in Baltimore, Maryland.
Methods. The HIV-1 RNA level was assessed each year from 1996 through 2010 at the date closest to 1 July for all patients in care and followed up in the Johns Hopkins HIV Clinical Cohort. The clinic population’s median HIV-1 RNA level and stratified threshold levels were plotted. The demographic characteristics of the population were also assessed over time.
Results. From 1996 (shortly after highly active antiretroviral therapy [HAART] was introduced) to 2010, the median HIV-1 RNA level decreased from 10 400 to <200 copies/mL. The proportion of patients with an HIV-1 RNA level >500 copies/mL decreased from 75% to only 16% during this same period. The population itself became older, had a higher proportion of women, and a lower proportion of patients with injection drug use as a transmission risk, but it was geographically stable. There was an increase in HAART use over time.
Discussion. Our results demonstrate the remarkable impact of increased use of and improved management with HAART in this urban HIV-infected population.
Highly active antiretroviral therapy (HAART) for human immunodeficiency virus (HIV) infection became available in 1995–1996, but the therapy was predominantly anchored by an unboosted protease inhibitor, was used frequently in persons who had previously received nonnucleoside reverse-transcriptase–inhibiting drugs, and was associated with significant side effects. Guidelines for when to start HAART have changed, beginning with use at higher CD4 T-cell counts, later recommending that patients not be treated until their CD4 T-cell count was <200 cells/mm3, and later <350 cells/mm3. Only in recent years have the guidelines recommended earlier initiation of HAART [1]. Over time, HAART has improved, becoming less toxic, and more efficacious. HIV-infected individuals are also less likely to have had previous antiretroviral drug exposure, and if they have prior exposure, the choices for subsequent therapy are broader and allow for selection of drugs for which they are likely to be naive. This should have a salutary effect on the HIV RNA level in a clinical population of HIV-infected individuals. In addition, if the HIV RNA level is decreased within the population, there may also be a decline in the risk of transmitting HIV to others, particularly by sexual contact [2–4].
In 1999, we published the results of a study from our institution showing that 44% of patients who received HAART in 1996–1998 achieved virologic suppression [5]. By early in the past decade, ritonavir-boosted protease inhibitors and efavirenz were in common use, and the prevalence of virologic suppression had improved. Data from our institution showed that 79% of those patients who received HAART in 2001–2002 achieved virologic suppression [6].
The Johns Hopkins HIV Service has provided HIV care to the majority of HIV-infected individuals who have sought care in Baltimore, Maryland, since the service opened in 1984. Its patients are principally from the Baltimore metropolitan region, an East Coast urban region with the fifth-highest incidence of HIV of US urban centers and in a state with the third-highest incidence of HIV infection in the United States [7]. The population has a high proportion of individuals who were infected through drug injection. We conducted an analysis of clinic-wide HIV-RNA levels over calendar time since HAART became generally available in 1996 in Baltimore.
METHODS
This analysis used data collected in the longitudinal Johns Hopkins HIV Clinical Cohort. This is a cohort of persons who received care from the Johns Hopkins HIV Service. All patients are approached to enroll in the cohort, and the rate of refusal is <0.5% [8].
We examined the HIV-1 RNA level collected closest to 1 July of each calendar year from 1996 through 2010. The window of time was 1 January to 1 July of each calendar year, but the value closest to 1 July was used in the analysis for each patient. Other information included demographic variables, CD4 T-cell counts, and whether the patient was receiving HAART at the time the HIV-1 RNA was measured.
The median and upper quartiles of HIV-1 RNA levels were computed for each calendar year for all individuals in care during that year. These levels were also stratified as <200, 200–1000, 1000–10 000, and >10 000 copies/mL. The demographics (age, sex, race, HIV transmission risk group, ZIP code of residence) and the CD4 T-cell count and the use of HAART at the time of HIV-1 RNA measurement were recorded for each calendar year. We compared the change in demographic and clinical characteristics of the population over calendar time to assess for changes in the population receiving care in the Johns Hopkins HIV Clinic. Comparisons were done by χ2 and t tests.
RESULTS
A total of 5290 patients were in care in the Johns Hopkins HIV Clinic during ≥1 calendar year between 1996 and 2010. Table 1 shows the demographic and clinical characteristics of the population by calendar time. The Johns Hopkins HIV Clinic population became older, with an increased proportion of women and associated heterosexual HIV transmission, these characteristics reflecting the changing demography of HIV infection in Maryland during this time. The ZIP code in which the patients resided did not change significantly over time, indicating that the clinic continued to care for the same population geographically. The CD4 T-cell count showed an increasing trend during this time period, consistent with the increase in suppressed HIV RNA levels, associated with the increase in the proportion of patients receiving HAART over time.
Table 1.
Demographic and Clinical Characteristics of the Sample Over Time
| Calendar year |
|||||||||||||||
| Characteristic | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
| Patients in care, no.a | 1304 | 1313 | 1504 | 1582 | 1735 | 1882 | 1975 | 1965 | 2010 | 1937 | 1891 | 1930 | 1968 | 1979 | 1983 |
| Age, median, years | 38 | 38 | 39 | 40 | 41 | 41 | 42 | 43 | 44 | 45 | 45 | 46 | 47 | 48 | 49b |
| Sex, % male | 71 | 67 | 66 | 66 | 66 | 65 | 65 | 64 | 64 | 64 | 64 | 65 | 65 | 65 | 65b |
| Race, % | |||||||||||||||
| Black | 73 | 76 | 76 | 75 | 74 | 74 | 74 | 73 | 73 | 74 | 74 | 74 | 74 | 73 | 73 |
| White | 25 | 23 | 23 | 23 | 24 | 24 | 25 | 25 | 25 | 24 | 24 | 24 | 23 | 24 | 24 |
| Transmission risk, %c | |||||||||||||||
| Injection drug use | 46 | 48 | 48 | 47 | 44 | 41 | 40 | 39 | 39 | 37 | 35 | 34 | 34 | 34 | 36b |
| Men who have sex with men | 34 | 31 | 29 | 30 | 30 | 29 | 29 | 28 | 28 | 28 | 28 | 28 | 29 | 28 | 28 |
| Heterosexual | 43 | 45 | 45 | 44 | 47 | 48 | 48 | 50 | 50 | 49 | 49 | 49 | 51 | 51 | 51b |
| ZIP code, % | |||||||||||||||
| Baltimore city | 85 | 85 | 85 | 83 | 83 | 82 | 81 | 81 | 81 | 81 | 81 | 81 | 80 | 80 | 80 |
| Other Maryland | 12 | 12 | 12 | 12 | 13 | 13 | 13 | 12 | 13 | 13 | 14 | 13 | 13 | 14 | 14 |
| CD4 T-cell count, median, cells/mm3 | 239 | 290 | 304 | 296 | 315 | 328 | 352 | 340 | 347 | 377 | 385 | 406 | 421 | 435 | 444b |
| Receiving HAART, %d | 22 | 51 | 58 | 66 | 69 | 71 | 70 | 70 | 75 | 77 | 79 | 80 | 83 | 84 | 85b |
Defined as attending ≥2 clinic visits during the year.
P < .05 for trend from 1996 to 2010.
Transmission categories are not mutually exclusive.
HAART, highly active antiretroviral therapy.
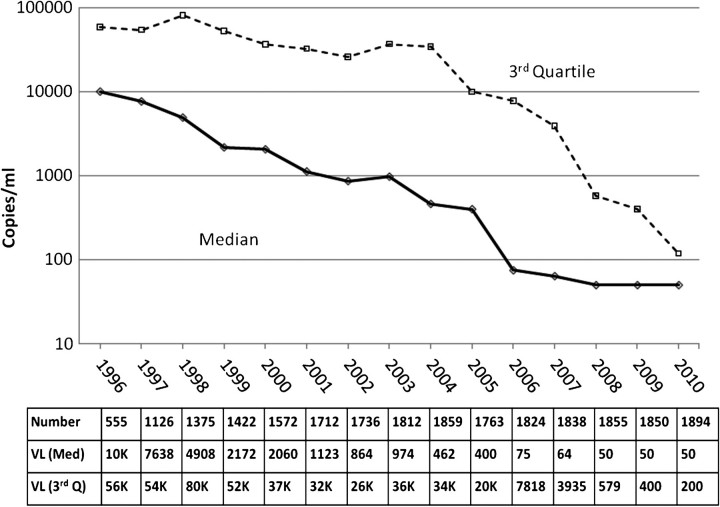
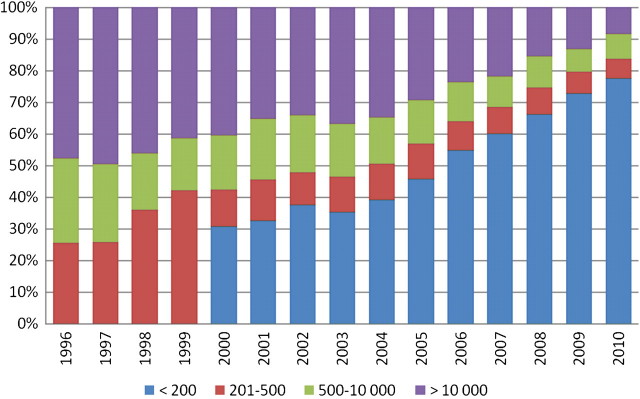
The HIV-1 RNA levels (median and upper quartiles) are shown in Figure 1. The number of patients who contributed HIV-1 RNA data is shown in the top row of the table within the figure. Particularly in earlier years, HIV-1 RNA levels were not always obtained on the entire clinic population. It can be seen that the median HIV-1 RNA level decreased from 10 000 copies/mL in 1996 to <200 copies/mL in 2006, and remained <200 copies/mL through 2010. The upper quartile declined from 80 000 copies/mL in 1996 to 500 copies/mL in 2008 and remained at this level through 2010. This demonstrates that only 25% of the patients in care had HIV-1 RNA levels of 500 copies/mL. Figure 2 depicts the HIV-1 RNA thresholds at each calendar year. The table within the figure shows the number of patients who had an HIV-1 RNA level and could contribute to this analysis.
Figure 1.
Line plot of median (Med) and upper- or third-quartile (Q) human immunodeficiency virus (HIV)–1 RNA levels from 1996 through 2010 in the Johns Hopkins HIV Clinical Cohort. K, thousand; VL, viral load.
Figure 2.
Bar chart of human immunodeficiency virus (HIV)–1 RNA thresholds from 1996 through 2010 in the Johns Hopkins HIV Clinical Cohort; threshold values are given in copies per milliliter.
Because patients who are less adherent to care and have higher HIV-1 NRA levels could selectively become unavailable for follow-up, we examined retention in care in 1996–2002 and 2003–2009. Retention was defined as a patient in care during one calendar year continuing in care the following calendar year. Retention was 86% in 1996–2002 and 94% in 2003–2009 overall (retention for injection drug users, 85% and 95%, respectively, for 1996–2002 and 2003–2009; for men who have sex with men, 86% and 94%; for heterosexuals, 86% and 93%).
DISCUSSION
Our clinic-wide analysis has shown that the burden of HIV-1 RNA has declined steadily since HAART was introduced in 1996 and that in the most recent year (2010), <17% of the patients had an HIV-1 RNA level of >500 copies/mL. This is a remarkable accomplishment that is probably due to improved antiretroviral drugs and changes in management, including the starting of antiretroviral therapy at less advanced immunosuppression. Proportionally fewer patients in care have HIV-1 RNA levels obtained in the earlier years, probably both because viral load testing was less available for these and because fewer of them were receiving HAART. Therefore, the HIV-1 NRA levels in the clinic may have even been higher than indicated by our data. Selective loss of patients who were less adherent to HIV care is unlikely to have affected our results differentially, because retention improved over time. We believe that our results emphasize that even in an inner urban HIV-infected population with a relatively high proportion of patients who were infected as a consequence of injection drug use, HAART can be highly successful. We note that our previous publications from our clinic focused only on those patients who received HAART [5, 6]. This current study analyzed data from the entire clinic population, irrespective of HAART use, further emphasizing both the utilization of HAART and its effectiveness in our clinical population. A recent study from British Columbia also showed an improvement in virologic suppression from 65% in 2000 to 87% in 2008 [9]. These results therefore reflect the increasing use of HAART and are a testament to the remarkable effectiveness of HAART in our patient population.
We posit that these data may also have implications for the transmission of HIV in the urban community from which the Johns Hopkins HIV Clinic draws its patients. Based on estimates of HIV infection in the United States from the Centers for Disease Control and Prevention, this may be as many as half of the patients who reside in this region [10, 11]. Studies have shown that low HIV RNA levels are associated with a decreased risk of sexual transmission of HIV [2–4]. HAART is effective in reducing HIV transmission by 92% in heterosexual HIV-discordant couples [12]. In more than half of our clinic population, HIV had been transmitted sexually, with transmission through injection drug use in the remainder. Although the association of lower HIV RNA levels with bloodborne HIV transmission is not well described, it is plausible that the risk of transmission by any mode would be reduced in the setting of a suppressed HIV RNA level. In support of this, a recent presentation suggests an ecologic association between a decline in HIV-RNA levels over time and the incidence of HIV in injection drug users [13]. Maryland and the city of Baltimore have had a particularly high prevalence and incidence of HIV infection during the past decade, although the most recently available data suggest that the incidence of new HIV cases may have stabilized or even declined slightly [2]. Whether a decline in prevalent community HIV RNA levels has contributed to the apparent decrease in the rate of new cases is unknown, but it is possible since other efforts to reduce transmission, such as condom use and clean needle exchange, have decreased in recent years owing to budget cuts.
We believe that our results emphasize the importance of early detection and early and successful referral for HIV care. Current guidelines recommend the use of HAART at higher CD4 T-cell counts, so that the earlier a person can receive HIV care, the more likely he or she is to be started on HAART [1]. Of course, many HIV-infected persons do not receive HIV care until more advanced immunosuppression [14, 15], so the challenge of detecting HIV infection and engaging the infected individual in HIV care remains. Efforts to promote universal HIV testing have been underway since 2006 [16], and it is not yet known how effective these have been in identifying HIV infection at earlier stages in the United States. Our findings do not minimize the continuing importance of other prevention interventions, such as condom use, use of clean needles, and diagnosis and treatment of genital ulcers and other sexually transmitted diseases. However it is known that these behavioral interventions can be of limited effectiveness because of poor adherence, and the treatment of HIV with antiretroviral therapy to suppress HIV-1 RNA is likely to be an important contributor to prevention of HIV transmission [17].
In summary, we have shown a dramatic decrease in the HIV-1 RNA level in a community of patients who receive care in a large urban region in the United States. Although these results to not reflect the entire community of HIV-infected individuals from our region, they are likely to represent a substantial proportion of those who are infected, and certainly a group of individuals that can be targeted with among whom an important intervention that may reduce HIV transmission.
Acknowledgments
Financial support. This work was supported by the National Institutes of Health (grants R01 DA11602, K24DA00432, and R01 AA16893).
Potential conflicts of interest. R. D. M. is a consultant to Bristol-Myers-Squibb and has institutional grant support from Bristol-Myers Squibb, GlaxoSmithKline, and Pfizer. J. G. B. : No no reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2011. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 14 March 2011. [Google Scholar]
- 2.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 4.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 5.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virological failure and adverse drug reactions. Ann Intern Med. 1999;131:81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 6.Moore RD, Keruly JC, Gebo KA, Lucas GM. An improvement in virologic response to HAART in clinical practice from 1996 through 2002. J Acquir Immune Defic Syndr. 2005;39:195–8. [PubMed] [Google Scholar]
- 7.Maryland Department of Health and Mental Hygiene. Maryland HIV/AIDS epidemiological profile. Fourth quarter 2009. Available at: http://dhmh.state.md.us?AIDS/Data&Statistics?marylandHIVEpiPRofile12-2009.pdf. Accessed 14 March 2011. [Google Scholar]
- 8.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr. 1998;17(Suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 9.Gill VS, Lima VD, Zhang W, et al. Improved virological outcomes in British Columbia concomitant with decreasing incidence of HIV type 1 drug resistance detection. Clin Infect Dis. 2010;50:98–105. doi: 10.1086/648729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Vital signs: HIV testing and diagnosis among adults—United States, 2001–2009. MMWR. 2010;59:1550–5. [PubMed] [Google Scholar]
- 11.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health AFF (Millwood) 2009;28:1677–87. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- 12.Donnell D, Baeten JD, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk G, Galai N, Astemborski J, et al. Decline in community viral load strongly associated with declining HIV incidence among IDU. Presented at the 18th Conference on Retroviruses and Opportunistic Infections Boston, MA, 27 February to 2 March 2011 (Abstract #484). Available at: http://www.retroconference.org/2011/Abstracts/42134.htm. Accessed 21 July 2011. [Google Scholar]
- 14.Keruly JC, Moore RD. Immune status at presentation to care has not improved among antiretroviral naïve persons from 1990 to 2006. Clin Infect Dis. 2007;45:1369–74. doi: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- 15.Altoff K, Gange SJ, Klein MB, et al. Late presentation for HIV care in the United States and Canada. Clin Infect Dis. 2010;50:1512–20. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 17.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]