Abstract
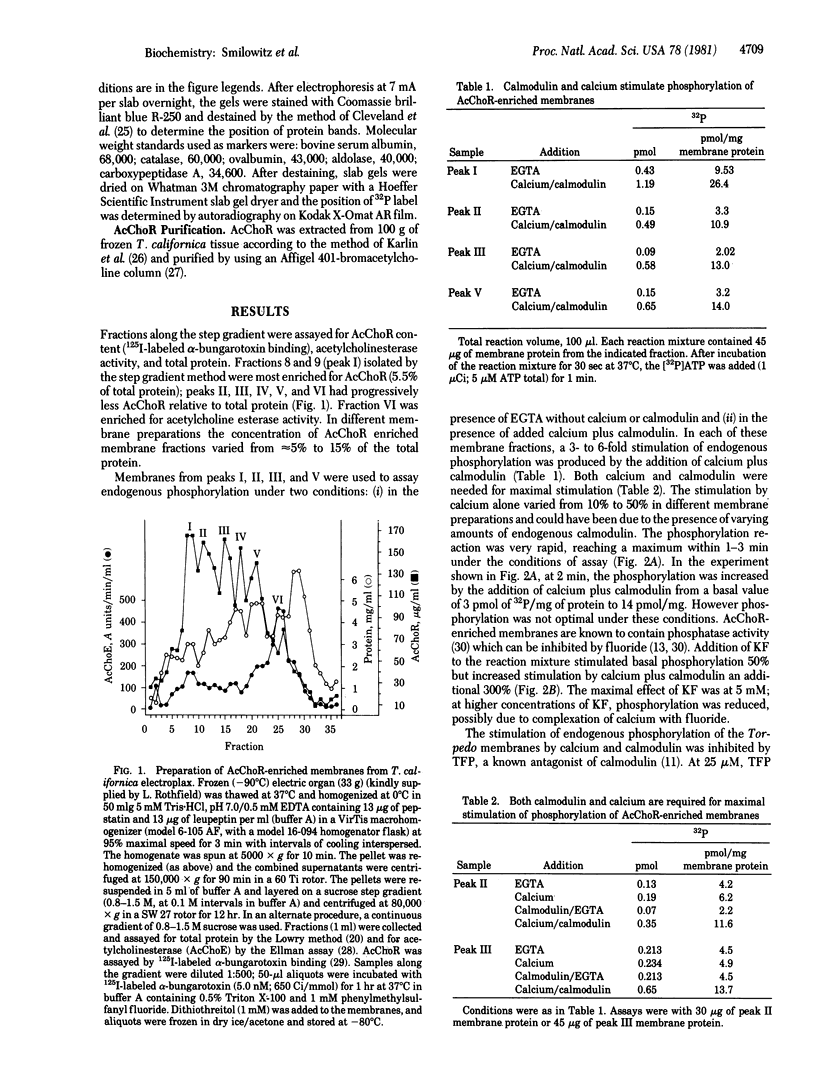
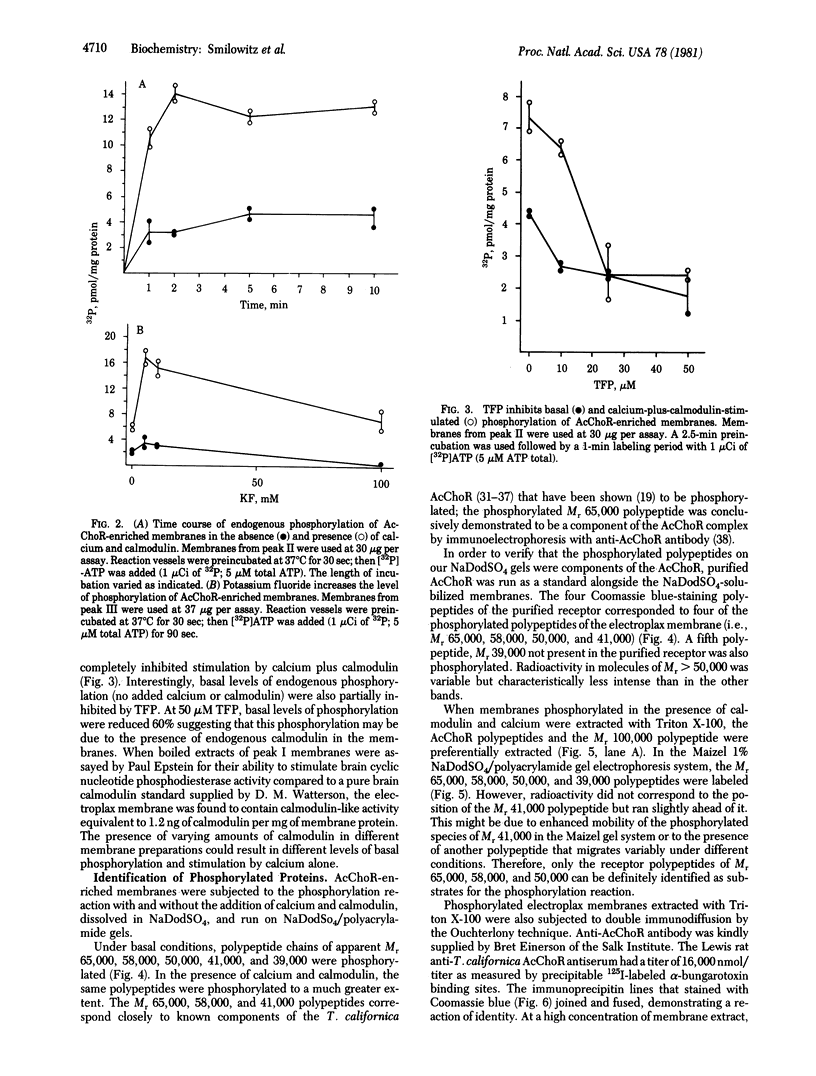
Acetylcholine receptor-enriched membranes prepared from frozen electric organ of Torpedo californica by differential centrifugation and density step gradient centrifugation were assayed for endogenous phosphorylation in the absence and presence of calmodulin and calcium. Each of the membrane fractions exhibited a 3- to 6-fold stimulation of endogenous phosphorylation by calcium and calmodulin. Both calcium and calmodulin were needed for maximal stimulation although calcium alone afforded a small, reproducible stimulation of endogenous phosphorylation. In the presence of fluoride, a phosphatase inhibitor, the calmodulin plus calcium stimulation was increased an additional 3-fold. The phosphorylation reaction was rapid, and maximal phosphorylation was achieved in 2 min. Stimulation of phosphorylation by calcium and calmodulin was completely inhibited by 25 microM trifluoperazine; at 50 microM it inhibited basal phosphorylation by 60%, suggesting that most of the basal phosphorylation may be due to the endogenous calmodulin present in our membrane preparation. NaDodSO4/polyacrylamide gel electrophoresis revealed that at least three of the phosphorylated species (both in the presence and in the absence of calcium and calmodulin) correspond to subunits of the purified acetylcholine receptor from T. californica (i.e., 65,000, 58,000, and 50,000 daltons) which are the beta, gamma, and delta subunits of the receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. C., Lambert J. J., Henderson E. G., Smilowitz H., Epstein P. M. Postsynaptic inhibition of neuromuscular transmission by trifluoperazine. J Pharmacol Exp Ther. 1981 Apr;217(1):44–50. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen J. B., Weber M., Huchet M., Changeux J. P. Purification from Torpedo marmorata electric tissue of membrane fragments particularly rich in cholinergic receptor protein. FEBS Lett. 1972 Oct 1;26(1):43–47. doi: 10.1016/0014-5793(72)80538-6. [DOI] [PubMed] [Google Scholar]
- Damle V. N., McLaughlin M., Karlin A. Bromoacetylcholine as an affinity label of the acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1978 Oct 30;84(4):845–851. doi: 10.1016/0006-291x(78)91661-3. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Rafto S. Comparison of the subunits of Torpedo californica acetylcholine receptor by peptide mapping. Biochemistry. 1979 Jan 23;18(2):301–307. doi: 10.1021/bi00569a011. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Diamond I. Phosphorylation of membrane proteins at a cholinergic synapse. Proc Natl Acad Sci U S A. 1977 Jan;74(1):263–267. doi: 10.1073/pnas.74.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Diamond I. Phosphorylation of acetylcholine receptor by endogenous membrane protein kinase in receptor-enriched membranes of Torpedo californica. Nature. 1977 Jun 9;267(5611):539–540. doi: 10.1038/267539a0. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Milfay D., Davis C. G., Diamond I. Protein phosphatase activity in acetylcholine receptor-enriched membranes. Biochem Biophys Res Commun. 1979 Apr 13;87(3):876–883. doi: 10.1016/0006-291x(79)92039-4. [DOI] [PubMed] [Google Scholar]
- Grab D. J., Berzins K., Cohen R. S., Siekevitz P. Presence of calmodulin in postsynaptic densities isolated from canine cerebral cortex. J Biol Chem. 1979 Sep 10;254(17):8690–8696. [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. The presence and functions of calmodulin in the postsynaptic density. Ann N Y Acad Sci. 1980;356:55–72. doi: 10.1111/j.1749-6632.1980.tb29599.x. [DOI] [PubMed] [Google Scholar]
- Karlin A., Weill C. L., McNamee M. G., Valderrama R. Facets of the structures of acetylcholine receptors from Electrophorus and Torpedo. Cold Spring Harb Symp Quant Biol. 1976;40:203–210. doi: 10.1101/sqb.1976.040.01.022. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Stroud R. M. Immunospecific identification and three-dimensional structure of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1979 Mar 5;128(3):319–334. doi: 10.1016/0022-2836(79)90091-3. [DOI] [PubMed] [Google Scholar]
- Krodel E. K., Beckman R. A., Cohen J. B. Identification of a local anesthetic binding site in nicotinic post-synaptic membranes isolated from Torpedo marmorata electric tissue. Mol Pharmacol. 1979 Mar;15(2):294–312. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C. T., Dedman J. R., Brinkley B. R., Means A. R. Localization of calmodulin in rat cerebellum by immunoelectron microscopy. J Cell Biol. 1980 May;85(2):473–480. doi: 10.1083/jcb.85.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Cooper J., Tzartos S. Acetylcholine receptors from Torpedo and Electrophorus have similar subunit structures. Biochemistry. 1980 Apr 1;19(7):1454–1458. doi: 10.1021/bi00548a029. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M., Hall Z. W. In situ labeling of Torpedo and rat muscle acetylcholine receptor by a photoaffinity derivative of alpha-bungarotoxin. J Biol Chem. 1980 Feb 25;255(4):1698–1703. [PubMed] [Google Scholar]
- Nelson N., Anholt R., Lindstrom J., Montal M. Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1980 May;77(5):3057–3061. doi: 10.1073/pnas.77.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Reed K., Vandlen R., Bode J., Duguid J., Raftery M. A. Characterization of acetylcholine receptor-rich and acetylcholinesterase-rich membrane particles from Torpedo californica electroplax. Arch Biochem Biophys. 1975 Mar;167(1):138–144. doi: 10.1016/0003-9861(75)90449-x. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Changeux J. P. Phosphorylation in vitro of membrane fragments from Torpedo marmorata electric organ. Effect on membrane solubilization by detergents. Eur J Biochem. 1980 Mar;105(1):51–62. doi: 10.1111/j.1432-1033.1980.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. A simple assay for the study of solubilized acetylcholine receptors. Anal Biochem. 1973 Apr;52(2):349–354. doi: 10.1016/0003-2697(73)90036-5. [DOI] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoake J. A., Song S. Y., Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Distribution and developmental changes of the enzyme and its protein activator in mammalian tissues and cells. Biochim Biophys Acta. 1974 Apr 25;341(2):402–411. doi: 10.1016/0005-2744(74)90233-2. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., O'Brien R. D. A comparison of the subunits of the acetylcholine receptor from electric eel and Torpedo californica. FEBS Lett. 1979 May 15;101(2):395–398. doi: 10.1016/0014-5793(79)81052-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Naka M., Hidaka H. Activation of myosin light chain kinase by trypsin. Biochem Biophys Res Commun. 1980 Jan 15;92(1):313–318. doi: 10.1016/0006-291x(80)91554-5. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Changeux J. P. Evidence for protein phosphorylation and dephosphorylation in membrane fragments isolated from the electric organ of Electrophorus electricus. FEBS Lett. 1977 Feb 15;74(1):71–76. doi: 10.1016/0014-5793(77)80755-2. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Sobel A., Changeux J. P. In vitro phosphorylation of the acetylcholine receptor. Nature. 1977 Jun 9;267(5611):540–542. doi: 10.1038/267540a0. [DOI] [PubMed] [Google Scholar]
- Volpi M., Sha'afi R. I., Epstein P. M., Andrenyak D. M., Feinstein M. B. Local anesthetics, mepacrine, and propranolol are antagonists of calmodulin. Proc Natl Acad Sci U S A. 1981 Feb;78(2):795–799. doi: 10.1073/pnas.78.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G., Frisman D., Taylor P. Affinity labeling of the subunits of the membrane associated cholinergic receptor. Mol Pharmacol. 1979 Mar;15(2):213–226. [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Westcott K. R., La Porte D. C., Storm D. R. Resolution of adenylate cyclase sensitive and insensitive to Ca2+ and calcium-dependent regulatory protein (CDR) by CDR-sepharose affinity chromatography. Proc Natl Acad Sci U S A. 1979 Jan;76(1):204–208. doi: 10.1073/pnas.76.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzemann V., Muchmore D., Raftery M. A. Affinity-directed cross-linking of membrane-bound acetylcholine receptor polypeptides with photolabile alpha-bungarotoxin derivatives. Biochemistry. 1979 Nov 27;18(24):5511–5518. doi: 10.1021/bi00591a039. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Siegel F. L. Purification of a calcium-binding phosphoprotein from pig brain. J Biol Chem. 1972 Jul 10;247(13):4180–4185. [PubMed] [Google Scholar]
- Wood J. G., Wallace R. W., Whitaker J. N., Cheung W. Y. Immunocytochemical localization of calmodulin and a heat-labile calmodulin-binding protein (CaM-BP80) in basal ganglia of mouse brain. J Cell Biol. 1980 Jan;84(1):66–76. doi: 10.1083/jcb.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]