Intermittent Preventive Treatment (IPTp) does not improve pregnancy outcomes in Muheza, Tanzania, where sulfadoxine-pyrimethamine-resistant parasites predominate, and may increase the odds of fetal anemia. As parasite resistance increases, the overall effect of IPTp may transition from net benefit to neutral or net harm.
Abstract
(See the article by Maiga et al, on pages 215–223, and editorial commentary by Leke and Taylor, on pages 231–233.)
Background. Millions of African women receive sulfadoxine-pyrimethamine (SP) as intermittent preventive treatment during pregnancy (IPTp) to avoid poor outcomes that result from malaria. However, parasites resistant to SP are widespread in parts of Africa, and IPTp may perversely exacerbate placental infections that contain SP-resistant parasites.
Methods. The study used a cross-sectional design. We determined IPTp use in a delivery cohort of 880 pregnant women in Muheza, Tanzania, by report and by plasma sulfa measurements, and we examined its effects on maternal and fetal delivery outcomes.
Results. In the overall cohort, IPTp was not associated with decreased odds of placental malaria or with increased mean maternal hemoglobin or mean birth weight. Unexpectedly, IPTp was associated with decreased cord hemoglobin level and increased risk of fetal anemia, which may be related to in utero SP exposure.
Conclusions. IPTp does not improve overall pregnancy outcomes in Muheza, Tanzania, where SP-resistant parasites predominate and may increase the odds of fetal anemia. As parasite resistance increases in a community, the overall effect of IPTp may transition from net benefit to neutral or net harm.
Malaria due to Plasmodium falciparum during pregnancy is a major cause of morbidity and mortality in sub-Saharan Africa. P. falciparum sequesters in placental vascular spaces [1], resulting in local inflammation [2] and poor delivery outcomes [3]. To improve outcomes, the World Health Organization (WHO) recommends using sulfadoxine-pyrimethamine (SP) as intermittent preventive treatment in pregnancy (IPTp) at the beginning of the second trimester, followed by at least 1 additional dose no less than 1 month later [4].
SP is a combination antifolate; sulfadoxine targets parasite dihyrofolate reductase (DHFR), and pyrimethamine targets dihydropteroate synthase (DHPS) [5]. Single-point mutations accumulate in an ordered fashion in dhfr and then dhps [5] and confer resistance. The dhps codon 581 (c581) mutation, which is among the last to accumulate, is associated with high level in vitro SP resistance [6] and treatment failure in children [7, 8]. Currently, the WHO concludes that IPTp remains effective in areas where the SP treatment failure rate in children reaches 50% [4].
We hypothesized that as resistance continues to accumulate IPTp may begin to fail. Furthermore, our earlier work from Muheza, Tanzania, which is a hotspot of malaria drug resistance, found that IPTp may exacerbate placental malaria (PM) where it fails to prevent infection [9], suggesting that IPTp may worsen some delivery outcomes. We examined pregnancy outcomes in women who did or did not receive IPTp as part of their routine antenatal care in an area where the 14-day parasitologic SP treatment failure rate in children was as high as 68% [7]. In this area, common resistance markers in dhfr and dhps approached saturation in placental infections except at c581, where the mean fraction of resistance alleles was 0.31 during this time period [9].
METHODS
Ethics Statement
The study was approved by both US and Tanzanian ethical review boards, and all women signed an informed consent.
Study Design
The study was a cross-sectional analysis of reported IPTp use in Muheza, Tanzania, where the control group comprised women who through their own actions or those of antenatal clinic (ANC) staff failed to receive IPTp. IPTp is standard of care in this community, and thus a placebo-controlled randomized trial was not ethically possible.
Clinical Cohort
Data and samples were gathered from a prospective birth cohort conducted from 2002 through 2005 in Muheza, Tanzania that has been described elsewhere [10]. Women were enrolled in the study when they delivered at Muheza Designated District Hospital. The analysis cohort (n = 880) excluded women with known or probable human immunodeficiency virus infection, chronic illness, or multiple gestation during the current pregnancy. Only the first child born to each woman during the study was included in the present analysis.
At enrollment, women were asked whether and when they had received SP for IPTp. The responses were recorded in the Case Report Form and were verified where possible with antenatal clinic cards on which administration of SP for IPTp was recorded by midwives. We concluded that a woman had received SP for IPTp if this was indicated by either source.
Sulfa compounds were assayed in maternal and cord plasma and confirmed the accuracy of reported IPTp use as previously described [9]. We estimate that our plasma sulfa assay (which has a sensitivity of 4 ug/mL) can detect sulfadoxine for up to 6 weeks after SP use [9, 11, 12]. Based on the assay data, women were further categorized as receiving early IPTp (a history of IPTp use but no detectable sulfa in plasma) or recent IPTp (a history of IPTp use and detectable sulfa).
Placental blood collection [1] and PM diagnosis [9] were performed as previously described. After delivery, maternal peripheral blood and cord blood were collected in citrate phosphate dextrose solution or ethylenediaminetetraacetic acid, respectively, and complete blood counts were obtained on a Cell-Dyne 1200 hematology analyzer (Abott Diagnostics). Maternal anemia was defined as a hemoglobin level <11 g/dL [13]; fetal anemia was defined as a hemoglobin level <12.5 g/dL [14]. Outliers were excluded from maternal and cord hemoglobin datasets using the 3σ rule [15]. Low birth weight (LBW) was defined as birth weight <2500 grams. Maternal age, parity, malaria transmission season, village, bed net use, and date of enrollment were recorded at delivery.
Statistical Analysis
The primary analysis examined the effect of no IPTp versus any IPTp (early and recent together). Covariates for descriptive statistics were evaluated with χ2 tests, except for maternal age and time, which were evaluated with Student’s ttests.
Primary outcomes were odds of PM, mean maternal hemoglobin level, mean birth weight, and mean cord hemoglobin level; secondary outcomes were odds of maternal anemia, LBW, and fetal anemia, and these were considered to be markers of clinically relevant change. To account for multiple comparisons based on 4 primary outcomes, the cut-off for significance was adjusted to P ≤ .013, using the Bonferroni correction method. All outcome analyses used linear or logistic regression models with robust standard errors to account for nonnormally distributed data.
Covariates modeled for adjustment included dichotomous variables (sex of infant, birth season based on incidence of parasitemia among 3–12-month-old infants (low [November to April] vs high malaria transmission [May to October]), and village setting [rural or semi-urban]), categorical variables (parity [primigravidae, secundigravidae, or multigravidae] and bed net use [no bed net, untreated bed net, insecticide-treated bed net, or unknown bed net use]), and continuous variables (maternal age and enrollment [measured as day after study start]). Adjusted models included all covariates described above.
Prior work from this cohort found that the effect of IPTp on parasite diversity and parasite density varied by whether sulfa was detectable in maternal peripheral plasma at delivery, a proxy for timing of last IPTp dose [9]. As a result, secondary exploratory analyses examined effects of IPTp timing (early vs recent) [9]. Early and recent IPTp were considered to be significantly different from each other when the postestimation test of equivalence yielded α ≤ 0.10.
Finally, we considered effect modification of IPTp by PM (for all outcomes other than PM) and parity, which have previously been related to malaria infection risk during early life [10, 16]. Interaction terms were considered significant when α ≤ 0.10.
All statistical analyses were conducted using Stata software, version 9.1 (Stata).
RESULTS
Demographic Characteristics of the Study Population
IPTp use was documented for 826 of 880 women in the cohort, including 108 (13.1%) who reported no IPTp and 718 (86.9%) who reported IPTp use. Of those who reported IPTp use, 607 (73.5%) were consistent with early use, and 111 (13.4%) were consistent with recent use.
Measured plasma sulfa levels were consistent with self-reported SP exposure (both IPTp and treatment doses): 136 of 137 plasma sulfa–positive women reported SP exposure, and 89 of 90 women who denied SP exposure were sulfa negative. These data argue that maternal report of SP exposure was accurate, and they increase our confidence in the validity of our results.
Women who received IPTp were significantly less likely than those who did not to live in an urban area (Table 1) and were more likely to enroll later in the study, reflecting increased use of IPTp between the first and last years of the study (from 67.2% to 92.0% of women). Women who received recent IPTp, compared with those who received early IPTp, were more likely to deliver during the high-transmission season (P = .05, by χ2 test) and were enrolled later in the study (P = .04, by χ2 test).
Table 1.
Baseline Characteristics by Intermittent Preventive Treatment During Pregnancy (IPTp) Use
| Covariate | No IPTp (n = 108) | Early IPTp (n = 607) | Recent IPTp (n = 111) | Pa |
| Female infant | 51 (47.2) | 317 (52.2) | 60 (54.0) | .31 |
| Birth season (high) | 61 (56.5) | 284 (46.8) | 63 (56.8) | .11 |
| Bed net use | .20 | |||
| None | 29 (26.9) | 190 (31.3) | 30 (27.0) | |
| Untreated | 45 (41.7) | 226 (37.2) | 37 (33.3) | |
| Treated | 11 (10.2) | 94 (15.5) | 23 (20.7) | |
| Unknown | 23 (21.3) | 97 (16.0) | 21 (18.9) | |
| Previous pregnancies | .17 | |||
| 0 | 34 (31.5) | 168 (27.7) | 33 (29.7) | |
| 1 | 30 (27.8) | 133 (21.9) | 24 (21.6) | |
| ≥2 | 44 (40.7) | 306 (50.4) | 54 (48.7) | |
| Village (urban) | 58 (53.7) | 269 (44.4) | 45 (40.5) | .05 |
| Maternal age, mean years (±SD) | 25.2 (5.7) | 26.3 (6.4) | 26.3 (6.5) | .07 |
| Days after study start, mean days (±SD) | 308.5 (331.0) | 483.5 (316.1) | 552.4 (348.4) | <.001 |
NOTE. Data are no. (%) of subjects unless otherwise indicated. Boldface type indicates statistical significance. SD, standard deviation.
P values reflect the difference between no IPTp and any IPTp.
IPTp Does Not Improve Overall Pregnancy Outcomes
IPTp was not associated with decreased odds of PM, nor was it associated with mean maternal hemoglobin level or birth weight (Table 2). Further, IPTp did not have any effect on the secondary outcomes of odds of maternal anemia or odds of LBW. Unexpectedly, IPTp was associated with decreased cord hemoglobin level and increased risk of fetal anemia. Recent versus early IPTp did not differ from each other in their prediction of any outcome.
Table 2.
Intermittent Preventive Treatment During Pregnancy (IPTp) Does Not Predict Reduced Risk of Poor Delivery Outcomes
| Outcome | No. of subjects (no. of subjects with event) | Effect size, OR (95% CI) | Pa | Adjusted effect size, OR (95% CI) | Pa |
| Placental malaria (n = 826) | |||||
| No IPTp | 108 (17) | Reference | Reference | ||
| IPTp | 718 (88) | 0.75 (0.43–1.31) | .31 | 0.93 (0.51–1.72) | .83 |
| Early | 607 (78) | 0.79 (0.45–1.40) | 0.99 (0.53–1.83) | ||
| Recent | 111 (10) | 0.53 (0.23–1.22) | 0.64 (0.27–1.55) | ||
| Maternal Hgb (n = 676)b | |||||
| No IPTp | 11.0 g/dL (4.0) | Reference | Reference | ||
| IPTp | 11.3 g/dL (3.9) | 0.2 g/dL (−0.6 to 1.1) | .61 | 0.1 g/dL (−0.9 to 1.0) | .92 |
| Early | 11.3 g/dL (3.9) | 0.3 g/dL (−0.6 to 1.2) | 0.1 g/dL (−0.8 to 1.1) | ||
| Recent | 10.8 g/dL (3.9) | −0.2 g/dL (−1.3 to 0.9) | −0.5 g/dL (−1.6 to 0.7) | ||
| Maternal anemia (n = 676) | |||||
| No IPTp | 97 (53) | Reference | Reference | ||
| IPTp | 579 (301) | 0.90 (0.58–1.39) | .63 | 0.99 (0.63–1.56) | .98 |
| Early | 489 (251) | 0.88 (0.57–1.36) | 0.96 (0.61–1.52) | ||
| Recent | 90 (50) | 1.04 (0.58–1.85) | 1.19 (0.66–2.16) | ||
| Birth weight (n = 826) | |||||
| No IPTp | 3210 g (497) | Reference | Reference | ||
| IPTp | 3198 g (423) | −12 g (−111 to 87) | .81 | −50g (−143 to 42) | .29 |
| Early | 3195 g (425) | −15 g (−114 to 85) | −52g (−145 to 42) | ||
| Recent | 3213 g (417) | 3 g (−119 to 125) | −42g (−160 to 75) | ||
| Low birth weight (n = 826) | |||||
| No IPTp | 108 (8) | Reference | Reference | ||
| IPTp | 718 (29) | 0.53 (0.23–1.19) | .12 | 0.71 (0.33–1.54) | .39 |
| Early | 607 (28) | 0.60 (0.27–1.37) | 0.82 (0.38–1.78) | ||
| Recent | 111 (1) | 0.11 (0.01–0.93) | 0.16 (0.02–1.23) | ||
| Cord Hgb (n = 685)b | |||||
| No IPTp | 14.3 g/dL (2.7) | Reference | Reference | ||
| IPTp | 13.6 g/dL (3.5) | −0.7 g/dL (−1.3 to −0.1) | .03 | −1.2 g/dL (−1.7 to −0.5) | <.001 |
| Early | 13.6 g/dL (4.5) | −0.7 g/dL (−1.3 to −0.1) | −1.2 g/dL (−1.8 to −0.5) | ||
| Recent | 13.7 g/dL (3.2) | −0.6 g/dL (−1.5 to 0.2) | −1.2 g/dL (−2.0 to −0.4) | ||
| Fetal anemia (n = 685) | |||||
| No IPTp | 96 (23) | Reference | Reference | ||
| IPTp | 589 (186) | 1.47 (0.89–2.42) | .14 | 1.91 (1.14–3.19) | .01 |
| Early | 495 (163) | 1.45 (0.94–3.58) | 2.01 (1.20–3.38) | ||
| Recent | 94 (23) | 1.03 (0.53–2.00) | 1.40 (0.71–2.78) |
NOTE. Boldface type indicates statistical significance. CI, confidence interval; Hgb, hemoglobin level; OR, odds ratio; SD, standard deviation.
P values reflect difference between no IPTp and any IPTp.
Values are mean (standard deviation).
We then examined effect modification of IPTp by PM status for all outcomes other than PM. PM status modified the effect of IPTp to predict birth weight (interaction term [IPTp × PM]: α = 0.09) and maternal hemoglobin level (interaction term [IPTp× PM]: α = 0.04). The effect of IPTp to reduce mean birth weight was stronger among infants born to PM-positve women compared with infants born to PM-negative women (Table 3). In contrast, IPTp was associated with an increase in maternal hemoglobin among PM-positive women but not among PM-negative women. There was no evidence of effect modification by parity.
Table 3.
Placental Malaria (PM) Status Modifies the Effect of Intermittent Preventive Treatment During Pregnancy (IPTp) to Predict Some Delivery Outcomes
| Outcome | PM negative, mean value (±SD) | Adjusted effect size | Pa | PM positive, mean value (±SD) | Adjusted effect size | Pa |
| Birth weight (α = 0.09) | ||||||
| No IPTp | 3224 g (521) | Reference | 3136 g (348) | Reference | ||
| IPTp | 3235 g (409) | −35 g (−138 to 69) | .51 | 2931 g (434) | −175 g (−402 to 53) | .13 |
| Early | 3340 g (407) | −29 g (−133 to 75) | 2893 g (423) | −224 g (−433 to −16) | ||
| Recent | 3212 g (419) | −67 g (−193 to 60) | 3225 g (420) | 111 g (−263 to 484) | ||
| Maternal hemoglobin level (α = 0.04) | ||||||
| No IPTp | 11.4 g/dL (4.2) | Reference | 9.3 g/dL (2.7) | Reference | ||
| IPTp | 11.3 g/dL (3.9) | −0.3 g/dL (−1.3 to 0.7) | .60 | 11.1 g/dL (3.8) | 2.1 g/dL (−0.1 to 4.2) | .06 |
| Early | 11.4 g/dL (4.0) | −0.2 g/dL (−1.2 to 0.9) | 11.1 g/dL (3.7) | 2.1 g/dL (−0.1 to 4.4) | ||
| Recent | 10.9 g/dL (3.8) | −0.8 g/dL (−2.1 to 0.4) | 10.7 g/dL (4.4) | 1.7 g/dL (−1.6 to 5.0) |
P values reflect difference between no IPTp and any IPTp.
Increasing Cord Sulfa Level Predicts Decreasing Cord Hemoglobin
Given the unexpected association between IPTp and decreased cord hemoglobin and increased risk of fetal anemia, we hypothesized that in utero SP exposure might suppress fetal hematopoiesis, similar to its effects in adults [17].
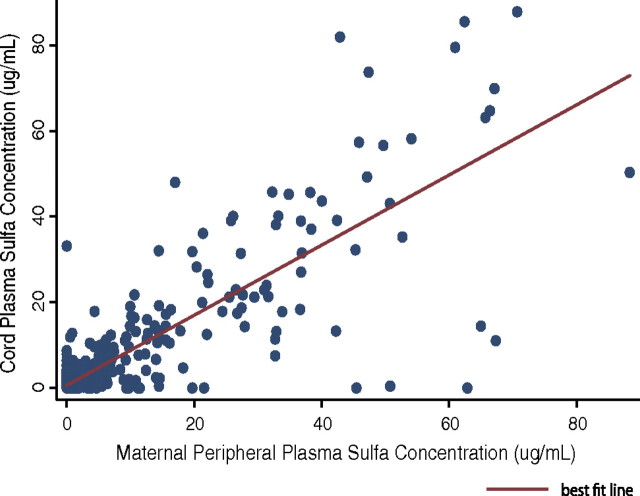
We first determined whether there was fetal exposure to SP from either IPTp or treatment doses by measuring cord plasma sulfa levels and comparing them to maternal plasma sulfa levels. Plasma sulfa measurements were available for 851 women and 847 cord samples. Sulfa was detected in the plasma of 141 (16.6%) of the women and 135 (15.9%) of the cord samples. Among positive samples, the mean concentration of sulfa was 22.7 μg/mL in maternal plasma and 21.9 μg/mL in cord plasma. When both maternal and cord samples were positive (n = 108), the sulfa concentrations were highly correlated (r = 0.77; P < .001), and the ratio of cord sulfa to maternal sulfa was 1.07 (interquartile range, 0.74–1.30). When negative samples were also considered (n = 821), the correlation between maternal and cord sulfa levels was strengthened (r = 0.84; P < .001) (Figure 1).
Figure 1.
Maternal peripheral plasma sulfa concentration is strongly associated with cord plasma sulfa concentration (r = 0.84; P < .001).
We then determined whether cord hemoglobin and red blood cell count were related to sulfa exposure. Among samples with detectable cord sulfa levels, sulfa concentration was inversely related to cord hemoglobin (Δ = −0.4 g/dL [95% confidence interval {CI}, −0.7 to 0.0] per 10 μg/mL increase in sulfa; P = .05) and red blood cell count (Δ = −1.1 × 105 [95% CI, −2.2 to 0.0] cells/μL per 10 μg/mL increase in sulfa; P = .05) (Figure 2).
Figure 2.
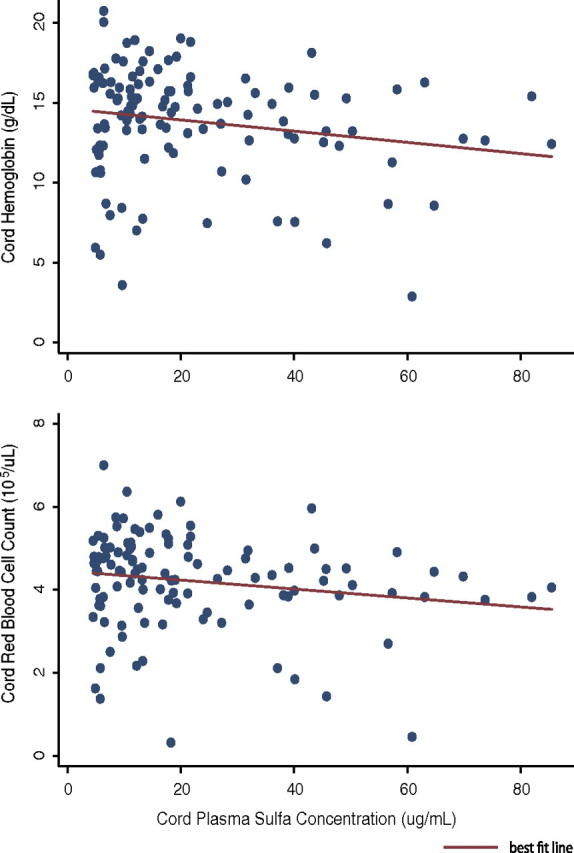
Increasing cord plasma sulfa concentration predicts decreasing cord hemoglobin level (−0.4 g/dL per 10 μg/mL increase in sulfa; P = .05) and decreasing cord red blood cell count (−1.1 × 105 RBC/μL per 10 μg/mL increase in sulfa; P = .05).
DISCUSSION
SP-IPTp did not improve overall pregnancy outcomes in our cohort from the community of Muheza, Tanzania, which is located in an area of widespread drug resistance. Instead, IPTp was associated with decreased cord hemoglobin levels and increased risk of fetal anemia.
Where parasites are susceptible to SP, IPTp reduces the prevalence of PM, maternal anemia, and LBW [18] and increases mean maternal hemoglobin level and birth weight [19]. A 2007 review concluded that IPTp remained beneficial in areas with high levels of SP resistance [20], but based this conclusion on communities where the parasitologic treatment failure rate in children had reached a maximum of 26% at day 14 [20]. By comparison, the 14-day treatment failure rate in children around Muheza, Tanzania, increased from 41% [21] to 68% [7] during the period of the current study, and parasite SP resistance alleles approached saturation [9].
Evidence of IPTp failure exists. We recently observed that placental infections were exacerbated in women who experienced failure of presumptive treatment and remained infected at delivery [9]. In addition, a 2008 study from Mozambique examined the effect of IPTp in the context of widespread insecticide-treated bed net use and found that IPTp had no effect on the prevalence of PM, maternal anemia, or LBW [22]. The absence of effect was attributed to the masking benefit of bed nets but may equally have been attributable to loss of SP efficacy in that area. Other randomized controlled studies of IPTp have variably found no reduction in PM [23, 24], maternal anemia [25], or LBW [13, 24, 25]. We hypothesize that the failure of IPTp to improve outcomes in previous trials may be the result of accumulating resistance in various communities.
The observations from the present study suggest that IPTp may have negligible overall benefits for the community of Muheza, Tanzania. In addition, the deleterious effects of IPTp on cord blood hemoglobin levels and fetal anemia were unexpected. Fetal anemia predisposes to infancy anemia [14, 26], which is a risk factor for infant mortality [27, 28]. Furthermore, recent work from Malawi has demonstrated an association between fetal anemia and shorter time to first illness during childhood (respiratory infection, malaria, or diarrhea), as well as a higher prevalence of persistent illness and a higher cumulative incidence of morbidity [29]. Fetal anemia may play a larger role in determining childhood morbidity and mortality than has previously been appreciated.
We found that sulfa was readily detectable in cord plasma and that levels correlated well with maternal plasma sulfa levels, suggesting considerable in utero exposure to SP. The ratio of cord-maternal sulfa that we describe is consistent with a report of SP used to treat congenital toxoplasmosis that found a mean fetal-maternal ratio of 0.97 (range, 0.65–1.16) [30]. In a second study of pregnant ewes given sulfamethoxazole (a related sulfonamide), sulfa was concentrated in fetal plasma as a result of decreased renal clearance of the primary metabolite by the fetus [31]. In addition, we observed an inverse relationship between cord sulfa concentration and both cord hemoglobin level and red blood cell count, supporting our hypothesis that in utero SP exposure may suppress hematopoiesis in the fetus. Future studies are needed to explore this possibility.
This study has several limitations. First, the dhfr-dhps quintuple mutant was ubiquitous, and nearly a third of parasites carried the additional mutation at dhps codon 581 [9]. This degree of drug resistance is unusually high, but it may nevertheless presage events elsewhere in Africa as drug-resistant parasites spread. Second, although a randomized placebo-controlled trial would have been the most rigorous study design to address our hypothesis, we used a cross-sectional approach. SP-IPTp is the standard of care in Tanzania, precluding a study that included a placebo control. Accordingly, our control group consisted of women who through their own actions or those of ANC staff failed to receive IPTp. Although we found and adjusted for multiple confounders, our results could be biased by unmeasured variables. Finally, our analysis of primary IPTp exposure was based on self-report. We validated our IPTp data with measured plasma sulfa levels, however, and found self-report to be very reliable.
Our data suggest that IPTp loses its benefits as drug resistance increases in an area. Models of drug resistance predict that benefits decay as drug-resistant parasites spread, eventually approaching zero. In contrast, our data suggest that continued mass administration of IPTp in an area of high resistance may move beyond zero and result in net harm. In addition, our data from previous work [9], as well as those from mouse models of competitive facilitation [32], imply that continued application of a failing drug regimen may exacerbate rather than alleviate infection and disease in some individuals. We hypothesize that above a threshold level of drug resistance the detrimental effects of a regimen will outweigh the beneficial effects, resulting in harm to the community. Our data suggest that Muheza is at or near such a threshold and that continued application of SP may be more harmful than beneficial.
Our findings of the potential for harm highlight a fundamental flaw in ongoing efforts to find effective new IPTp regimens, which are compared with SP [33]. Such trials may find that new IPTp regimens confer benefits when compared with SP, but may still not be able to conclude that they are better than placebo, increasing the risk of Type I error. More generally, many studies of new interventions for the treatment of malaria or other diseases do not include a placebo control group. Instead, new interventions are evaluated against older interventions. Evidence that a new intervention is better than an older, harmful intervention does not justify the conclusion that it is better than placebo. This key implication of our data should inform future prophylaxis and treatment trials for malaria.
Once a policy of mass drug administration has been implemented, clinical equipoise makes placebo-controlled trials problematic, and the opportunity to confirm potential harm in a community is limited. Cross-sectional observational studies similar to our own should be undertaken at other sites that have varying degrees of resistance and PM prevalence. These might yield an improved model of IPTp efficacy that could predict the appropriate time to remove this failing drug regimen. Separately, this study provides the impetus for future work using placebo-controlled trials of IPTp to ensure that mass drug administration is conferring benefits and not harm.
The WHO recently concluded that priorities for future research were the identification of “when SP should be replaced with a more effective antimalarial” (p. 5) and “the efficacy of SP-IPTp against increasing prevalent quintuple pfdhr/dhps mutant P. falciparum” [4 p. 5]. In addition, the WHO concluded that increasing resistance demands the “evaluation of the in vivo therapeutic and protective efficacy of SP in asymptomatic pregnant women, and its correlation with molecular markers of SP resistance, and the continuous monitoring of effectiveness of SP-IPTp” [4 p. 6]. This study directly addresses the recommendations of the WHO and is the first to examine IPTp efficacy in an area where the SP treatment failure rate in children exceeds 50% [7, 21] and where molecular markers of resistance approach saturation [9]. We find strong evidence that IPTp is failing in Muheza, arguing for urgent reevaluation of IPTp in areas of widespread resistance.
Acknowledgments
We thank the women and infants who participated in this study.
The content is solely the responsibility of the Preventive Treatment of Malaria authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Financial support. This work was supported by the Bill & Melinda Gates Foundation (grant 29202); the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (grant 1364); the US National Institutes of Health Fogarty International Center (FIC) (grant D43 TW005509); the National Institute of Allergy and Infectious Diseases (R01AI52059 to P. E. D.); and by the National Heart, Lung, and Blood Institute (NIH Fellowship 1F30HL096298 to W. E. H.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 2.Garnham PCC. The placenta in malaria with special reference to reticulo-endothelial immunity. Trans R Soc Trop Med Hyg. 1938;32:13–22. [Google Scholar]
- 3.Poespoprodjo JR, Fobia W, Kenangalem E, et al. Adverse pregnancy outcomes in an area where multidrug-resistant plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46:1374–81. doi: 10.1086/586743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Technical Expert Group meeting on intermittent preventive treatment in pregnancy (IPTp) Geneva, Switzerland: WHO Headquarters; 11–13 July. 2007. [Google Scholar]
- 5.Gregson A, Plowe CV. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev. 2005;57:117–45. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Brooks DR, Wang P, Read M, Watkins WM, Sims PF, Hyde JE. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur J Biochem. 1994;224:397–405. doi: 10.1111/j.1432-1033.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- 7.Gesase S, Gosling RD, Hashim R, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One. 2009;4:e4569. doi: 10.1371/journal.pone.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jelinek T, Kilian AH, Kabagambe G, von Sonnenburg F. Plasmodium falciparum resistance to sulfadoxine/pyrimethamine in Uganda: correlation with polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes. Am J Trop Med Hyg. 1999;61:463–6. doi: 10.4269/ajtmh.1999.61.463. [DOI] [PubMed] [Google Scholar]
- 9.Harrington WE, Mutabingwa TK, Muehlenbachs A, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci U S A. 2009;106:9027–32. doi: 10.1073/pnas.0901415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutabingwa TK, Bolla MC, Li JL, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fansidar product information. Roche pharmaceuticals 08/16/04. http://www.rocheusa.com/products/fansidar/pi.pdf. Accessed 16 August 2004. [Google Scholar]
- 12.Morris CJ. The determination of sulphanilamide and its derivatives. Biochem J. 1941;35:952–9. doi: 10.1042/bj0350952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parise ME, Ayisi JG, Nahlen BL, et al. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59:813–22. doi: 10.4269/ajtmh.1998.59.813. [DOI] [PubMed] [Google Scholar]
- 14.le Cessie S, Verhoeff FH, Mengistie G, Kazembe P, Broadhead R, Brabin BJ. Changes in haemoglobin levels in infants in Malawi: effect of low birth weight and fetal anaemia. Arch Dis Child Fetal Neonatal Ed. 2002;86:F182–7. doi: 10.1136/fn.86.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pukelsheim F. The three sigma rule. Am Statistician. 1994;48:88–91. [Google Scholar]
- 16.Pullan RL, Bukirwa H, Staedke SG, Snow RW, Brooker S. Plasmodium infection and its risk factors in eastern Uganda. Malar J. 2010;9:2. doi: 10.1186/1475-2875-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drugdex evaluations: sulfadoxine/pyrimethamine. Micromedex 20. Available at: http://www.thomsonhc-com.offcampus.lib.washington.edu/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/D5D607/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/512A26/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.IntermediateToDocumentLink?docId=0872&contentSetId=31&title=SULFADOXINE%2FPYRIMETHAMINE&servicesTitle=SULFADOXINE%2FPYRIMETHAMINE. Accessed 13 June 2011. [Google Scholar]
- 18.Peters PJ, Thigpen MC, Parise ME, Newman RD. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 2007;30:481–501. doi: 10.2165/00002018-200730060-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kayentao K, Kodio M, Newman RD, et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. 2005;191:109–16. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- 20.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–16. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 21.Mutabingwa TK, Anthony D, Heller A, et al. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005;365:1474–80. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- 22.Menendez C, Bardaji A, Sigauque B, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One. 2008;3:e1934. doi: 10.1371/journal.pone.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhoeff FH, Brabin BJ, Chimsuku L, Kazembe P, Russell WB, Broadhead RL. An evaluation of the effects of intermittent sulfadoxine-pyrimethamine treatment in pregnancy on parasite clearance and risk of low birthweight in rural Malawi. Ann Trop Med Parasito. 1998;92:141–50. doi: 10.1080/00034989859979. [DOI] [PubMed] [Google Scholar]
- 24.Hommerich L, von Oertzen C, Bedu-Addo G, et al. Decline of placental malaria in southern Ghana after the implementation of intermittent preventive treatment in pregnancy. Malar J. 2007;6:144. doi: 10.1186/1475-2875-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gies S, Coulibaly SO, Ouattara FT, Ky C, Brabin BJ, D'Alessandro U. A community effectiveness trial of strategies promoting intermittent preventive treatment with sulphadoxine-pyrimethamine in pregnant women in rural Burkina Faso. Malar J. 2008;7:180. doi: 10.1186/1475-2875-7-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brabin B. Fetal anaemia in malarious areas: its causes and significance. Ann Trop Paediatr. 1992;12:303–10. doi: 10.1080/02724936.1992.11747589. [DOI] [PubMed] [Google Scholar]
- 27.van Eijk AM, Ayisi JG, Ter Kuile FO, et al. HIV, malaria, and infant anemia as risk factors for postneonatal infant mortality among HIV-seropositive women in Kisumu, Kenya. J Infect Dis. 2007;196:30–7. doi: 10.1086/518441. [DOI] [PubMed] [Google Scholar]
- 28.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131(2 Suppl 2):636S–45S. doi: 10.1093/jn/131.2.636S. discussion 46S–48S. [DOI] [PubMed] [Google Scholar]
- 29.Kalanda B, Verhoeff F, le Cessie S, Brabin J. Low birth weight and fetal anaemia as risk factors for infant morbidity in rural Malawi. Malawi Med J. 2009;21:69–74. doi: 10.4314/mmj.v21i2.44553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trenque T, Marx C, Quereux C, et al. Human maternofoetal distribution of pyrimethamine-sulphadoxine. Br J Clin Pharmacol. 1998;45:179–80. doi: 10.1046/j.1365-2125.1998.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vree TB, Reekers-Ketting JJ, Nouws JF, Arts TH. Placental transfer and renal clearance of sulphamethoxazole and its metabolite N4-acetylsulphamethoxazole in a pregnant ewe. J Vet Pharmacol Ther. 1983;6:77–81. doi: 10.1111/j.1365-2885.1983.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 32.Wargo AR, Huijben S, de Roode JC, Shepherd J, Read AF. Competitive release and facilitation of drug-resistant parasites after therapeutic chemotherapy in a rodent malaria model. Proc Natl Acad Sci U S A. 2007;104:19914–9. doi: 10.1073/pnas.0707766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman RD, Parise ME, Slutsker L, Nahlen B, Steketee RW. Safety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan Africa. Trop Med Int Health. 2003;8:488–506. doi: 10.1046/j.1365-3156.2003.01066.x. [DOI] [PubMed] [Google Scholar]