In a community-based cohort study of injection drug users in Baltimore, Maryland, incarceration was the most important risk factor for loss of virologic control within 6 months after achieving HIV suppression in response to antiretroviral therapy.
Abstract
Background. Incarceration may lead to interruptions in antiretroviral therapy (ART) for persons receiving treatment for human immunodeficiency virus (HIV) infection. We assessed whether incarceration and subsequent release were associated with virologic failure for injection drug users (IDUs) who were previously successfully treated with ART.
Methods. ALIVE is a prospective, community-based cohort study of IDUs in Baltimore, Maryland. IDUs receiving ART during 1998–2009 who successfully achieved an HIV RNA level below the limit of detection (<400 copies/mL) were followed up for development of virologic failure at the subsequent semiannual study visit. Logistic regression with generalized estimating equations was used to assess whether incarceration was independently associated with virologic failure.
Results. Of 437 HIV-infected IDUs who achieved undetectable HIV RNA for at least one study visit, 69% were male, 95% were African-American, and 40% reported at least one incarceration during follow-up. Virologic failure occurred at 26.3% of visits after a median of 6 months since achieving undetectable HIV RNA. In multivariate analysis accounting for demographic characteristics, drug use, and HIV disease stage, brief incarceration was strongly associated with virologic failure (adjusted odds ratio, 7.7; 95% confidence interval, 3.0–19.7), although incarceration lasting >30 days was not (odds ratio, 1.4; 95% confidence interval, .8–2.6).
Conclusions. Among IDUs achieving viral suppression while receiving ART, virologic failure occurred with high frequency and was strongly associated with brief incarceration. Efforts should be made to ensure continuity of care both during and after incarceration to improve treatment outcomes and prevent viral resistance in this vulnerable population.
Antiretroviral therapy (ART) suppresses replication of human immunodeficiency virus (HIV), leading to improved survival of HIV-infected persons [1–3] and diminished transmission of HIV to others [4]. After virologic suppression is achieved, however, data from clinical trials show that 10%–20% of patients will experience virologic failure within the first year of treatment [5–7]. Observational studies from clinical cohorts suggest that the incidence of virologic failure is even higher in clinical practice [8–11]. Because virologic failure can lead to development of drug resistance, limit future treatment options, and increase risk of clinical events, it is important for clinicians to recognize patients at increased risk of virologic failure.
In the United States, HIV-infected individuals are more frequently incarcerated than is the general population. In one year, an estimated 22%–31% of HIV-infected Americans passed through a correctional facility [12]. By disrupting access to usual sources of care for arrestees, incarceration may cause interruptions in treatment for HIV infection and other infectious diseases [13, 14]. In this way, incarceration may be an important but often overlooked cause of treatment failure for individuals receiving ART. To address this issue, we used data from a prospective, community-based cohort study to assess whether incarceration is an independent predictor of virologic failure among patients who had achieved virologic suppression in response to ART.
METHODS
Study Population
Participants in this study were current and former HIV-infected injection drug users (IDUs) in Baltimore, Maryland, who were recruited into a community-based cohort study of the natural history of HIV-1 infection. As described elsewhere [15], the AIDS Linked to the Intravenous Experience (ALIVE) study began to prospectively follow up 2946 IDUs in 1988. Participants were aged ≥18 years, were free of clinical AIDS at the time of enrollment, and reported injection drug use during the preceding 11 years. Recruitment of additional IDUs occurred during 1994–1995, 1998, 2000, and 2005–2008. The study was approved by the institutional review board at the Johns Hopkins Bloomberg School of Public Health, and all participants provided written informed consent.
Individuals were included in the present analysis if they (1) were HIV seropositive or experienced seroconversion during follow-up, (2) contributed specimens for evaluation of quantitative HIV RNA from January 1998 through December 2009, and (3) had at least one study visit at which the HIV RNA level was below the limit of detection (LOD; <400 copies/mL). From 1998 through 2009, 767 HIV-infected IDUs were followed up in ALIVE and attended at least 2 study visits. We excluded 321 IDUs from the primary analysis, because they never achieved virologic suppression during follow-up. An additional 9 individuals who had persistently undetectable HIV RNA in the absence of ART were considered to be elite controllers and were also excluded. The remaining 437 participants comprise the study sample for the main analysis of virologic failure. The excluded participants reported incarceration more frequently than did those who were included in the analysis (at 15% vs 8% of visits). They were also more likely to be unemployed, have advanced HIV infection, and to be using drugs and alcohol than were participants included in the analysis (P < .05 for each comparison).
Ascertainment of Incarceration Status
Beginning in 1998, self-reported data regarding sensitive topics, such as drug use, sexual practices, illegal activities, and incarceration, were captured using audio computer-assisted self-interview (ACASI). At each study visit, participants were prompted to report whether and how many times they were incarcerated for at least 7 days and the total length of incarceration since their previous study visit. We defined brief incarceration as admission to a correctional facility for >7 days but <30 days and prolonged incarceration as report of incarceration lasting ≥30 days. These intervals were selected to distinguish pretrial detention in jails, which are usually brief, from longer prison stays, in which inmates are more likely to regularly receive medical care and discharge planning.
Laboratory Testing
A clinical examination and phlebotomy were performed at every semiannual study visit to collect clinical data relevant to HIV infection and for laboratory testing of HIV disease markers, respectively. Plasma HIV RNA levels were quantified using reverse-transcriptase polymerase chain reaction (Roche Molecular Systems) according to manufacturer’s specifications. The minimal detectable HIV RNA level was 400 copies/mL. CD4 lymphocyte counts were measured using whole blood staining methods and flow cytometry.
Statistical Analysis
The primary unit of analysis was visit pairs. We defined virologic failure as an increase in HIV RNA level from below the limit of detection at the first visit (<400 copies/mL) to any value >400 copies/mL at the follow-up visit. Because intermittent ART use is common among IDUs [16–18], we considered every visit when a participant’s HIV RNA was undetectable to represent a new period at risk for the outcome, meaning virologic failure could occur multiple times for a single person. To identify factors independently associated with virologic failure, we performed univariate and multivariate logistic regression analyses with generalized estimating equations with robust variance estimates to account for intra-subject correlation resulting from repeated measurements. Primary exposures of interest were brief and prolonged incarceration, assessed at the second visit of each pair. This reflected incarceration occurring any time within 6 months before assessment of the outcome. Potential confounders of this association were also assessed at the second visit and included age, sex, race, homelessness, active injection and noninjection drug use, methadone maintenance, and CD4+ cell count. Variables associated with virologic failure at a level P < .05 and those considered a priori to be potential confounders were included the multivariate model.
Acknowledging that transient, clinically insignificant elevations in HIV RNA level can occur in patients successfully receiving ART, we performed a sensitivity analysis in which virologic failure was defined as an increase in HIV RNA level to >1000 copies/mL, rather than 400 copies/mL. Identical statistical models were used to estimate odds ratios (ORs) after recoding the response variable to reflect this higher threshold. This allowed us to determine the proportion of cases of virologic failure that were attributable to potentially insignificant viremic blips and to assess whether any association between incarceration and virologic failure remained significant under a more robust definition of the outcome.
Antiretroviral Therapy Use and Health Care Utilization
Because discontinuation of ART predictably leads to HIV rebound, participant report of ART use is expected to be highly correlated with virologic suppression. Therefore, if the association between incarceration and virologic failure was completely mediated by discontinuation of ART, we would expect that controlling for ART use would eliminate the observed association between incarceration and the outcome. To investigate this possibility, we compared multivariate models with and without ART included as a covariate. We hypothesized that attendance at a primary care visit soon after incarceration could modify the effect of incarceration on virologic outcome and assessed this possibility by including in the multivariate model an interaction term composed of incarceration and HIV clinic attendance.
Risk Behavior Assessment
We assessed whether participants engaged in HIV transmission risk behaviors, including sharing injection equipment or attending so-called “shooting galleries” (public drug-injecting venues), during the 6 months before every study visit. The frequency of these behaviors was compared between visits when recent incarceration was and was not reported. We further explored differences in these behaviors according to whether IDUs had achieved HIV suppression in response to ART.
RESULTS
Study Population and Frequency of Incarceration
In the final study sample of 437 IDUs, the median age was 43 years, 94.5% were African American, 68.6% were male, 80.4% were unemployed, and 58.1% were actively injecting drugs at the time of the first study visit. The data set included 2075 paired visits during which the HIV RNA level was below the limit of detection at the first visit. Overall, the median time elapsed between the initial and follow-up visit was 6.0 months (interquartile range [IQR], 5.8–6.5 months). When an incarceration was reported, the median time between visits was 7.6 months (IQR, 5.9–10.5 months). Over a median of 6.6 years of follow-up during 1998–2009, 175 (40%) of 437 participants reported at least 1 incident incarceration; 115 (26.3%) reported >1 incarceration. The median duration of incarceration was 119 days (IQR, 61–170 days). Brief incarcerations (7–30 days) accounted for 19% of all reported incarcerations. Table 1 shows a comparison of characteristics of participants who reported any incarceration during follow-up with those in persons who were never incarcerated.
Table 1.
Participant Characteristics by Ever/Never Incarceration Among Injection Drug Users (IDUs) Achieving Virologic Suppression (n = 437)
| Never incarcerated during follow-up (n = 255) | Incarcerated at least once during follow-up (n = 182) | |
| Male | 158 (62.0) | 142 (78.0) b |
| African-American | 241 (94.5) | 172 (94.5) |
| Age (median, IQR) | 45 (40–49) | 42 (38–46) b |
| Unemployed | 206 (81.4) | 142 (78.9) |
| Finished high school | 103 (40.7) | 65 (35.7) |
| Homeless | 38 (15.0) | 48 (26.7) b |
| CD4 count (cells/μL) b | ||
| ≥350 | 96 (42.7) | 63 (40.7) |
| 200–349 | 75 (33.3) | 51 (32.9) |
| <200 | 54 (24.0) | 41 (26.5) |
| HIV RNA level (copies/mL) b | ||
| ≤400 | 98 (43.2) | 44 (28.2) |
| 401–10 000 | 44 (19.4) | 44 (28.2) |
| >10 000 | 85 (37.4) | 68 (43.6) |
| Injection drug use during past 6 months b | ||
| None | 133 (52.4) | 49 (27.2) |
| Occasional | 68 (26.8) | 68 (37.8) |
| Daily | 53 (20.9) | 63 (35.0) a |
| Alcohol use during the past 6 months b | ||
| None | 136 (53.8) | 79 (44.9) |
| Occasional | 102 (40.3) | 68 (38.6) |
| Daily | 15 (5.9) | 29 (16.5) a |
| Receiving methadone maintenance | 59 (23.2) | 12 (6.6) a |
| Incarcerated during follow-up | ||
| Never incarcerated | 255 (100.0) | 0 (0.0) |
| Incarcerated once | 0 (0.0) | 65 (35.7) |
| Multiple incarcerations | 0 (0.0) | 117 (64.3) |
Data are reported as no. (%) unless otherwise indicated. Data obtained from first study visit after January 1, 1998, unless otherwise specified. Abbreviation: IQR, interquartile range.
Significant difference between groups (α = .05) using χ2 test.
Significant difference between groups (α = .05) using t test.
Predictors of Virologic Failure
Virologic failure occurred at 546 (26.3%) of follow-up visits. Virologic failure occurred at 53.3% of follow-up visits when an incarceration was reported, compared with 24.8% of visits when no incarceration was reported, yielding an overall OR of 2.4 (95% confidence interval [CI], 1.5–4.0). When incarcerations were categorized as either brief (7–30 days) or prolonged (>30 days), unadjusted ORs for virologic failure were 6.5 (95% CI, 2.1–19.8) and 1.7 (1.0–3.0), respectively. Most cases of virologic failure were documented within 7 months after the first visit (78%), and nearly all occurred within 12 months (94%). Factors associated with virologic failure in univariate and multivariate analysis are shown in Table 2. Statistically significant univariate associations were detected between virologic failure and younger age, homelessness, failing to obtain high school education, lower CD4 cell count, and alcohol, cocaine, and active injection drug use. Use of nonstandard ART regimens was associated with increased virologic failure, but there was no difference between protease inhibitor–based and nonnucleoside reverse-transcriptase inhibitor–based regimens. In multivariate analysis, brief incarceration remained a strong and statistically significant predictor of virologic failure (OR, 7.7; 95% CI, 3.0–19.7), but prolonged incarceration was no longer statistically significant (OR, 1.4; 95% CI, .8–2.6). Other factors that remained significantly associated with virologic failure in the final model were female sex, alcohol use, lower CD4 cell count, and visits occurring during later years of the study.
Table 2.
Factors Associated With Virologic Failure Among 437 Injection Drug Users (IDUs) Achieving Virologic Suppression in ALIVE
| Variable | Unadjusted OR | 95% CI | Adjusted ORa | 95% CI |
| Incarceration | ||||
| Not incarcerated | 1 | 1 | ||
| Incarcerated 7–30 days | 6.5 | 2.1–19.8 | 7.7 | 3.0–19.7 |
| Incarcerated 30+ days | 1.7 | 1.0–3.0 | 1.4 | .8–2.6 |
| Sociodemographic characteristics | ||||
| Age (per 5-year increase) | 0.7 | .7–.8 | 0.9 | .8–1.0 |
| Female sex | 1.1 | .9–1.5 | 1.4 | 1.0–1.8 |
| African-American race | 0.9 | .5–1.6 | 0.7 | .4–1.4 |
| Employed | 0.8 | .6–1.0 | ||
| Health insurance | 0.6 | .4–.8 | ||
| Homeless | 1.6 | 1.1–2.3 | ||
| At least HS education | 0.7 | .5–1.0 | ||
| Calendar year | 0.9 | .8–.9 | 0.9 | .8–.9 |
| HIV related | ||||
| CD4 cell count | ||||
| ≥350 | 1 | 1 | ||
| 200–349 | 1.8 | 1.4–2.3 | 2.0 | 1.5–2.9 |
| <200 | 4.3 | 3.2–5.9 | 4.5 | 3.3–6.2 |
| Type of ART regimen | ||||
| PI- or NNRTI-based regimen | 1 | |||
| 3 NRTI or other non-standard | 1.6 | 1.0–2.8 | ||
| Outpatient visit within past 3 mo. | 0.8 | .6–1.0 | ||
| Substance abuse | ||||
| Injection drug use | ||||
| None | 1 | |||
| Occasional | 1.7 | 1.3–2.2 | ||
| Daily | 2.0 | 1.4–2.8 | ||
| Any alcohol use | 1.5 | 1.2–1.9 | 1.5 | 1.2–2.0 |
| Any cocaine use | 1.5 | 1.1–1.9 | ||
| Methadone maintenance | 1.0 | .8–1.3 | ||
Abbreviations: HS, high school; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Adjusted for previous incarceration, age, race, sex, calendar year, CD4 cell count, alcohol use.
Sensitivity Analysis
To evaluate the assumption that a single elevation in HIV RNA level to >400 copies/mL constitutes virologic failure, we repeated the aforementioned analyses with use of a more conservative definition of the outcome. When virologic failure was defined as an HIV RNA level >1000 copies/mL, the number of events observed during follow-up was reduced from 546 to 411 (27%–21% of visits). Under the new outcome criterion, brief incarceration remained significantly associated with virologic failure (OR, 4.1; 95% CI, 1.6–6.9). A trend toward increased virologic failure with prolonged incarceration appeared stronger in this revised model, but did not reach statistical significance (OR, 1.7; 95% CI, .9–2.3).
Antiretroviral Therapy Use and Health Care Utilization
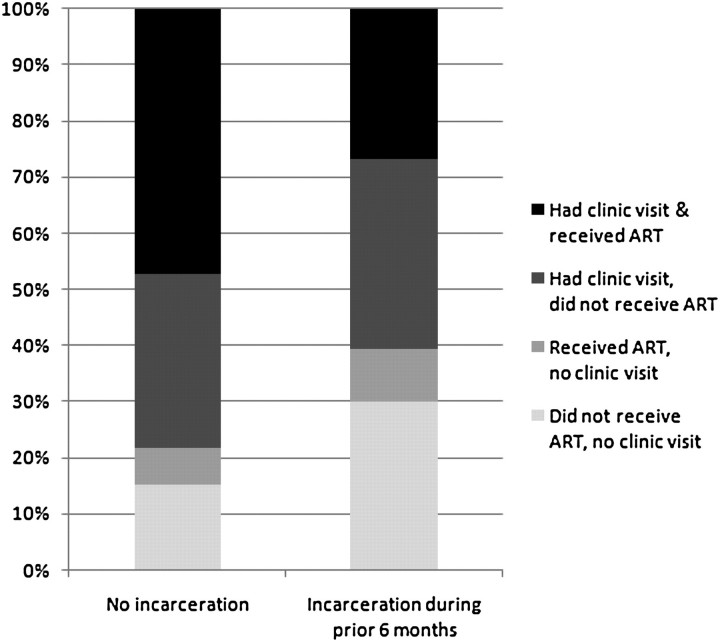
Attendance at HIV-related clinic appointments and use of ART differed by incarceration status, although emergency department visits and hospitalizations did not. Participants reported both ART use and attendance at a clinic visit at 47% of follow-up visits overall, whereas both ART and clinic attendance were reported at only 27% of follow-up visits when recent incarceration was reported (Figure 1). Report of a clinic visit within 6 months was associated with decreased odds of virologic failure in univariate analysis (OR, .8; 95% CI, .6–1.0), but this was not statistically significant when adjusting for other variables. A test for interaction showed that the effect of incarceration on virologic failure did not differ according to whether a participant attended an outpatient visit within 6 months (P = .4 for interaction term). Comparison of multivariate models including and excluding ART use showed that adjustment for ART only minimally attenuated the association between brief incarceration and virologic failure (adjusted OR, 5.9; 95% CI, 2.0–17.4) and did not significantly change the OR for prolonged incarceration (adjusted OR, 1.3; 95% CI, .7–2.4).
Figure 1.
Recent antiretroviral therapy (ART) use and outpatient clinic attendance according to incarceration history for 437 injection drug users (IDUs) in ALIVE (2075 study visits).
HIV Transmission Risk Behaviors
Compared with visits when no incarceration was reported, ALIVE participants were more than twice as likely to report sharing syringes or needles at visits after a reported incarceration (11.8% vs 23.7%; P = .002). Shooting gallery attendance had an even stronger association with recent incarceration (1.6% vs 9.1%; P < .001). The proportion reporting sharing works or attending shooting galleries was similar among visits when the HIV RNA level remained suppressed and those when virologic failure had occurred.
DISCUSSION
In this cohort of mostly African-American, HIV-infected IDUs, virologic failure was common—1 of 4 persons who had achieved virologic suppression experienced treatment failure within 6 months. This high frequency of treatment failure adds to previous research showing that IDUs are less likely to initiate ART [19], are more likely to discontinue or modify their ART regimen [20], and have inferior immunologic and virologic response to ART [21], compared with individuals who do not use drugs. We extend these findings by showing that incarceration significantly increases the risk of short-term virologic failure. In particular, brief incarceration <30 days increases the risk of treatment failure by >7-fold.
The observation that incarceration is associated with increased risk of virologic failure is consistent with previous findings documenting a multifaceted detrimental effect of incarceration on ART effectiveness. Although ART can be successfully administered in prison settings [22], studies of released inmates indicate that a large proportion fail to continuously access ART during the months after release [13] and that the immunologic and virologic benefits of ART achieved in prisons are often not sustained when inmates return to the community [23, 24]. A community-based study of HIV-infected IDUs in Vancouver found that recent incarceration was associated with a nearly 5-fold increased odds of discontinuation of ART [14]. Other studies in the same setting showed that, for IDUs who initiate ART, incarceration is linked to poor adherence and decreased odds of achieving virologic suppression [25, 26]. Similarly, we previously reported that ALIVE participants were less likely to have an immunologic and virologic response if they reported an incarceration within 6 months after initiating ART [21]. Our findings add to this literature by showing that incarceration appears to confer increased risk of viral rebound among IDUs that had achieved viral suppression before incarceration, even when adjusting for continuous use of ART.
Brief incarceration, which likely represents jail stays, was the strongest predictor of virologic failure of all the covariates that we considered, increasing the odds of failure by >7-fold. This finding is consistent with the hypothesis that jail stays, rather than imprisonment, confer the highest risk of interruption of HIV care and subsequent treatment failure. Pretrial detention centers are often described as hectic environments where over-crowding, insufficient communication with care providers, and unpredictable lengths of stay make delivery of health care challenging. In the Baltimore city jail, for example, the mean length of stay is 38 days but ranges from several hours to >2 years [27]. The first days after arrest may be complicated by intoxication or withdrawal from substances, which may cause treatment for chronic medical problems to be overlooked. Prisons, conversely, tend to be better equipped to identify inmates infected with HIV and to deliver appropriate care. Many inmates receive ART for the first time in prison [28], and treatment of comorbid psychiatric and substance use disorders in prison may facilitate better levels of ART adherence than some IDUs sustain in the community setting.
Several important limitations should be considered when interpreting our findings. Because all the data were collected at 6-month intervals, we cannot reliably determine the temporal sequence of virologic failure and incarceration. Published research indicates that ART interruption occurs frequently after incarceration [23, 24]. Treatment interruption and subsequent virologic failure occurring before incarceration, such as in the context of heavy drug use and associated criminal behavior, is an alternatively plausible scenario that deserves further study. An important question not addressed by this study is whether virologic failure resulted from development of drug resistance, because data describing antiretroviral resistance mutations are not currently available in ALIVE. Future studies should evaluate whether incarceration increases the risk of drug resistance, and strategies should be developed to minimize this possibility.
Another limitation is that our assessment of incarceration, ART use, and other behavioral variables was obtained via self report. It is possible that incarceration was underreported by participants who did not experience virologic failure, in which case, our results may be biased away from the null. However, we do not have reason to suspect that error in self report would differentially affect participants with or without virologic failure, and therefore, the strong association that we observed is unlikely to be spurious.
Beyond the negative effects for HIV-infected individuals who are incarcerated, loss of virologic control after incarceration may facilitate increased transmission of HIV. There is increasing recognition of an association between higher population mean viral load (community viral load) and incidence of HIV infection [29, 30]. This finding may be of particular importance in IDU populations. Our data suggest that incarceration leads to higher HIV load among IDUsand that it is a marker of increased behaviors that place others at risk for HIV infection. Our findings of a >2-fold increase in needle sharing and shooting gallery attendance after incarceration add support to previous studies showing high-risk injecting in former prisoners [31, 32]. High-risk sexual behavior [33–36] and increased rates of sexually transmitted infections [37, 38] have also been documented among recent inmates. Future investigations should evaluate the contribution of loss of virological suppression among recently incarcerated persons to community viral load estimates.
Development of interventions or policy changes aimed at promoting optimal HIV care for IDUs and prisoners requires a more complete understanding of how incarceration negatively influences the effectiveness of ART. Our data show that incarceration is a strong marker of increased vulnerability to virologic failure for IDUs receiving ART. Further research should strive to elucidate the relevant mechanisms and to evaluate potential interventions to address these disparities. Recent trials have shown substantial success of discharge planning and intensive case-management in promptly linking prison releases to HIV care [39]. Unfortunately, these programs represent the exception rather than the norm and face greater challenges with expansion into jails from prison settings. Consistent with recent National Institute of Health initiatives [40], our study findings highlight that improved strategies to identify and successfully link HIV-injected inmates to appropriate HIV care are urgently needed.
Acknowledgments
We thank Lisa McCall, for project management, and the ALIVE staff and participants, without whom this work would not be possible.
Financial support. This study was supported by National Institutes of Health, National Institutes of Drug Abuse (grants R01DA04334 and R01DA12568); and National Center for Research Resources (grant KL2RR025006-03 to R. W.).
Potential conflicts of interest. G. K. has received payment from GSK and Merck for consultancy on viral hepatitis. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Vlahov D, Galai N, Safaeian M, et al. Effectiveness of highly active antiretroviral therapy among injection drug users with late-stage human immunodeficiency virus infection. Am J Epidemiol. 2005;161:999–1012. doi: 10.1093/aje/kwi133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–9. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Gulick RM, Lalama CM, Ribaudo HJ, et al. Intensification of a triple-nucleoside regimen with tenofovir or efavirenz in HIV-1-infected patients with virological suppression. AIDS. 2007;21:813–23. doi: 10.1097/QAD.0b013e32805e8753. [DOI] [PubMed] [Google Scholar]
- 6.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296:769–81. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 7.Flexner C, Tierney C, Gross R, et al. Comparison of once-daily versus twice-daily combination antiretroviral therapy in treatment-naive patients: results of AIDS clinical trials group (ACTG) A5073, a 48-week randomized controlled trial. Clin Infect Dis. 2010;50:1041–52. doi: 10.1086/651118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–8. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 10.Mocroft A, Devereux H, Kinloch-de-Loes S, et al. Immunological, virological and clinical response to highly active antiretroviral therapy treatment regimens in a complete clinic population. Royal Free Centre for HIV Medicine. AIDS. 2000;14:1545–2. doi: 10.1097/00002030-200007280-00010. [DOI] [PubMed] [Google Scholar]
- 11.Robbins GK, Daniels B, Zheng H, Chueh H, Meigs JB, Freedberg KA. Predictors of antiretroviral treatment failure in an urban HIV clinic. J Acquir Immune Defic Syndr. 2007;44:30–7. doi: 10.1097/01.qai.0000248351.10383.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammett TM, Harmon MP, Rhodes W. The burden of infectious disease among inmates of and releasees from US correctional facilities, 1997. Am J Public Health. 2002;92:1789–94. doi: 10.2105/ajph.92.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baillargeon J, Giordano TP, Rich JD, et al. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301:848–57. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr T, Marshall A, Walsh J, et al. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17:539–49. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 15.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 16.Taffe P, Rickenbach M, Hirschel B, et al. Impact of occasional short interruptions of HAART on the progression of HIV infection: results from a cohort study. AIDS. 2002;16:747–55. doi: 10.1097/00002030-200203290-00010. [DOI] [PubMed] [Google Scholar]
- 17.d'Arminio Monforte A, Cozzi-Lepri A, Phillips A, et al. Interruption of highly active antiretroviral therapy in HIV clinical practice: results from the Italian Cohort of Antiretroviral-Naive Patients. J Acquir Immune Defic Syndr. 2005;38:407–16. doi: 10.1097/01.qai.0000147529.57240.b0. [DOI] [PubMed] [Google Scholar]
- 18.Kavasery R, Galai N, Astemborski J, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009;50:360–6. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celentano DD, Galai N, Sethi AK, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–15. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 20.Morris JD, Golub ET, Mehta SH, Jacobson LP, Gange SJ. Injection drug use and patterns of highly active antiretroviral therapy use: an analysis of ALIVE, WIHS, and MACS cohorts. AIDS Res Ther. 2007;4:12. doi: 10.1186/1742-6405-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta SH, Lucas G, Astemborski J, Kirk GD, Vlahov D, Galai N. Early immunologic and virologic responses to highly active antiretroviral therapy and subsequent disease progression among HIV-infected injection drug users. AIDS Care. 2007;19:637–45. doi: 10.1080/09540120701235644. [DOI] [PubMed] [Google Scholar]
- 22.Springer SA, Friedland GH, Doros G, Pesanti E, Altice FL. Antiretroviral treatment regimen outcomes among HIV-infected prisoners. HIV Clin Trials. 2007;8:205–12. doi: 10.1310/hct0804-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson BL, Wohl DA, Golin CE, Tien HC, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Rep. 2005;120:84–8. doi: 10.1177/003335490512000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38:1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 25.Palepu A, Tyndall MW, Chan K, Wood E, Montaner JS, Hogg RS. Initiating highly active antiretroviral therapy and continuity of HIV care: the impact of incarceration and prison release on adherence and HIV treatment outcomes. Antivir Ther. 2004;9:713–9. [PubMed] [Google Scholar]
- 26.Small W, Wood E, Betteridge G, Montaner J, Kerr T. The impact of incarceration upon adherence to HIV treatment among HIV-positive injection drug users: a qualitative study. AIDS Care. 2009;21:708–14. doi: 10.1080/09540120802511869. [DOI] [PubMed] [Google Scholar]
- 27.Walsh N. Baltimore behind bars: how to reduce the jail population, save money and improve public safety. Washington, DC: Justice Policy Institute; 2010. Available at: http://www.justicepolicy.org/images/upload/10-06_REP_BaltBehindBars_MD-PS-AC-RD.pdf. Accessed 26 June 2011. [Google Scholar]
- 28.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood E, Li K, Small W, Montaner JS, Schechter MT, Kerr T. Recent incarceration independently associated with syringe sharing by injection drug users. Public Health Rep. 2005;120:150–6. doi: 10.1177/003335490512000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milloy MJ, Buxton J, Wood E, Li K, Montaner JS, Kerr T. Elevated HIV risk behaviour among recently incarcerated injection drug users in a Canadian setting: a longitudinal analysis. BMC Public Health. 2009;9:156. doi: 10.1186/1471-2458-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan MR, Doherty IA, Schoenbach VJ, Taylor EM, Epperson MW, Adimora AA. Incarceration and high-risk sex partnerships among men in the United States. J Urban Health. 2009;86:584–601. doi: 10.1007/s11524-009-9348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MR, Wohl DA, Weir SS, et al. Incarceration and risky sexual partnerships in a southern US city. J Urban Health. 2008;85:100–13. doi: 10.1007/s11524-007-9237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacGowan RJ, Margolis A, Gaiter J, et al. Predictors of risky sex of young men after release from prison. Int J STD AIDS. 2003;14:519–23. doi: 10.1258/095646203767869110. [DOI] [PubMed] [Google Scholar]
- 36.Werb D, Kerr T, Small W, Li K, Montaner J, Wood E. HIV risks associated with incarceration among injection drug users: implications for prison-based public health strategies. J Public Health (Oxf) 2008;30:126–32. doi: 10.1093/pubmed/fdn021. [DOI] [PubMed] [Google Scholar]
- 37.Morrow KM Project START Study Group. HIV, STD, and hepatitis risk behaviors of young men before and after incarceration. AIDS Care. 2009;21:235–43. doi: 10.1080/09540120802017586. [DOI] [PubMed] [Google Scholar]
- 38.Hammett TM. Sexually transmitted diseases and incarceration. Curr Opin Infect Dis. 2009;22:77–81. doi: 10.1097/QCO.0b013e328320a85d. [DOI] [PubMed] [Google Scholar]
- 39.Wohl DA, Scheyett A, Golin CE, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15:356–64. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuehn BM. Inmates with HIV. JAMA. 2010;304:2232. [Google Scholar]