Abstract
PURPOSE
We aimed to test the hypothesis that subjective angiographic endpoints during transarterial chemoembolization (TACE) of hepatocellular carcinoma (HCC) exhibit consistency and correlate with objective intraprocedural reductions in tumor perfusion as determined by quantitative four dimensional (4D) transcatheter intraarterial perfusion (TRIP) magnetic resonance (MR) imaging.
MATERIALS AND METHODS
This prospective study was approved by the institutional review board. Eighteen consecutive patients underwent TACE in a combined MR/interventional radiology (MR-IR) suite. Three board-certified interventional radiologists independently graded the angiographic endpoint of each procedure based on a previously described subjective angiographic chemoembolization endpoint (SACE) scale. A consensus SACE rating was established for each patient. Patients underwent quantitative 4D TRIP-MR imaging immediately before and after TACE, from which mean whole tumor perfusion (Fρ) was calculated. Consistency of SACE ratings between observers was evaluated using the intraclass correlation coefficient (ICC). The relationship between SACE ratings and intraprocedural TRIP-MR imaging perfusion changes was evaluated using Spearman’s rank correlation coefficient.
RESULTS
The SACE rating scale demonstrated very good consistency among all observers (ICC = 0.80). The consensus SACE rating was significantly correlated with both absolute (r = 0.54, P = 0.022) and percent (r = 0.85, P < 0.001) intraprocedural perfusion reduction.
CONCLUSION
The SACE rating scale demonstrates very good consistency between raters, and significantly correlates with objectively measured intraprocedural perfusion reductions during TACE. These results support the use of the SACE scale as a standardized alternative method to quantitative 4D TRIP-MR imaging to classify patients based on embolic endpoints of TACE.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 3rd leading cause of cancer death in the world, and its incidence in the United States continues to increase [1]. Although surgical resection and liver transplantation represent potentially curative treatments, only 10–15% of patients are candidates [2]. Transarterial chemoembolization (TACE) is the most common locoregional intervention for patients with unresectable HCC, and produces survival benefits [3, 4]. There is no consensus, however, regarding the optimal procedural endpoint [5]. Under-embolization may lead to inadequate treatment [6], while over-embolization may induce liver failure or potentially tumor angiogenesis [7–10].
A similar dilemma, determining the optimal level of coronary artery reperfusion following an acute myocardial infarction, was addressed with the Thrombolysis In Myocardial Infarction (TIMI) flow grading scale [11]. Modeled after TIMI, a subjective angiographic chemoembolization endpoint (SACE) scale has been established to describe angiographic endpoints of TACE [12]. Although the SACE scale has demonstrated clinical utility by predicting survival following TACE [13], the lack of validation by an objective perfusion method remains a limitation and requires further investigation.
The availability of an integrated magnetic resonance/interventional radiology (MR-IR) suite affords a unique opportunity to conduct this by evaluating the SACE scale’s relationship with simultaneously measured objective MR perfusion changes. Intraprocedural transcatheter intraarterial perfusion (TRIP) MR imaging collects continuous dynamic contrast enhanced (DCE) MR images immediately following arterial injection of contrast agent [14, 15]. Because TRIP-MR imaging uses small amounts of gadolinium injected directly into a branch of the hepatic artery, serial injections can be performed during chemoembolization, allowing for quantitative calculation of intraprocedural changes in physiologic tumor perfusion (Fρ) [16]. However, the relationship between SACE-monitored TACE endpoints and fully quantitative TRIP-MR imaging measurements remain untested and was the motivation behind the present study. We hypothesize that this angiographic rating scale correlates with quantitative intraprocedural reductions in tumor perfusion during TACE. If so, then angiographic SACE ratings could potentially offer a simplified approach to assess physiologic embolic endpoints, and be used as a standardized intraprocedural endpoint to guide TACE.
SUBJECTS AND METHODS
Clinical Setting and Patients
This study was approved by the institutional review board and complied with the Health Insurance Portability and Accountability Act. Between September 2008 to December 2009, 19 consecutive patients with surgically unresectable HCC who underwent TACE in an integrated MR-IR suite were enrolled. During this time, approximately 80 total patients underwent TACE at our institution. Of these, approximately 25% were excluded from the study because the target tumor was not well-defined by cross-sectional imaging. The remaining patients were ultimately excluded because of logistical limitations associated with scheduling the integrated MR-IR suite, which is used to accommodate multiple research studies at our institution. Of the 19 patients ultimately enrolled in our study, one patient was excluded from analysis because catheter position was altered following pre-TACE TRIP-MR imaging but before injection of chemoembolic material. Characteristics of the remaining 18 patients are presented in Table 1. Of the 18 patients included in the study, three had received prior locoregional treatment. Two patients each received one round of treatment with radiofrequency ablation (RFA). A third patient had previously received two treatments with TACE. Patients were non-invasively diagnosed with HCC based on characteristic imaging findings in the setting of cirrhosis [17]. Treatment was chosen at a weekly institutional multi-disciplinary tumor conference. All patients met a modified set of inclusion and exclusion criteria established by Brown et al [18], including age > 18 years, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, Child-Pugh class A or B disease, focal or multifocal HCC with measurable disease, no contraindications to MR imaging, and informed consent. Exclusion criterion were life expectancy < 6 months, ECOG performance status > 2, Child-Pugh class C, uncorrectable coagulopathy with International Normalized Ratio > 1.5, total bilirubin level > 4.0 mg/dL, serum creatinine > 2.0 mg/dL, uncorrectable platelet count < 50,000/μL, or contraindications to MR imaging. Patients with portal vein thrombosis were enrolled if superselective segmental chemoembolization was technically feasible [19].
Table 1.
Baseline Patient Characteristics
| Gender | |
|---|---|
| Male | 13 (72) |
| Female | 5 (28) |
| Age | |
| Average ± SD (y) | 63 ± 10 |
| Child Pugh Class | |
| A | 13 (72) |
| B | 5 (28) |
| Okuda Classification | |
| 1 | 9 (50) |
| 2 | 9 (50) |
| ECOG Performance Status | |
| 0 | 4 (22) |
| 1 | 13 (72) |
| 2 | 1 (6) |
| Tumor Morphology | |
| Unifocal | 9 (50) |
| Multifocal | 9 (50) |
| Maximum Diameter of Largest Target Tumor | |
| Average ± SD (cm) | 4.4 ± 3.2 |
| <4 cm | 11 (61) |
| ≥ 4cm | 7 (39) |
| Portal Vein Thrombosis | |
| Yes | 3 (17) |
| No | 15 (83) |
| Tumor Necrosis | |
| 0% | 11 (61) |
| 1–50% | 5 (28) |
| >50% | 2 (11) |
| Distribution Embolized | |
| Right Lobar | 2 (11) |
| Left Lobar | 2 (11) |
| Segment 2/3 | 2 (11) |
| Segment 4 | 2 (11) |
| Segment 5/8 | 6 (33) |
| Segment 6/7 | 4 (22) |
Note. —Values in parentheses are percentages; ECOG = Eastern Cooperative Oncology Group
MR-IR Unit
A dedicated MR-IR suite (Miyabi; Siemens, Erlangen, Germany) containing an Artis-dTA flat-panel digital subtraction angiography (DSA) system integrated with a 1.5-T Espree MR scanner via a moving table was used for all chemoembolization procedures.
TACE
Five board-certified attending interventional radiologists specializing in interventional oncology (average experience > 10 years) performed TACE using the same technique. Arterial access was gained via the common femoral artery. Selective catheterization of the lobar or segmental hepatic artery supplying the targeted tumor was performed with a 2.8-F microcatheter (Renegade Hi-Flo; Boston Scientific, Natick, Massachusetts), which was coaxially inserted over a 0.016-inch-diameter guide wire (Headliner; Terumo, Tokyo, Japan). DSA was performed with injection of iohexol (Omnipaque 300; Amersham Health, Princeton, New Jersey) using a power injector. Injection rates were typically 1–3 mL/s, with a total amount of 6–10 mL injected. These values were adjusted to best depict blood flow to the targeted tumor while minimizing reflux into non-target liver segments.
After selective catheter placement, patients were transferred to the MR scanner on the sliding table. With the catheter position unchanged, baseline pre-TACE TRIP-MR imaging was performed. Patients were then transferred back to the IR angiography table for TACE. A 1:1 mixture of emulsifying contrast agent (Ethiodol; Savage Laboratories, Melville, New York) and a three-drug chemotherapy regimen consisting of cisplatin 100 mg, doxorubicin 30 mg, and mitomycin-C 30 mg was injected in 1–3 mL aliquots under direct fluoroscopic observation. Chemotherapy infusion was continued until antegrade blood flow slowed. In other words, when the TACE operator subjectively observed that either the intensity or velocity of the radio-opaque chemotherapy-Ethiodol emulsion decreased relative to the beginning baseline, he stopped injecting. TACE was then completed by injection of 300 – 500-μm or 500 –700-μm-diameter Embospheres (Biosphere Medical, Rockland, Mass) mixed with iohexol until a stasis or sub-stasis endpoint of antegrade blood flow was achieved per the discretion of the treating physician. Stasis was considered to be achieved when no iohexol from the injection could be visualized in the arteriole directly feeding the tumor. Sub-stasis was considered to be achieved when some residual iohexol could still be visualized in the arteriole directly feeding the tumor. Typically, a sub-stasis endpoint was chosen for lobar treatments or when the patient was likely to undergo a follow-up treatment session. After chemoembolization, completion arteriography was conducted via manual injection, rather than power injection, to minimize reflux of iohexol and injected chemoembolic material into non-target areas. To accomplish this, the operator injected iohexol by hand until he noted angiographic evidence of retrograde reflux, at which point he ceased injection. Aliquots of less than 5 mL were typically used before reflux was noted. Patients were then transferred back to the MR scanner for post-TACE TRIP-MR imaging. After scanning, patients were transferred back to the DSA unit where all vascular access devices were removed, and hemostasis was achieved.
MR Imaging
Following patient transfer to the MR scanner, we performed two dimensional multislice axial and coronal T2-weighted turbo spine-echo (TSE) and axial T1-weighted gradient recall echo (GRE) sequence localization studies at expiration position. This protocol was repeated after each between-unit transfer to ensure consistent selection of optimal slice positions for subsequent TRIP-MR imaging. Quantitative TRIP-MR imaging was performed including [16]: (a) three dimensional (3D) baseline longitudinal relaxation rate (R1) mapping using TR/TE = 4.0/1.72 msec, variable flip angle (VFA) GRE = 2°, 9°, 15°, 19°, three averages, 192 × 108 × 24 to 192 × 132 × 24 matrix, 400–450 mm field of view, 5 mm interpolated partition thickness, and 670 Hz/pixel bandwidth, (b) 3D targeted radiofrequency field (B1) mapping using TSE reduced field of view catalyzed double-angle method using TR/TE = 400/12 msec, excitation/compensation FA = 60°/120° and 120°/60°, refocusing FA = 180°, catalyzation chain pulse FA = 90°, 660 Hz/pixel bandwidth, 128 × 28 × 16 matrix, and (c) 4D TRIP dynamic R1 mapping using the same parameters as the VFA method, at a single angle (15°) dynamic GRE method co-registered to the baseline R1 maps with the targeted 3D imaging volume consecutively acquired at a 2.1 second sampling rate.
Five seconds after 4D dynamic R1 image acquisition, the interventional radiologist manually injected 5–10 mL of 20% gadopentetate dimeglumine solution (Gd-DTPA) (Magnevist; Berlex, Montvill, NJ) evenly over 5 seconds directly into the catheter placed in the selected branch of the hepatic artery. 5 mL of Gd-DTPA was used when catheters were placed in a segmental hepatic artery branch, and 10mL was used when catheters were placed in a lobar hepatic artery branch. These volumes were empirically chosen because they minimized Gd-DTPA reflux during our institution’s previous TRIP-MR imaging experiences. Before and after TACE, each patient underwent the same TRIP-MR imaging scan and contrast agent injection protocol.
Data Analysis
SACE
Fluoroscopic angiographic series images before and after TACE were recorded and stored in a picture archiving and communications system (PACS). In batch fashion, these images were independently presented to three board certified interventional radiologists with over 20 combined years of experience in interventional oncology. These raters, blinded to the results of the TRIP-MR imaging measurements, were asked to rate the TACE angiographic endpoint according to the previously established SACE scale (Table 2). In cases of multiple tumors, only the largest targeted tumor was considered when assigning a SACE rating. Additionally, a consensus SACE rating was assigned to each patient based on that patient’s majority SACE rating (i.e. the rating assigned most frequently to the patient).
Table 2.
Subjective Angiographic Chemoembolization Endpoint (SACE) Rating Scale
| Level | Antegrade Arterial Flow | Tumor Blush |
|---|---|---|
| I | Normal | Normal/reduced |
| II | Reduced | Reduced |
| III | Reduced | Eliminated |
| IV | Eliminated | Eliminated |
TRIP-MR imaging
TRIP-MR imaging series were exported first to a Siemens Argus computer workstation, and then to a separate desktop workstation with MatLab imaging processing software (Mathworks, Natick, Massachusetts). Using MatLab, a time-contrast agent concentration curve was measured at all voxel positions, from which we calculated Fρ at all voxel positions [16] and constructed perfusion maps for each tumor (Figure 1). The quantitative perfusion analysis was based on first-pass distributed parameter (DP) modeling [16]. With targeted intrahepatic arterial injection of the contrast agent, this first-pass tracer kinetic modeling of TRIP-MR imaging considers only single arterial input rather than complex arterial and portal dual-input within the liver. Despite the DP model-based quantification being more computationally intensive than semi-quantitative model-free methods, it may give a better reflection of the microcirculation than semi-quantitative methods and conventional compartment models [20]. Based on localization scans, we selected all slices in which the targeted tumor was well visualized. In patients with multiple tumors, only the largest targeted tumor, corresponding to the tumor used for SACE rating, was used for TRIP-MR imaging analysis. An attending interventional radiologist with over 15 years of experience in abdominal MR imaging drew a region of interest (ROI) encompassing the entire tumor within each selected slice. For each slice, we calculated the mean tumor Fρ. A weighted average of the mean tumor Fρ values over the selected slices was used to reflect the Fρ for the entire targeted tumor before TACE. After TACE, an identical method was used to calculate Fρ for the targeted tumor. This allowed calculation of both the absolute and percent intraprocedural reduction of Fρ during TACE.
Fig. 1.
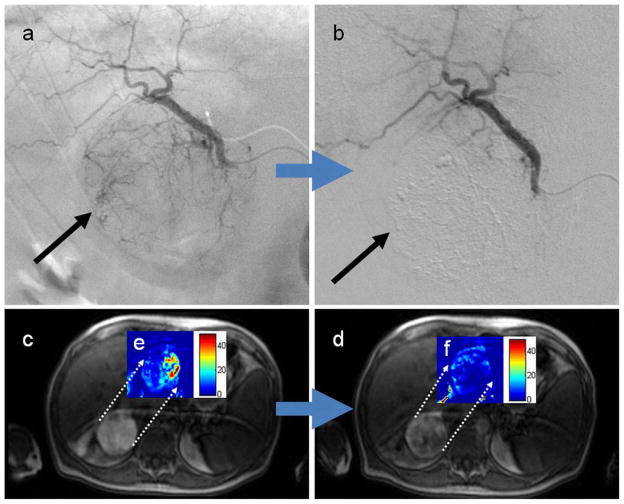
Representative images of a 75 year old man with hepatitis C virus complicated by right lobe (segment VI) HCC. On the basis of angiographic images before (a) and after (b) chemoembolization, each of the three operators categorized this procedure as SACE 3, indicating reduced antegrade blood flow and eliminated tumor blush (black arrow). Intra-procedural TRIP-MR images from the same patient before (c) and after (d) chemoembolization demonstrate reduced arterial enhancement to the tumor. Dynamic gadolinium enhancement data was used to construct color perfusion maps for each tumor immediately before and after TACE. These pre-TACE (e) and post-TACE (f) perfusion maps were then cropped around each tumor, and then superimposed over their respective TRIP-MR image. The color bar to the right of each superimposed perfusion map depicts perfusion (Fρ) in units of mL/min/100mL. Weighted average calculations over all slice positions demonstrate a 6.43 mL/min/100mL perfusion reduction over the whole tumor, which corresponds to 63% perfusion reduction from pre-chemoembolization levels.
Statistical Analysis
Changes in intraprocedural tumor perfusion during TACE were assessed with a paired t-test. Intraclass correlation coefficient (ICC) was used to evaluate the consistency of SACE ratings across the three raters. Spearman’s rank correlation coefficient was used to assess the relationship between SACE ratings and quantitative TRIP-MR imaging perfusion reductions. Finally, analysis of variance (ANOVA) was used to determine whether groups defined on the basis of SACE ratings differed in the measured quantitative perfusion reductions. A P-value less than 0.05 was considered to be significant.
RESULTS
TRIP- MR Imaging Perfusion Reductions
TACE was technically successful in all 19 patients. However the catheter position was adjusted between pre-TACE TRIP-MR imaging and injection of chemoembolic material in one patient. This patient was therefore excluded from further analysis. There were no complications (such as vasospasm, bleeding, or infection) incurred. Intraprocedural quantitative TRIP-MR imaging was successfully completed in all patients and demonstrated intraprocedural tumor perfusion reductions during TACE. Perfusion maps indicated clear reduction in blood flow to the tumor within the targeted vascular territory: mean absolute intraprocedural perfusion reduction (± SD) of 10.62 mL/min/100mL ± 7.71 (P < 0.001) and a mean percent intraprocedural perfusion reduction (± SD) of 66.5% ± 25.4 (P < 0.001).
SACE Ratings
ICC values indicated good to very good consistency between each pair of raters (0.73, 0.81, 0.84), as well as very good consistency over all three raters (0.80). Overall, 11 of the 18 patients received unanimous SACE ratings. None of the patients received completely disparate SACE ratings.
Correlation
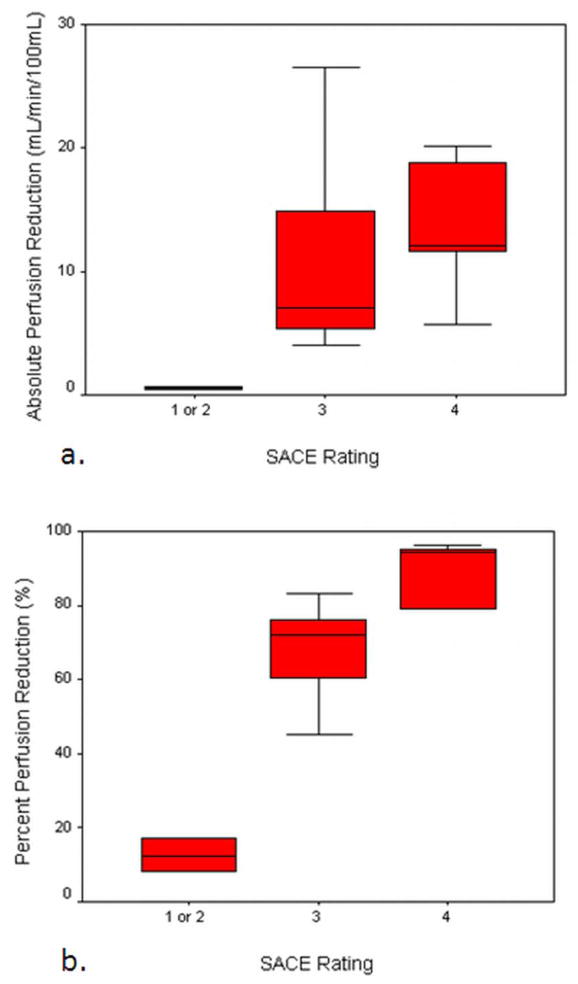
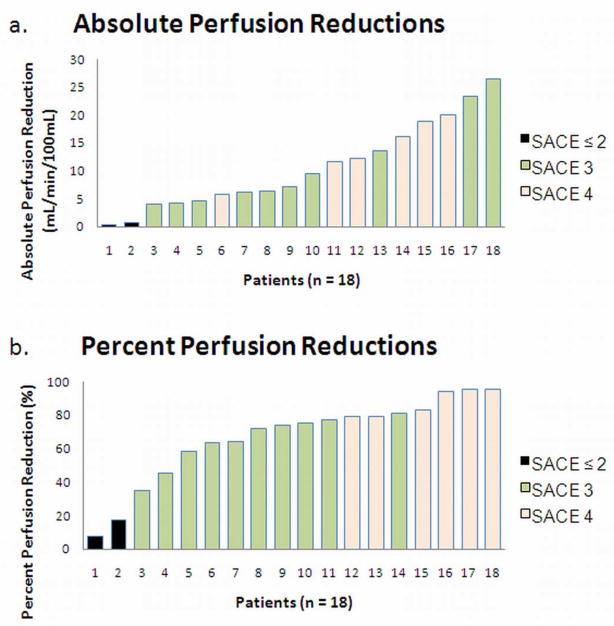
Table 3 presents the Spearmen correlation coefficients between individual reader and consensus SACE ratings and both the absolute and percentage perfusion reduction. Consensus SACE ratings produced a Spearman correlation coefficient of 0.54 (P = 0.022) for absolute perfusion reduction, and 0.85 (P < 0.001) for percent perfusion reduction. A similar pattern was seen for individual raters, as each rater exhibited a higher correlation coefficient with percent intraprocedural perfusion reduction (Rater 1: 0.69, P = 0.001; Rater 2: 0.83, P < 0.001; Rater 3: 0.81, P < 0.001) than absolute intraprocedural perfusion reduction (Rater 1: 0.43, P = 0.076; Rater 2: 0.62, P = 0.006; Rater 3: 0.41, P = 0.095). The consensus SACE ratings are displayed with both absolute perfusion reduction and percent perfusion reduction (Figure 2). SACE level 4 associated with the highest mean intraprocedural perfusion reduction, while SACE levels 1 and 2 were associated with the lowest mean intraprocedural perfusion reduction. SACE level 3 had the largest range of quantitative intraprocedural perfusion reductions, and exhibited some overlap with SACE level 4 with regard to absolute intraprocedural perfusion reduction. When considering percent intraprocedural perfusion reduction, however, only one SACE level 3 patient overlapped with the range of SACE level 4 patients (Figure 3). Due to the small number of patients with SACE 1 or 2 ratings, these patients were grouped together for ANOVA analysis. When patients were classified by SACE ratings and compared for intraprocedural perfusion reductions, significant or nearly significant group differences were identified (percent reduction: P < 0.001; absolute reduction: P = 0.117) (Table 4).
Table 3.
Spearman Correlation Coefficients for Each Interventional Radiologist
| Rater 1 | Rater 2 | Rater 3 | Consensus SACE Rating | |
|---|---|---|---|---|
| Absolute Perfusion Reduction | 0.43 (P = 0.076) | 0.62 (P = 0.006) | 0.41 (P = 0.095) | 0.54 (P = 0.022) |
| Percent Perfusion Reduction | 0.69 (P = 0.001) | 0.83 (P < 0.001) | 0.81 (P < 0.001) | 0.85 (P < 0.001) |
Fig. 2.
Box and whisker plot displaying the distribution of absolute (a) and percent (b) intraprocedural perfusion reductions grouped according to each patient’s consensus SACE rating. Patients with SACE ratings 1 or 2 were merged together and treated as a single group. Groups defined by SACE levels failed to show significant differences in absolute intraprocedural perfusion reduction (P = 0.117), but differed significantly in percent intraprocedural perfusion reduction (P < 0.001).
Fig. 3.
Patients were ranked from least to greatest according to their tumor’s absolute (a) and percent (b) intraprocedural perfusion reduction. Intraprocedural perfusion reduction values for each individual patient are plotted in the color corresponding to their consensus SACE rating. Patients with SACE ratings 1 or 2 were merged together and treated as a single group. Consensus SACE ratings were significantly correlated with both absolute intraprocedural perfusion reduction (r = 0.54, P = 0.022) and percent intraprocedural perfusion reduction (r = 0.85, P < 0.001).
Table 4.
Comparison of Quantitative TRIP-MR imaging Measurements Across SACE Levels
| SACE Level ≤ 2 | SACE Level 3 | SACE Level 4 | P* | |
|---|---|---|---|---|
| Absolute Reduction (mL/min/100mL ± SD) | 0.56 ± 0.19 | 11.06 ± 7.88 | 13.69 ± 5.89 | 0.117 |
| Percent Reduction (% ± SD) | 12.50 ± 6.36 | 66.09 ± 15.15 | 88.60 ± 8.79 | < 0.001 |
P values determined by one way analysis of variance (ANOVA)
DISCUSSION
Our results demonstrate a correlation between the angiographic classifications of the SACE scale and quantitative physiologic reductions in intraprocedural tumor perfusion during TACE as measured by quantitative TRIP-MR imaging. Our study also shows that interventional radiologists have very good inter-rater consistency in classifying angiographic endpoints using SACE criteria.
Correlations between SACE ratings and percent intraprocedural perfusion reductions were generally higher than those for absolute reduction. This may be due to the wide range of baseline tumor vascularity and perfusion [21]. Analyzing percent intraprocedural perfusion reductions, rather than absolute, effectively normalizes for variable baseline tumor vascularity. Another factor to consider is that the SACE scale itself represents a relative, rather than absolute angiographic assessment of perfusion change during TACE.
There remains no consensus regarding the ideal embolization endpoint of TACE. Inadequate embolization may incompletely treat targeted tumors [6]. Alternatively, over-embolization may accelerate the onset of liver failure [7] or promote disease progression by increasing expression of angiogenic proteins [8–10]. A previous study has suggested using the SACE scale in targeting an intermediate level embolic endpoint during TACE [13]. However, the SACE scale is, by definition, completely subjectively based, and therefore lacks any objective measurable component. This lack of objective validation of the SACE scale constitutes a significant limitation, and represents a barrier to using the SACE scale as a procedural guide during TACE. Our study aimed to address this limitation by examining whether embolic endpoints classified by the SACE scale correlate with objectively measured perfusion reductions.
TRIP-MR imaging is a clinically applied method of measuring intraprocedural perfusion during TACE [14, 15, 22, 23], and is able to intraprocedurally quantify the physiologic tumor perfusion [16]. Quantitative TRIP-MR imaging, therefore, provides us with a valuable quantitative modality to measure reductions in intraprocedural tumor perfusion. The relationship between this quantitative technique, and the subjective SACE rating scale, however, remains unknown and was the focus of this study. Our results support the hypothesis that angiographic embolic endpoints and quantitative intraprocedural perfusion changes are correlated. This result suggests that categorical SACE ratings may serve as a reasonable surrogate for objectively measured tumor perfusion reductions.
A previous study attempting to correlate subjective angiographic endpoints with TRIP-MR imaging did not detect a correlation between the SACE scale and perfusion reductions measured by TRIP-MR imaging [12]. However, that study measured perfusion using a semi-quantitative, non physiologic, arbitrary unit [14]. Since then, refinements in TRIP-MR imaging post-processing have allowed the calculation of a fully quantitative and physiologic measurement of perfusion [16]. This improvement in the protocol and accuracy of TRIP-MR imaging may explain the improvement in correlation between the SACE scale and objective measurements of the current study.
There are, however, limitations to using the SACE scale which objective methods such as TRIP-MR imaging can overcome. First, although the SACE scale is able to separate low level embolization, from intermediate level embolization, from high level embolization endpoints, it exhibits poor resolution. For example, SACE 3 embolic endpoints in our study ranged from 35%–81% perfusion reductions. Second, the SACE scale is limited in its correlation with absolute perfusion reductions for many of the reasons mentioned above. Therefore the SACE scale cannot replace objective measurements when greater precision is required to describe embolic endpoints, or when absolute, and not percent, perfusion reductions are required.
There are several limitations to this study. Quantitative TRIP-MR imaging has yet to be validated against a reference standard in humans. However, its evaluation in an animal study and its successful ongoing clinical application support its use to investigate the potential utility of the SACE rating scale. Nevertheless, correlative studies with pathologic data are necessary in order to validate TRIP-MR imaging as an accurate tool to measure arterial tumor perfusion. Our analysis required the manual placement of ROIs around each tumor, which was subject to potential operator variability. To minimize this variability, the same attending radiologist drew the ROIs for all patients in our study. Additionally, in order to prevent this potential bias from affecting the results of our study, this radiologist was completely blinded to, and isolated from, the SACE classification process. This type of operator variability may be further reduced through the use of automated approaches to determine lesion edges [24]. Future quantitative studies should look to incorporate this automated technique, thereby eliminating the reliance on subjective manual ROI placement. Our study did not stratify patients according to tumor grade. It has been reported that tumor grade correlates with tumor blood flow [25]. However, tumor grade should likely not affect the correlative value between angiographic endpoints and quantitative perfusion reductions. Nevertheless, future studies quantifying tumor perfusion should take tumor grade into account. Requiring the patient to hold his/her breath, resulting in motion, during MR data acquisition could also represent a potential limitation. We tried to minimize this effect by acquiring B1, baseline R1, and dynamic R1 maps for TRIP-MR imaging in multiple, shorter breath holds. In addition, acquiring images at exhalation position has also minimized motion artifact relative to acquiring images at inhalation position. Future advances in motion correction and image co-registration algorithms will be needed to fully alleviate this limitation. The current study does not incorporate any follow up imaging or histopathologic data. However, the purpose of the study was to investigate the correlation between quantitative perfusion reductions (TRIP-MR imaging) and subjectively observed embolic endpoints (SACE scale). Therefore follow up tumor data (imaging or histopathologic) was not recorded. However the progression of this perfusion measurement technology will certainly require future studies investigating its correlation with tumor response/progression by utilizing follow up imaging or histopathologic data. Another potential limitation is that absolute tumor perfusion as measured by intraarterial TRIP-MR imaging is noticeably lower than previously reported tumor perfusion data as measured by CT perfusion studies with intravenous contrast. A number of potential explanations include the positioning of an intraarterial catheter, assessment of only a single blood vessel supply distal to the catheter tip, and averaging perfusion over the whole tumor which may often times contain regions of necrosis or relative hypoperfusion [16]. Nevertheless, slight differences in absolute perfusion measurement as compared to CT perfusion studies would not affect percent perfusion reductions, which the current study finds to be highly correlated with subjective embolic endpoints. Finally, it is important to appreciate that quantitative TRIP-MR imaging and the SACE scale can only assess perfusion in the distribution of blood vessels distal to the position of the catheter. Detection of perfusion to the tumor from collateral supply requires repositioning of the catheters within these collateral vessels.
In conclusion, we found that the SACE rating scale exhibited very good consistency between raters and highly correlated with quantitative perfusion reductions as measured by using intraprocedural TRIP-MR imaging. These results support the use of the SACE rating scale as an angiographic rating scale to classify embolization endpoints during TACE. Future investigations should validate the SACE rating scale against a perfusion reference standard, as well as continue to investigate its relationship with long term clinical outcomes following TACE.
Acknowledgments
FUNDING:
-NIH R01 CA 126809
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Marcos-Alvarez A, Jenkins RL, Washburn WK, et al. Multimodality treatment of hepatocellular carcinoma in a hepatobiliary specialty center. Arch Surg. 1996;131:292–298. doi: 10.1001/archsurg.1996.01430150070014. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 5.Brown DB, Geschwind JF, Soulen MC, Millward SF, Sacks D. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol. 2009;20:S317–323. doi: 10.1016/j.jvir.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda M, Maeda S, Shibata J, et al. Transcatheter arterial chemotherapy with and without embolization in patients with hepatocellular carcinoma. Oncology. 2004;66:24–31. doi: 10.1159/000076331. [DOI] [PubMed] [Google Scholar]
- 7.Geschwind JF, Ramsey DE, Cleffken B, et al. Transcatheter arterial chemoembolization of liver tumors: effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovasc Intervent Radiol. 2003;26:111–117. doi: 10.1007/s00270-002-2524-6. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N, Ishii M, Ueno Y, et al. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver. 1999;19:25–31. doi: 10.1111/j.1478-3231.1999.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 9.Xiong ZP, Yang SR, Liang ZY, et al. Association between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:386–390. [PubMed] [Google Scholar]
- 10.Rhee TK, Young JY, Larson AC, et al. Effect of transcatheter arterial embolization on levels of hypoxia-inducible factor-1alpha in rabbit VX2 liver tumors. J Vasc Interv Radiol. 2007;18:639–645. doi: 10.1016/j.jvir.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 11.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowski RJ, Wang D, Gehl J, et al. A comparison of chemoembolization endpoints using angiographic versus transcatheter intraarterial perfusion/MR imaging monitoring. J Vasc Interv Radiol. 2007;18:1249–1257. doi: 10.1016/j.jvir.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Jin B, Wang D, Lewandowski RJ, et al. The impact of chemoembolization endpoints on survival in hepatocellular carcinoma patients. Am J Roentgenol. 2010 doi: 10.2214/AJR.10.4770. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson AC, Wang D, Atassi B, et al. Transcatheter intraarterial perfusion: MR monitoring of chemoembolization for hepatocellular carcinoma--feasibility of initial clinical translation. Radiology. 2008;246:964–971. doi: 10.1148/radiol.2463070725. [DOI] [PubMed] [Google Scholar]
- 15.Gaba RC, Wang D, Lewandowski RJ, et al. Four-dimensional transcatheter intraarterial perfusion MR imaging for monitoring chemoembolizationof hepatocellular carcinoma: preliminary results. J Vasc Interv Radiol. 2008;19:1589–1595. doi: 10.1016/j.jvir.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Jin B, Lewandowski RJ, et al. Quantitative 4D transcatheter intraarterial perfusion MRI for monitoring chemoembolization of hepatocellular carcinoma. J Magn Reson Imaging. 2010;31:1106–1116. doi: 10.1002/jmri.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2006;17:225–232. doi: 10.1097/01.RVI.0000195330.47954.48. [DOI] [PubMed] [Google Scholar]
- 19.Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–1659. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 20.Thng CH, Koh TS, Collins DJ, Koh DM. Perfusion magnetic resonance imaging of the liver. World J Gastroenterol. 16:1598–1609. doi: 10.3748/wjg.v16.i13.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimatsu S, Inoue Y, Ibukuro K, Suzuki S. Hypovascular hepatocellular carcinoma undetected at angiography and CT with iodized oil. Radiology. 1989;171:343–347. doi: 10.1148/radiology.171.2.2539607. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Bangash AK, Rhee TK, et al. Liver tumors: monitoring embolization in rabbits with VX2 tumors--transcatheter intraarterial first-pass perfusion MR imaging. Radiology. 2007;245:130–139. doi: 10.1148/radiol.2451061689. [DOI] [PubMed] [Google Scholar]
- 23.Lewandowski RJ, Tepper J, Wang D, et al. MR imaging perfusion mismatch: a technique to verify successful targeting of liver tumors during transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2008;19:698–705. doi: 10.1016/j.jvir.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Monsky WL, Raptopoulos V, Keogan MT, et al. Reproducibility of linear tumor measurements using PACS: comparison of caliper method with edge-tracing method. Eur Radiol. 2004;14:519–525. doi: 10.1007/s00330-003-2027-0. [DOI] [PubMed] [Google Scholar]
- 25.Asayama Y, Yoshimitsu K, Nishihara Y, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol. 2008;190:W28–34. doi: 10.2214/AJR.07.2117. [DOI] [PubMed] [Google Scholar]