Abstract
BACKGROUND
The objective was to assess the effect of timing of postpartum levonorgestrel-releasing IUD insertion on breastfeeding continuation.
STUDY DESIGN
Women interested in using a levonorgestrel IUD postpartum were randomized to immediate postplacental insertion (postplacental group) or insertion 6–8 weeks after vaginal delivery (delayed group). Duration and exclusivity of breastfeeding were assessed at 6–8 weeks, 3 months, and 6 months postpartum. Only women who received an IUD were included in this analysis.
RESULTS
Breastfeeding was initiated by 32/50 (64%) of women receiving a postplacental IUD and 27/46 (58.7%) of women receiving a delayed IUD (p=0.59). More women in the delayed group compared to the postplacental group continued to breastfeed at 6–8 weeks (16/46 vs 15/50, p=0.62), 3 months (13/46 vs 7/50, p=0.13), and 6 months postpartum (11/46 vs 3/50, p=0.02). The results did not differ when only women who initiated breastfeeding or only primiparous women with no prior breastfeeding experience were analyzed.
CONCLUSIONS
Immediate postplacental insertion of the levonorgestrel IUD is associated with shorter duration of breastfeeding and less exclusive breastfeeding. Further studies are needed of the effects of early initiation of progestin-only methods on women’s lactation experience.
Keywords: contraception, intrauterine devices, breast feeding, lactation, levonorgestrel, postpartum period
1. Introduction
Breastfeeding has numerous benefits for women and their children, including immunologic protection and nutrition for the infant as well as protection from breast cancer, diabetes, and cardiovascular disease for the woman [1, 2]. Exclusive breastfeeding for at least six months is recommended by the American College of Obstetricians and Gynecologists [2], the American Academy of Pediatrics [3], and the World Health Organization [4]. Unfortunately, although 72% of children in the most recent National Survey of Children’s Health had ever been breastfed, only 38% were breastfed for 6 or more months [5]. One goal of Healthy People 2010 was to increase the percentage of women who breastfeed at 6 months to 50%, and at one year to 25% [6]. It is therefore important to examine factors that may affect early discontinuation of breastfeeding.
Because progesterone withdrawal may be the stimulus that initiates lactogenesis, administration of progestin-only methods shortly after delivery could theoretically inhibit or alter lactation [7]. Depot medroxyprogesterone acetate (DMPA) provides the highest systemic level of progestin as compared to other progestin-only methods. There are limited studies examining administration of DMPA prior to hospital discharge in breastfeeding women, although existing studies have not shown detrimental effects on breastfeeding [8–10] or infant growth or development [11, 12]. However, these studies used inappropriate control groups by comparing DMPA to nonhormonal controls [8, 9] or prior lactation experience [10], did not investigate timing of DMPA initiation [8, 9], and did not look at long-term continuation rates of DMPA [8]. Most importantly, women who choose DMPA may be very different than women who choose nonhormonal postpartum contraceptive options, so use of the latter group as a comparator is incorrect. A more appropriate control group would be women who desire DMPA but wait 4–6 weeks for administration.
One study comparing breastfeeding outcomes in women receiving the LNG-IUD and the Copper T 380A did not find a negative effect of the LNG-IUD on lactation; however, IUD insertions for both groups were performed 6–8 weeks postpartum [13]. No studies of the effect of immediate postpartum insertion of the levonorgestrel IUD (LNG-IUD) on lactation could be identified. Thus, the effect of initiation of progestin-only contraception immediately postpartum on lactation compared to delayed initiation is unclear.
The purpose of this analysis was to compare breastfeeding continuation among women who enrolled in a randomized trial comparing postplacental LNG-IUD insertion (within 10 min after expulsion of the placenta) and delayed insertion 6–8 weeks postpartum.
2. Materials and methods
This study was a secondary analysis of a trial approved by the University of Pittsburgh Institutional Review Board and performed at Magee-Womens Hospital, Pittsburgh, PA. The primary study examined 6-month utilization of the LNG-IUD when placed postplacentally after vaginal delivery compared to delayed insertion 6–8 weeks postpartum. The study protocol, demographics, and outcomes were previously described [14]. Briefly, we enrolled pregnant women aged 18 years and over who anticipated undergoing a vaginal delivery and were interested in using the LNG-IUD for postpartum contraception. All subjects gave written informed consent prior to enrollment. If a subject asked about breastfeeding and LNG-IUD usage, she was counseled that the LNG-IUD would not affect breastfeeding. Women received routine prenatal care and were delivered by their primary obstetrician (clinic or private practice) or midwife.
Subjects were randomized equally to postplacental or delayed IUD insertion when they presented to the hospital in anticipation of delivery. The primary obstetrician or midwife informed the investigator on-call of the subject’s admission and the investigator opened the next sequentially numbered opaque sealed envelope containing the group assignment of immediate postplacental or delayed IUD insertion. A statistician not involved with the clinical conduct of the study prepared the envelopes using computer-generated random allocations in a permutated blocks. Additional criteria were assessed intrapartum for post-enrollment exclusion. These included: 1) cesarean delivery; 2) clinical diagnosis of chorioamnionitis or treatment for presumed chorioamnionitis; 3) sexually transmitted infection during pregnancy diagnosed after enrollment without a subsequent negative test of cure; 4) rupture of membranes for more than 24 h; 5) postpartum hemorrhage; 6) rupture of membranes at less than 34 weeks gestation; 7) subject no longer desired a LNG-IUD; or 8) precipitous delivery such that the investigators were unable to begin placement of the LNG-IUD within 10 min of placental delivery or if the investigators were not notified of the subject’s labor and delivery.
Postplacental insertion was performed using the standard LNG-IUD inserter under transabdominal ultrasound guidance, with scheduled follow-up visits at 6–8 weeks and 6 months post-delivery and a phone contact at 3 months. Subjects eligible for delayed IUD insertion were prescribed postpartum contraception by their primary obstetrician or midwife until the 6–8 week post-delivery visit. Following insertion, all subjects had a phone contact at 3 months and a visit at 6 months post-delivery. Study staff were blinded to subjects’ randomization assignments when performing the 3-month and 6-month evaluations.
At all follow-up assessments, subjects were asked whether they initiated breastfeeding after delivery and whether they were still breastfeeding. Women who were breastfeeding were asked about exclusivity of breastfeeding. Women who had discontinued breastfeeding were asked how many weeks they breastfed after delivery. The primary outcome of this secondary analysis was continuation of breastfeeding at 6 months in women eligible for LNG-IUD insertion. A secondary outcome was duration of exclusive breastfeeding.
Statistical analysis was performed using Stata 10 (StataCorp LP, College Station, TX). Only women who received an IUD through the study were included. The analysis used modified intention-to-treat data, which included all subjects randomized to postplacental or delayed IUD insertion who were eligible for placement (i.e., did not meet post-enrollment ineligibility criteria). For the primary analysis, subjects lost to follow-up were analyzed as not breastfeeding. Multivariable logistic regression was used to examine the association between timing of IUD placement and the odds of breastfeeding at 6 months while controlling for age, race, nulliparity, and education (high school or less versus greater than high school education). In a second model, receipt of DMPA was added as a covariate. In addition, we performed an analysis limited to the population of women who initiated breastfeeding and an analysis limited to primiparous women. The D’Agostino test of skewness-kurtosis was used to evaluate normality. The Mann-Whitney U test was used for nonparametric continuous variables. Chi-square or Fisher’s exact tests were used for comparisons of categorical variables as appropriate. Exact binomial 95% confidence intervals (CI) were calculated for breastfeeding continuation at each timepoint. Survival analysis (time to breastfeeding discontinuation) was analyzed using the log-rank test. For the survival analysis, subjects lost to follow-up were censored at the last known contact.
3. Results
This prospective trial was conducted between May 2007 and October 2008. This analysis includes the 102 women who had uncomplicated vaginal deliveries and were eligible for LNG-IUD insertion (Fig. 1). One subject in the postplacental group did not receive her IUD at time of delivery and five subjects in the delayed group did not follow up for IUD placement; these women were excluded from this analysis. Thus, 50 subjects in the postplacental group and 46 subjects in the delayed group were included in this analysis.
Fig. 1.
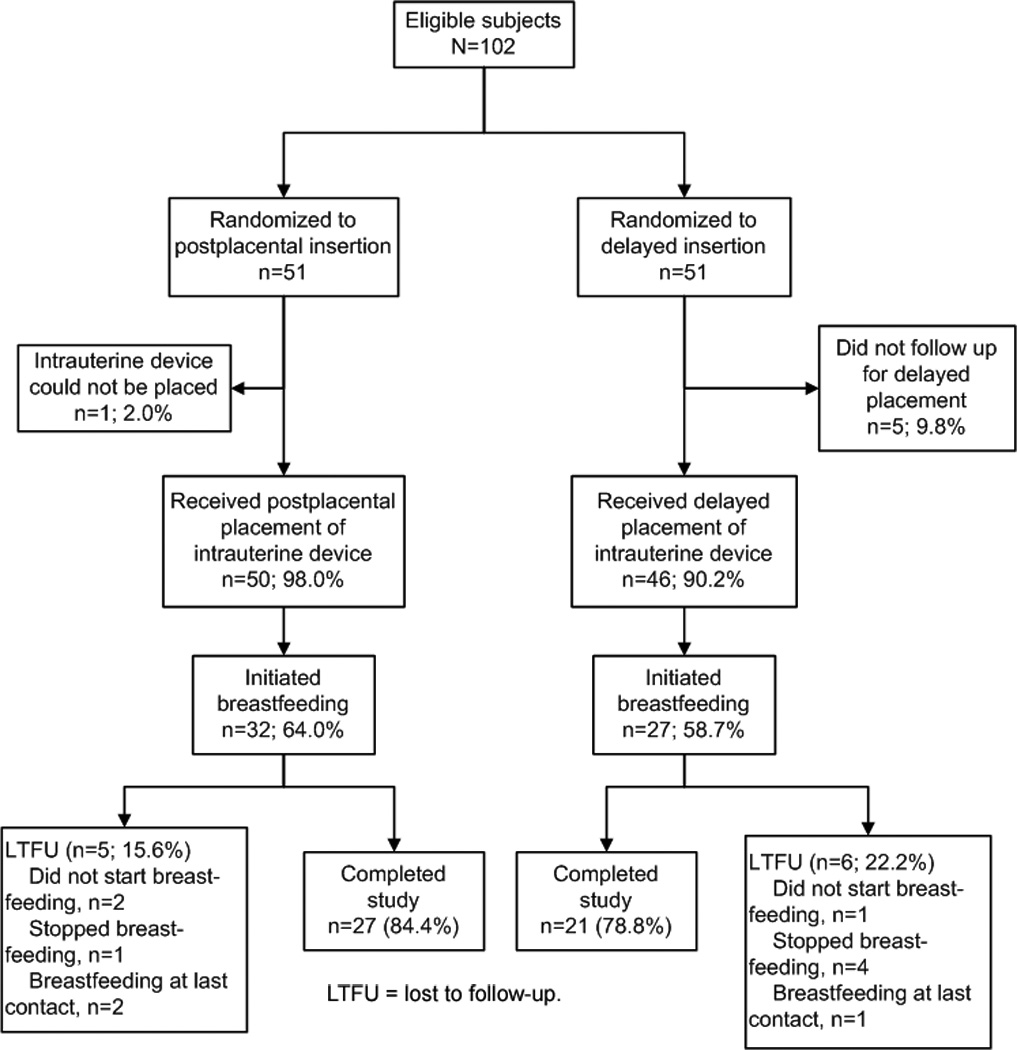
Flow of participants.
Breastfeeding was initiated by 32/50 (64%, 95% CI 49.2, 77.1) of women receiving a postplacental IUD and 27/46 (58.7%, 95% CI 43.2, 73.0) of women receiving a delayed IUD (p=0.59). There were no significant differences in the sociodemographic characteristics of women randomized to each group who initiated breastfeeding (Table 1).
Table 1.
Sociodemographic characteristics of women who initiated breastfeeding*
| Postplacental | Delayed | p-value | ||
|---|---|---|---|---|
| N=32 | N=27 | |||
| n(%) | n (%) | |||
| Age (mean, SD) | 24.8 ±5.2 | 24.7 ±4.4 | 0.90 | |
| Gravidity | 1 | 13 (40.6) | 10 (37.0) | 0.84 |
| 2 | 7 (21.9) | 5 (18.5) | ||
| 3 | 7 (21.9) | 5 (18.5) | ||
| 4 or more | 5 (15.6) | 7 (25.9) | ||
| Parity | 0 | 14 (43.8) | 15 (55.6) | 0.07 |
| 1 | 11 (34.4) | 6 (22.2) | ||
| 2 | 6 (18.8) | 1 (3.7) | ||
| 3 or more | 1 (3.1) | 5 (18.5) | ||
| Race | White | 18 (56.3) | 14 (51.9) | 0.76 |
| African-American | 13 (40.6) | 11 (40.7) | ||
| Other | 1 (3.1) | 2 (7.4) | ||
| Hispanic | 2 (6.3) | 2 (7.4) | >0.99 | |
| Education | ≤ High school | 13 (40.6) | 10 (37.0) | 0.78 |
| ≥ College | 19 (59.4) | 17 (63.0) | ||
| Work outside home or student | 23 (71.9) | 20 (74.1) | 0.85 | |
| Insurance | Medicaid | 21 (65.6) | 20 (74.1) | 0.48 |
| Private | 11 (34.4) | 7 (25.9) | ||
| Caregiver | Clinic | 25 (78.1%) | 22 (81.5%) | 0.89 |
| Private | 5 (15.6%) | 3 (11.1%) | ||
| Midwife | 2 (6.3%) | 2 (7.4%) | ||
Percentages may not add up to 100% due to rounding.
The median duration of lactation was 5 weeks (range 0.5, 27) in the postplacental group and 8.5 weeks (range 0.1, 43) in the delayed insertion groups, p=0.06. Breastfeeding rates declined over 6 months of follow-up, both when evaluating all women randomized and only those women who initiated breastfeeding (Table 2). More women in the delayed group compared to the postplacental group continued to breastfeed at 6–8 weeks (16/46 vs 15/50, p=0.62), 3 months (13/46 vs 7/50, p=0.13), and 6 months postpartum (11/46 vs 3/50, p=0.02). The results did not differ when only women who initiated breastfeeding or only primiparous women with no prior breastfeeding experience were analyzed.
Table 2.
Breastfeeding continuation by timing of IUD placement
| All subjects randomized, n (%) | Subjects who initiated breastfeeding, n (%) | |||||
|---|---|---|---|---|---|---|
| Postplacental | Delayed | p- | Postplacental | Delayed | p- | |
| N=50 | N=46 | value | N=32 | N=27 | value | |
| 6–8 weeks postpartum | ||||||
| Any breastfeeding | 15 (30.0%) | 16 (34.8%) | 0.62 | 15 (46.9%) | 16 (59.3%) | 0.34 |
| 95% CI 17.9, 44.6 | 95% CI 21.4, 50.2 | 95% CI 29.1,65.3 | 95% CI 38.8, 77.6 | |||
| Exclusive breastfeeding | 4 (8.0%) | 10 (21.7%) | 0.08 | 4 (12.5%) | 10 (37.0%) | 0.04 |
| 95%CI2.2, 19.2 | 95% CI 10.9, 36.4 | 95% CI 3.5, 29.0 | 95% CI 19.4, 57.6 | |||
| 3 months postpartum | ||||||
| Any breastfeeding | 7 (14.0%) | 13 (28.3%) | 0.09 | 7 (21.9%) | 13 (48.2%) | 0.03 |
| 95% CI 5.8, 26.7 | 95% CI 16.0, 43.5 | 95% CI 9.3, 40.0 | 95% CI 28.7, 68.1 | |||
| Exclusive breastfeeding | 1 (2.0%) | 9 (19.6%) | 0.006 | 1 (3.1%) | 9 (33.3%) | 0.004 |
| 95% CI 0.05, 10.6 | 95% CI 9.4, 33.9 | 95% CI 0.08, 16.2 | 95% CI 16.5, 54.0 | |||
| 6 months postpartum | ||||||
| Any breastfeeding | 3 (6.0%) | 11 (23.9%) | 0.02 | 3 (9.4%) | 11 (40.7%) | 0.006 |
| 95% CI1.3, 16.5 | 95% CI 12.6, 38.8 | 95% CI 2.0, 25.0 | 95% CI 22.4, 61.2 | |||
| Exclusive breastfeeding | 1 (2.0%) | 6 (13.0%) | 0.05 | 1 (3.1%) | 6 (22.2%) | 0.04 |
| 95% CI 0.05, 10.6 | 95% CI 4.9, 26.3 | 95% CI 0.08, 16.2 | 95% CI 8.6, 42.3 | |||
IUD = intrauterine device; CI = confidence interval
Although power is limited by the small number of outcomes, multivariable logistic regression was performed to see if any factors were strongly correlated with breastfeeding at 6 months (Table 3). Although not statistically significant, African-American race and less than high school education both tended to be negatively associated with breastfeeding at 6 months (p=0.06).
Table 3.
Results of multivariable logistic regression for breastfeeding at 6 months for all women*
| Variable | Odds ratio (95% CI) | p-value |
|---|---|---|
| Delayed IUD insertion | 9.76 (1.97,48.4) | 0.005 |
| Age** | 1.04 (0.90, 1.19) | 0.62 |
| African-American race | 0.22 (0.05, 1.07) | 0.06 |
| Nulliparity | 0.42 (0.09, 1.99) | 0.27 |
| Greater than high school education | 6.80 (0.95, 48.7) | 0.06 |
IUD = intrauterine device; CI = confidence interval.
Odds ratios shown reflect adjustment for all variables shown in table.
OR per additional year of age.
Of women randomized to delayed insertion, six women were given DMPA for interim contraception prior to their 6–8 week visit, four of whom initiated breastfeeding. The remainder of the women were using condoms, abstinence, withdrawal, or no contraception. When DMPA use was included in the multivariable logistic regression analysis, results were similar and DMPA was not associated with breastfeeding continuation at 6 months.
When using the log-rank test to analyze the time to discontinuation of breastfeeding for all subjects (Fig. 2A) and for primiparous women only (Fig. 2B), we found that timing of IUD insertion tended to be associated with rates of breastfeeding discontinuation (p=0.06 and p=0.04, respectively).
Fig. 2.
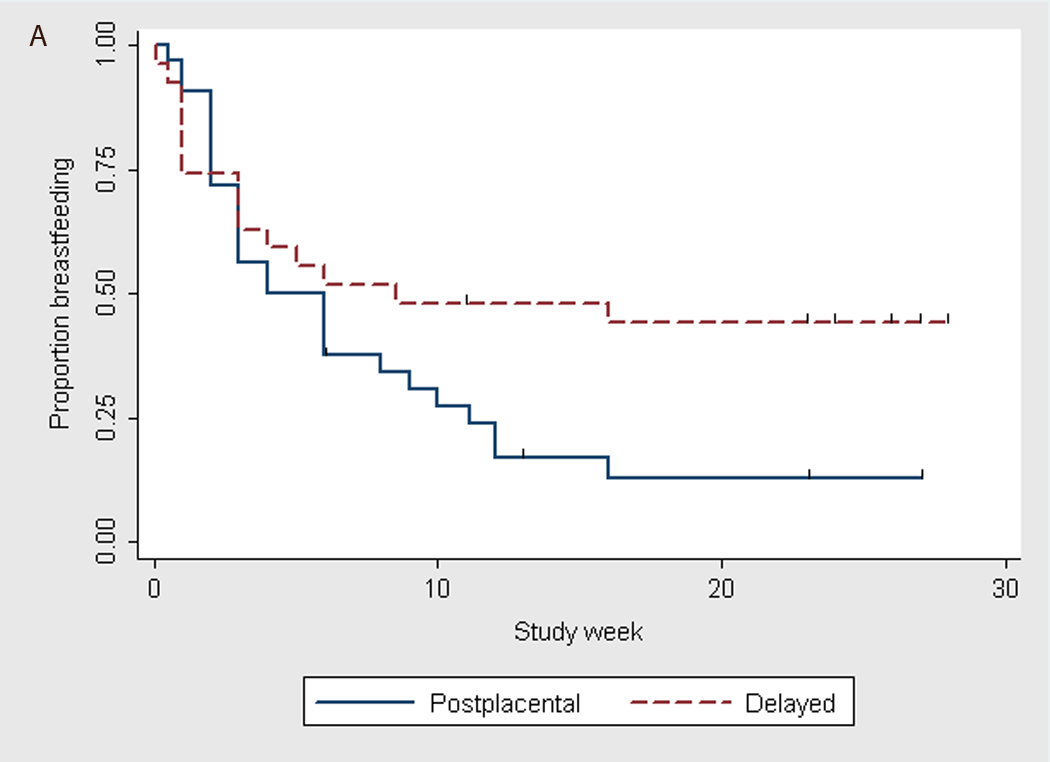
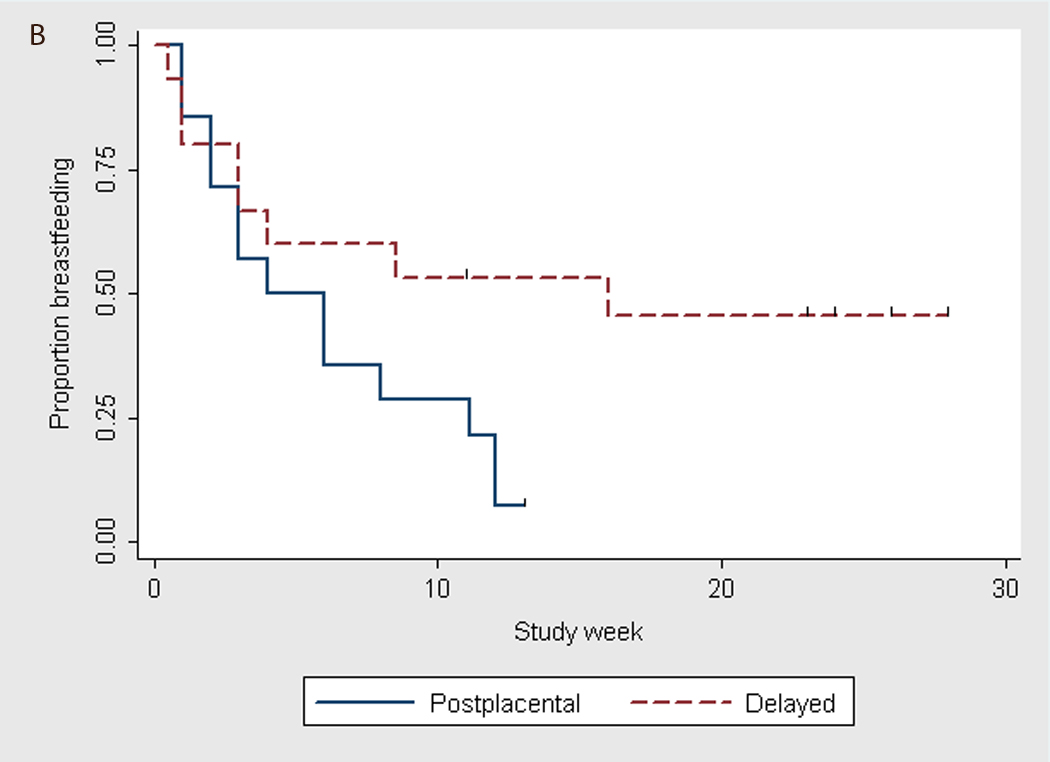
Time to breastfeeding discontinuation by timing of levonorgestrel IUD placement (Kaplan-Meier graphs). A) All subjects*; * p–value = 0.06 using log-rank test. B) Primiparas*; * p–value = 0.04 using log-rank test.
4. Discussion
This study found that women who underwent LNG-IUD placement 6–8 weeks postpartum were more likely to breastfeed at 6 months than women who had an IUD placed immediately after delivery, irrespective of parity. Similarly, when IUD insertion was delayed, there was a trend towards increased duration of exclusive breastfeeding. The same findings were seen when analyzing only women who initiated breastfeeding.
Breastfeeding has multiple benefits for both infant and maternal health. These include reducing rates of infant infection, hospitalization for respiratory disease [15], and death [16], and reducing maternal risk of ovarian [17] and breast cancer [18]. In addition, women who do not breastfeed have been found to be at higher risk of diabetes [19, 20] and cardiovascular disease [21, 22]. Given the importance of breastfeeding for both infant and maternal health [1], care must be taken to ensure that efforts to avoid unintended pregnancy do not adversely affect women’s ability to breastfeed their children.
While the World Health Organization advises against early postpartum use of hormonal contraception [23], DMPA is commonly administered to US women before they are discharged from the hospital [8, 9] and has been recommended immediately postpartum by other experts [24]. In the United States, postpartum administration of progestin-only methods are considered category 2 (advantages outweigh risks) for the Centers for Disease Control Medical Eligibility Criteria for Contraceptive Use [25]. Studies of progestin-only methods including progestin-only pills, DMPA, norethisterone enanthate injections, and levonorgestrel implants have not found detrimental effects on infant growth or development [11, 12] or adverse impact on breastfeeding [8–10]. However, these studies were not randomized trials, and thus may be subject to residual confounding. In addition, some studies did not investigate timing of DMPA initiation [8, 9] or long-term continuation rates of DMPA [8]. Most importantly, women who choose DMPA differ from women who choose non-hormonal contraception, so use of the latter group as a comparator is problematic.
The findings of this study call into question the administration of progestin-containing contraceptives to women in the early postpartum period. This effect on lactation is biologically plausible since if administration of progestin-only methods shortly after delivery inhibits lactogenesis, delayed onset of lactation could lead to decreased exclusive breastfeeding. Given our finding that breastfeeding was sustained longer and possibly with greater exclusivity when LNG-IUD insertion was delayed until 6–8 weeks postpartum, and previous findings that immediate postpartum IUD placement may increase rates of IUD expulsion [26], immediate postpartum insertion of a LNG-IUD may not be an optimal strategy for postpartum contraception unless women are unlikely to return for IUD placement or are not planning on breastfeeding.
While this study benefits from the use of a rigorous, randomized, blinded, and controlled design, it is limited by the fact that infant feeding practices were self-reported. In addition, this was a secondary analysis of a study that was not specifically designed to look at breastfeeding continuation. Thus, differences in self-reports of infant feeding practices are unlikely to differ by timing of IUD insertion. Bias against breastfeeding in the women randomized to postplacental insertion is also unlikely. Although women were not asked about their breastfeeding intentions prior to enrollment in the study, they were counseled that the LNG-IUD would not affect breastfeeding if questions were raised during the consent process about effects on breastfeeding. Furthermore, women initiated breastfeeding at similar rates in both the postplacental and delayed groups. However, multiple factors play a role in breastfeeding initiation and continuation, such as short hospital stays, hospital policies, partner and family support, social and cultural attitudes, breastfeeding difficulties, parity, and return to work [2, 27]. Thus, the findings of this study may not be generalizable to populations and settings with higher rates of breastfeeding support, initiation, and continuation.
Six subjects in this study were given DMPA as interim contraception prior to their postpartum visit, of whom four women initiated breastfeeding. Although use of interim DMPA was not found to be associated with differences in breastfeeding continuation in this study, the number of women receiving DMPA in the delayed group was small. Given the frequency with which DMPA is used as a postpartum contraceptive in this country, further studies are needed on effects of early postpartum initiation of progestin-containing contraceptive methods. In addition, a randomized trial specifically designed to examine breastfeeding continuation and exclusivity in women receiving postplacental or delayed LNG-IUDs that includes assessment of other factors that contribute to breastfeeding initiation and continuation would be helpful in determining whether the findings of this study are replicable.
In conclusion, this study indicates that breastfeeding women who delay placement of a LNG-IUD until 6–8 weeks postpartum are more likely to continue breastfeeding for 6 months and may be more likely to continue exclusive breastfeeding.
Acknowledgments
Funding: Funds to conduct the study were received from an anonymous foundation. The study was conducted in a Clinical and Translational Research Center that is supported by NCRR CTSA Grant 1 UL1 RR024153.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Poster presentation at Reproductive Health 2009, Los Angeles, CA, Sept 30-Oct 3, 2009.
Registered on www.clinicaltrials.gov, ID# NCT00476021.
References
- 1.Stuebe AM, Schwarz EB. The risks and benefits of infant feeding practices for women and their children. J Perinatol. 2010;30:155–162. doi: 10.1038/jp.2009.107. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. Breastfeeding: maternal and infant aspects. Special report from ACOG. ACOG Clin Rev. 2007;12:1S–16S. [Google Scholar]
- 3.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Report of the expert consultation on the optimal duration of exclusive breastfeeding. Geneva, Switzerland: World Health Organization; 2002
- 5.Centers for Disease Control and Prevention. Breastfeeding trends and updated national health objectives for exclusive breastfeeding--United States, birth years 2000–2004. MMWR. 2007;56:760–763. [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. Healthy People 2010. 2nd edition. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- 7.Kennedy KI, Short RV, Tully MR. Premature introduction of progestin-only contraceptive methods during lactation. Contraception. 1997;55:347–350. doi: 10.1016/s0010-7824(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 8.Halderman LD, Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol. 2002;186:1250–1256. doi: 10.1067/mob.2002.123738. [DOI] [PubMed] [Google Scholar]
- 9.Hannon PR, Duggan AK, Serwint JR, Vogelhut JW, Witter F, DeAngelis C. The influence of medroxyprogesterone on the duration of breast-feeding in mothers in an urban community. Arch Pediatr Adolesc Med. 1997;151:490–496. doi: 10.1001/archpedi.1997.02170420060010. [DOI] [PubMed] [Google Scholar]
- 10.Guiloff E, Ibarra-Polo A, Zanartu J, Toscanini C, Mischler TW, Gomez-Rogers C. Effect of contraception on lactation. Am J Obstet Gynecol. 1974;118:42–45. doi: 10.1016/s0002-9378(16)33643-2. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Progestogen-only contraceptives during lactation: II. Infant development. World Health Organization, Task Force for Epidemiological Research on Reproductive Health; Special Programme of Research, Development, and Research Training in Human Reproduction. Contraception. 1994;50:55–68. [PubMed] [Google Scholar]
- 12.World Health Organization. Progestogen-only contraceptives during lactation: I. Infant growth. World Health Organization Task Force for Epidemiological Research on Reproductive Health; Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1994;50:35–53. [PubMed] [Google Scholar]
- 13.Shaamash AH, Sayed GH, Hussien MM, et al. A comparative study of the levonorgestrel-releasing intrauterine system Mirena versus the Copper T380A intrauterine device during lactation: breast-feeding performance, infant growth and infant development. Contraception. 2005;72:346–351. doi: 10.1016/j.contraception.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery. Obstet Gynecol. 2010;116:1079–1087. doi: 10.1097/AOG.0b013e3181f73fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachrach VR, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med. 2003;157:237–243. doi: 10.1001/archpedi.157.3.237. [DOI] [PubMed] [Google Scholar]
- 16.Vennemann MM, Bajanowski T, Brinkmann B, et al. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics. 2009;123:e406–e410. doi: 10.1542/peds.2008-2145. [DOI] [PubMed] [Google Scholar]
- 17.Jordan SJ, Siskind V, A CG, Whiteman DC, Webb PM. Breastfeeding and risk of epithelial ovarian cancer. Cancer Causes Control. 2010;21:109–116. doi: 10.1007/s10552-009-9440-x. [DOI] [PubMed] [Google Scholar]
- 18.Stuebe AM, Willett WC, Xue F, Michels KB. Lactation and incidence of premenopausal breast cancer: a longitudinal study. Arch Intern Med. 2009;169:1364–1371. doi: 10.1001/archinternmed.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 20.Gunderson EP, Jacobs DR, Jr, Chiang V, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010;59:495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974–982. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz EB, McClure CK, Tepper PG, et al. Lactation and maternal measures of subclinical cardiovascular disease. Obstet Gynecol. 2010;115:41–48. doi: 10.1097/AOG.0b013e3181c5512a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Medical eligibility criteria for contraceptive use. Geneva, Switzerland: World Health Organization; 2009
- 24.Speroff L, Mishell DR., Jr The postpartum visit: it's time for a change in order to optimally initiate contraception. Contraception. 2008;78:90–98. doi: 10.1016/j.contraception.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010: adapted from the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th edition. MMWR Recomm Rep. 2010;59:1–86. [Google Scholar]
- 26.Kapp N, Curtis KM. Intrauterine device insertion during the postpartum period: a systematic review. Contraception. 2009;80:327–336. doi: 10.1016/j.contraception.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Scott JA, Binns CW. Factors associated with the initiation and duration of breastfeeding: a review of the literature. Breastfeed Rev. 1999;7:5–16. [PubMed] [Google Scholar]


