Abstract
BACKGROUND:
In patients chronically infected with the hepatitis C virus (HCV), it is not established whether viral outcomes or health-related quality of life (HRQoL) differ between individuals treated at academic or community centres.
METHODS:
In the present observational study, adults with chronic HCV were treated with peginterferon alfa-2a 180 μg/week plus ribavirin at 45 Canadian centres (16 academic, 29 community). The primary efficacy end point was sustained virological response (SVR). Other outcome measures included HRQoL (assessed using the 36-item Short-Form Health Survey), heath resource use, and workplace productivity and absences within a 60-day interval.
RESULTS:
In treatment-naive patients infected with HCV genotype 1, significantly higher SVR rates were achieved in those treated at academic (n=54) compared with community (n=125) centres (52% versus 32% [P=0.01]), although rates of dosage reduction and treatment discontinuation were similar across settings. SVR rates among patients infected with genotype 2/3 were similar between academic (n=59) and community (n=100) centres (64% versus 67% [P=0.73]). Following antiviral therapy, patients with genotype 1 who achieved an SVR (n=67) had significantly higher mean scores on the physical (P=0.005) and mental components of the 36-item Short-Form Health Survey (P=0.043) compared with those without an SVR (n=111). In contrast, HRQoL scores were similar in HCV genotype 2/3 patients with and without an SVR. There were no differences in workplace productivity or absences between patients with and without an SVR. The most frequently used health care resources by all patients were visits and phone calls to hepatitis nurses, and general practice or walk-in clinics.
CONCLUSION:
Patients infected with HCV genotype 1 achieved higher SVR rates when treated at academic rather than community centres in Canada. The reasons for this difference require additional investigation.
Keywords: Health-related quality of life, Hepatitis C virus, Peginterferon, Ribavirin, Treatment
Abstract
HISTORIQUE :
Chez les patients atteints d’une infection chronique par le virus de l’hépatite C (VHC), on ne sait pas si les issues virales ou la qualité de vie liée à la santé (QVLS) diffèrent entre les individus traités dans des centres universitaires ou communautaires.
MÉTHODOLOGIE :
Dans la présente étude d’observation, les adultes atteints d’un VHC chronique ont été traités à l’aide de 180 μg/semaine de peginterféron alfa-2a associés à de la ribavirine dans 45 centres canadiens (16 centres universitaires, 29 centres communautaires). Des réponses virologiques soutenues (RVS) constituaient le paramètre ultime d’efficacité primaire. Les autres mesures d’issue incluaient la QVLS (évaluée au moyen du questionnaire cours sur la santé en 36 questions), l’utilisation des ressources de santé ainsi que la productivité et les absences en milieu de travail dans un intervalle de 60 jours.
RÉSULTATS :
Chez les patients naïfs aux traitements infectés par le VHC du génotype 1, on constatait des taux de RVS considérablement plus élevés s’ils étaient traités dans un centre universitaire (n=54) plutôt que dans un centre communautaire (n=125) (52 % par rapport à 32 % [P=0,01]), même si le taux de réduction de la dose et d’arrêt du traitement était similaire entre les établissements. Le taux de RVS chez les patients infectés par les génotypes 2 et 3 était similaire entre les centres universitaires (n=59) et communautaires (n=100) (64 % par rapport à 67 % [P=0,73]). Après l’antivirothérapie, les patients ayant un génotype 1 qui avaient obtenu une RVS (n=67) obtenaient des indices moyens considérablement plus élevés aux éléments physiques (P=0,005) et mentaux du questionnaire court sur la santé en 36 questions (P=0,043) que ceux sans RSV (n=111). Par contre, les indices de QVLS étaient similaires chez les patients des génotypes 2 et 3, avec ou sans RVS. On ne constatait aucune différence dans la productivité ou les absences au travail des patients avec ou sans RVS. Les ressources de santé les plus utilisées par tous les patients étaient les visites et les appels aux infirmières spécialisées en hépatite, les cabinets de médecine générale ou les cliniques sans rendez-vous.
CONCLUSION :
Les patients infectés par le VHC de génotype 1 obtenaient des taux de RVS plus élevés lorsqu’ils étaient traités dans un centre universitaire plutôt que dans un centre communautaire au Canada. Il faudra d’autres recherches pour établir les raisons de cette différence.
Chronic hepatitis C virus (HCV) is an indolent infection that impairs health-related quality of life (HRQoL), reduces work productivity and increases health care costs (1–3). Left untreated, the disease can progress to cirrhosis, end-stage liver disease, hepatocellular carcinoma and death. The combination of pegylated interferon (peginterferon) and ribavirin has a well-established efficacy and safety profile, and is the current standard of care for treatment of patients with chronic HCV (4,5). The objective of treatment is eradication of HCV infection and prevention of long-term morbidity and mortality. Sustained virological response (SVR), defined as the absence of serum HCV RNA six months after completion of treatment, is durable and associated with regression of hepatic fibrosis and a reduced risk of liver-related morbidity and mortality (6–8).
Nearly 1% of Canadians (an estimated 210,000 to 275,000 individuals) have chronic HCV infection (9) and are at risk of serious long-term complications (10,11). Modelling studies (9) and, more recently, population-based reports (12) suggest that we are in the midst of an epidemic of HCV-related complications. Although effective treatment is available, it was initially offered only through specialist clinics in Canada. To extend the benefits of treatment to the largest possible population and optimize outcomes on a nationwide scale, treatment outside of tertiary care settings is necessary. However, it is not clear whether similar outcomes, including viral eradication and other health-related benefits, are achieved in tertiary academic centres compared with community settings. The APPROACH (A Prospective study of Peginterferon alfa-2a and Ribavirin: Outcomes Assessment in Chronic Hepatitis C patients) study was undertaken to evaluate the efficacy and safety of treatment with peginterferon alfa-2a plus ribavirin in diverse clinical settings across Canada. Specifically, we aimed to compare SVR rates achieved in academic and community centres, and to assess the impact of treatment on HRQoL, work productivity and resource use.
METHODS
The APPROACH study was a multicentre, observational study conducted at 45 Canadian centres including 16 academic/tertiary centres and 29 community clinics. Sites were identified as either academic or community treatment centres by the study investigators. The first patient enrolled on April 19, 2006, and the final patient visit occurred on October 30, 2008.
Study population
Eligible patients were adults at least 18 years of age with serological evidence of chronic HCV infection including detectable serum HCV RNA. Treatment-naive and -experienced patients were included in the study. Patients with any of the following characteristics were excluded: coinfection with HIV or hepatitis B virus; decompensated cirrhosis (Child-Pugh class B or C); signs or symptoms of hepatocellular carcinoma; history of autoimmune disease including coexistent autoimmune hepatitis; a history of a severe psychiatric disorder; uncontrolled thyroid disease; hemoglobinopathy; serum creatinine >177 μmol/L; or receipt of any investigational drug within six weeks of enrollment. Pregnant and breastfeeding women, and male partners of women who were pregnant were also excluded. Women were required to undergo a negative pregnancy test before treatment, and both women and male partners of fertile women were required to use two forms of effective contraception during treatment and for six months after treatment.
Study design
All patients received peginterferon alfa-2a 180 μg/week plus ribavirin (Pegasys RBV [Roche Canada]) according to the standard of care and the Canadian product monograph. Treatment duration was based on HCV genotype (genotype 2/3: 24 weeks; non-2/3 gentoype: 48 weeks).
Assessments and outcome measures
All patients were assessed at baseline, week 12 of treatment, the end of the 24- or 48-week treatment period, and 24 weeks following the end of treatment (study week 48 or 72). At each visit, serum HCV RNA level was determined by real-time polymerase chain reaction assay (COBAS Ampliprep/COBAS TaqMan HCV test [detection limit 50 IU/mL] [Roche Diagnostics North America, USA]). HRQoL was measured using version 2 of the 36-item Short-Form Health Survey (SF-36). In addition, workplace absences, workplace productivity and health resource use during the previous 60 days was assessed with a questionnaire designed specifically for the present study (available from the authors upon request). Resource use included visits to emergency departments, general practitioners/walk-in clinics, specialists, nurses and counsellors/psychologists as well as hospitalizations. Patients completed all assessments, including HRQoL, before being informed of their HCV RNA status and liver biochemistry at each visit (ie, blinded to their most recent virological and biochemical results at the time the form was completed).
The primary efficacy end point was SVR in HCV treatment-naive patients, defined as undetectable serum HCV RNA 24 weeks after the end of treatment. End-of-treatment virological response was defined as undetectable HCV RNA at the end of planned treatment (ie, week 24 or 48). Relapse was defined as detection of HCV RNA at the end of untreated follow-up in patients who demonstrated an end-of-treatment virological response. Secondary outcome measures included the SF-36 physical and mental component scores, and estimated monthly health resource use. Safety end points included all serious adverse events (SAEs); as well as adverse events (AEs) leading to dose reductions or treatment discontinuations.
Statistical analyses
All patients who received at least one dose of study medication were included in the efficacy analyses. Those with missing HCV RNA results at the end of follow-up were considered to not have achieved an SVR according to the intention-to-treat principle. Between-group comparisons were performed using Fisher’s exact test for categorical variables and Wilcoxon’s rank-sum test (for two group comparisons) or the Kruskal-Wallis test (for three or more groups) for continuous variables. A two-sided P<0.05 was considered to be statistically significant. Due to the exploratory and descriptive nature of the study, no corrections were made to account for multiple testing.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and the tenets of good clinical practice. The protocol and all amendments were approved by the research ethics boards of the participating institutions. All patients provided informed written consent before undergoing any study procedure.
RESULTS
Patient characteristics
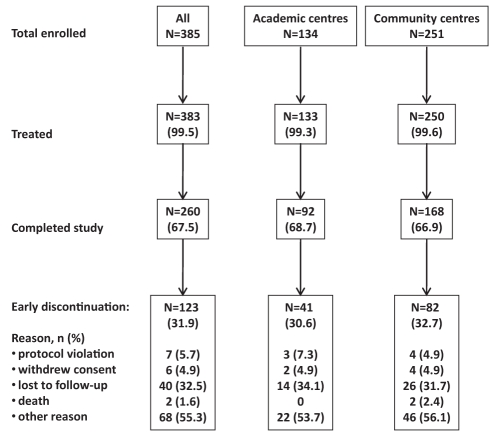
A total of 385 patients were enrolled, of whom 383 received at least one dose of study medication, and 260 completed the study (Figure 1). The baseline characteristics and treatment assignments of the included patients according to treatment setting (academic versus community centre) are presented in Table 1. Overall, the majority of patients had HCV genotype 1 (53%), were male (66%), Caucasian (86%), treatment naive (91%) and enrolled at a community treatment centre (65%). In general, patients treated at academic or community centres had similar baseline characteristics including age, body mass index and Caucasian race. However, patients enrolled at community centres had a lower mean HCV RNA level at baseline compared with those treated at academic centres (2.3 million IU/mL versus 3.1 million IU/mL [P=0.0002]). A higher proportion of patients enrolled at community centres had previously undergone liver biopsy (64% versus 56% of patients at academic centres [P=0.15]); a similar proportion of these individuals had cirrhosis (10% versus 14%).
Figure 1).
Flow of participants through the trial according to type of treatment centre (ie, academic versus community)
TABLE 1.
Baseline characteristics of the study cohort according to treatment setting
|
Treatment setting |
||
|---|---|---|
| Academic (n=133) | Community (n=250) | |
| Age, years | 46.2±9.1 | 46.0±10.1 |
| Male sex | 93 (69.9) | 160 (64.0) |
| Race | ||
| Caucasian | 114 (85.7) | 215 (86.0) |
| Black | 5 (3.8) | 4 (1.6) |
| Asian | 6 (4.5) | 9 (3.6) |
| First Nations | 4 (3.0) | 9 (3.6) |
| Other | 4 (3.0) | 13 (5.2) |
| Weight, kg | 80.8±18.1 | 79.1±16.7 |
| Body mass index, kg/m2 | 27.6±5.4 | 27.0±4.9 |
| Treatment history | ||
| Naive | 118 (88.7) | 229 (91.6) |
| Previously treated | 15 (11.3) | 21 (8.4) |
| Breakthrough | 1 (0.8) | 1 (0.4) |
| Relapse | 3 (2.3) | 11 (4.4) |
| Nonresponse | 9 (6.8) | 7 (2.8) |
| Unknown | 2 (1.5) | 2 (0.8) |
| HCV RNA level*, ×103 IU/mL | 3132±4130 | 2336±4864 |
| Alanine aminotransferase, U/L | 93.5±72.0 | 108.5±79.6 |
| Previous liver biopsy | 58 (43.6) | 90 (36.0) |
| Fibrosis stage | ||
| Missing | 21 (36.2) | 17 (18.9) |
| F0 | 5 (8.6) | 6 (6.7) |
| F1 | 8 (13.8) | 21 (23.3) |
| F2 | 9 (15.5) | 25 (27.8) |
| F3 | 5 (8.6) | 8 (8.9) |
| F4 | 8 (13.8) | 9 (10.0) |
| Unknown | 2 (3.4) | 4 (4.4) |
| HCV genotype | ||
| 1 | 65 (48.9) | 138 (55.2) |
| 2 | 27 (20.3) | 51 (20.4) |
| 3 | 36 (27.1) | 56 (22.4) |
| 4 | 5 (3.8) | 4 (1.6) |
| 6 | 0 | 1 (0.4) |
| Assigned treatment duration, weeks | ||
| 24 | 62 (46.6) | 106 (42.4) |
| 48 | 71 (53.4) | 144 (57.6) |
| Assigned ribavirin dose, mg/day | ||
| 600 | 1 (0.8) | 1 (0.4) |
| 800 | 54 (40.6) | 90 (36.0) |
| 1000 | 28 (21.1) | 59 (23.6) |
| 1200 | 50 (37.6) | 100 (40.0) |
Data presented as mean ± SD or n (%).
P=0.002 for comparison between patients enrolled at academic verus community centres. HCV Hepatitis C virus
SVR
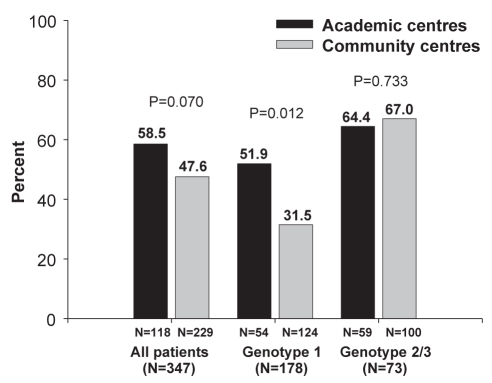
The rates of SVR among treatment-naive patients according to treatment setting are illustrated in Figure 2. Overall, 51% (178 of 347) of patients achieved an SVR including 38% (67 of 178), 74% (54 of 73) and 59% (51 of 86) of patients with HCV genotypes 1, 2 and 3, respectively. Including previously treated patients, the overall SVR rate was slighter higher among those enrolled at academic (n=133) versus community (n=250) centres (59% versus 48%), although this difference did not reach statistical significance (P=0.07). Differences in SVR rates between settings were attributable to a significant difference among patients with HCV genotype 1 enrolled at academic versus community centres (52% versus 32% [P=0.012]). SVR rates were similar among patients with HCV genotype 2/3 treated at academic and community centres (64% versus 67% [P=0.73]).
Figure 2).
Sustained virological response rates in treatment-naive patients according to type of treatment centre (ie, academic versus community)
HRQoL
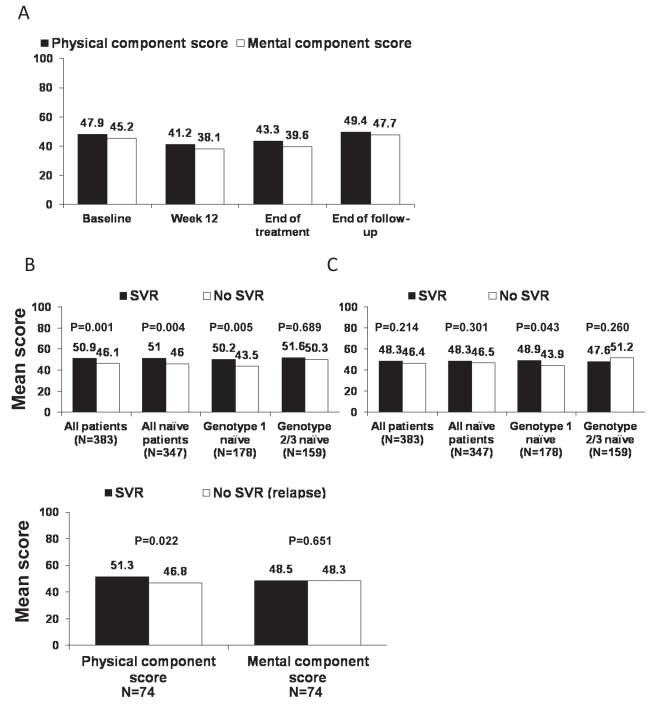
Figure 3A illustrates the mean scores for the physical and mental components of the SF-36 for the overall population at baseline and through the end of follow-up. On both domains, HRQoL was impaired at treatment week 12 and the end of treatment when compared with baseline and the end of follow-up. The impact of achieving an SVR on HRQoL is illustrated in Figures 3B to 3D. Mean physical component scores at the end of follow-up were significantly higher in patients who achieved an SVR in the overall population (P=0.001 versus patients without an SVR), in treatment-naive patients (P=0.004) and in treatment-naive patients with HCV genotype 1 (P=0.005). Differences among treatment-naive patients with HCV genotypes 2 and 3 were not statistically significant (Figure 3B). Mean mental component scores at the end of follow-up were significantly higher among patients with HCV genotype 1 who achieved an SVR (P=0.043 versus patients without an SVR), but not among other subgroups (Figure 3C). When the analysis of HRQoL was restricted to patients who were HCV RNA negative at the end of treatment (Figure 3D), the difference in physical (but not mental) component scores between patients with and without an SVR (ie, relapsers) remained significant (P=0.022).
Figure 3).
Impact of antiviral therapy on health-related quality of life (36-item Short-Form Health Survey). A Mean physical and mental component scores at baseline, during treatment and at the end of follow-up in all treated patients (n=383). B Mean physical component score at the end of follow-up according to sustained virological response (SVR) status. C Mean mental component score at the end of follow-up according to SVR status. D Mean physical and mental component scores at the end of follow-up according to SVR status in treatment-naive patients who were hepatitis C virus RNA negative at the end of treatment (n=245)
Work productivity
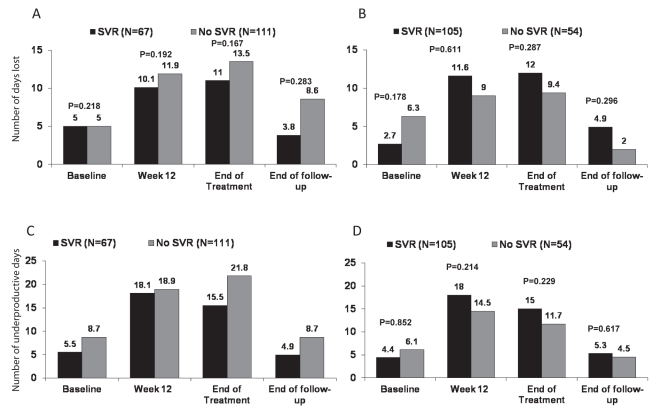
The numbers of work days lost and days considered underproductive within the preceding 60 days are presented for patients according to SVR status and HCV genotype in Figures 4A to 4C. The number of days lost and unproductive increased during treatment and then returned toward baseline at the end of follow-up. There were no statistically significant differences in these outcomes between patients with and without an SVR at any time point during the study, including when analyzed separately according to genotype.
Figure 4).
Impact of antiviral therapy on work productivity in the previous 60 days among treatment-naive patients according to sustained virological response (SVR) status. A Days lost in the previous 60 days in patients with hepatitis C virus (HCV) genotype 1. B Days lost in the previous 60 days in patients with HCV genotype 2/3. C Underproductive days in the previous 60 days in patients with HCV genotype 1. D Underproductive days in the previous 60 days in patients with HCV genotype 2/3
Health resource use
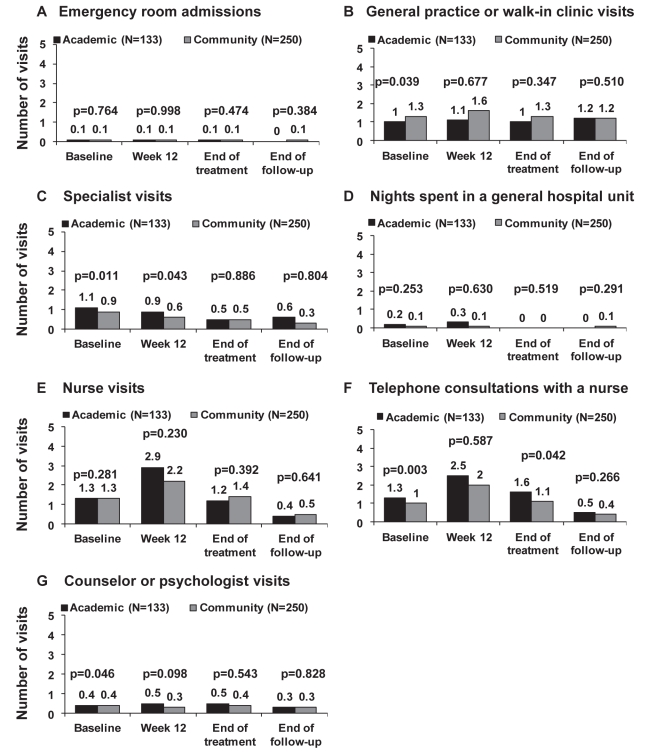
Health resource use according to treatment setting during the previous 60 days measured at baseline, treatment week 12, the end of treatment and at the end of follow-up is shown in Figures 5A to 5G. The most frequently used resources were visits and telephone calls to hepatitis nurses, and visits to general practice or walk-in clinics. Compared with baseline, a higher number of patients consulted or telephoned a nurse during treatment, particularly at week 12 at both academic and community centres (Figures 5E and 5F). Conversely, a lower number of patients consulted or telephoned a nurse at the end of follow-up. Patients treated at academic centres had significantly more telephone consultations with nurses than those treated at community centres, particularly at baseline and the end of treatment. These patients were also more likely to visit a specialist within 60 days of the baseline visit and treatment week 12 (Figure 5C). In contrast, the frequency of visits to general practice or walk-in clinics was similar throughout the study period and did not differ between treatment settings (Figure 5B). Emergency room visits and hospitalizations were uncommon; no patients spent a night in an intensive care unit.
Figure 5).
Patient resource use in the preceding 60 days according to treatment at academic or community centres. A Emergency room admissions. B General practice or walk-in clinic visits. C Specialist visits. D Nights spent in a general hospital unit. E Nurse visits. F Telephone consultations with a nurse. G Counsellor or psychologist visits
Safety
Approximately 10% of patients withdrew from treatment because of AEs or laboratory abnormalities at both academic and community treatment centres (Table 2). The incidence of SAEs (11.8% versus 5.1% [P=0.04]), including those considered directly related to treatment (7.4% versus 2.5% [P=0.06]), was higher at community compared with academic centres. The incidence of laboratory abnormalities, including anemia, neutropenia and thrombocytopenia, was also slightly higher at community centres, although these differences were not statistically significant. While dosage reductions of peginterferon alfa-2a and ribavirin were similar between treatment settings, more patients treated in academic centres discontinued these treatments for any reason including lack of response, AEs and laboratory abnormalities.
TABLE 2.
Adverse events (AEs), laboratory abnormalities and dosage modifications
|
Treatment setting |
||
|---|---|---|
| Academic (n=133) | Community (n=250) | |
| Withdrawal from treatment for AEs and/or laboratory abnormalities | 14 (10.5) | 25 (10.0) |
| Serious AEs | 6 (5.1) | 27 (11.8) |
| Serious AEs related to study medication* | 3 (2.5) | 17 (7.4) |
| Deaths | 0 | 2 (0.8) |
| Laboratory abnormalities | ||
| Hemoglobin <100 g/L | 12 (9.0) | 29 (11.6) |
| Neutrophils <0.75×109/L | 13 (9.8) | 32 (12.8) |
| Platelets <50×109/L | 4 (3.0) | 14 (5.6) |
| Dosage reductions | ||
| Peginterferon alfa-2a | 6 (4.5) | 11 (4.4) |
| Ribavirin | 15 (11.3) | 22 (8.8) |
| Discontinuation† | ||
| Peginterferon alfa-2a | 82 (61.7) | 122 (48.8) |
| Ribavirin | 81 (60.9) | 124 (49.6) |
Data presented as n (%).
Possibly, probably or definitely related to study drug in the opinion of the investigator;
Includes patients discontinued for any reason including lack of response, adverse events or laboratory abnormalities
Two deaths occurred during the study: a 51-year-old man with HCV genotype 3 died from hepatocellular carcinoma 61 days after discontinuing treatment, and a 43-year-old man with HCV genotype 1 died from a myocardial infarction 12 days after discontinuing treatment. Neither death was deemed to be related to treatment in the opinion of the investigators.
DISCUSSION
The overall SVR rate achieved in treatment-naive patients with HCV in our study (51%) compares favourably with those reported in the international registration trials of peginterferon and ribavirin (54% to 56% [13,14]), and is somewhat higher than reported in large American studies (40% to 44% [15,16]). The reasons for the apparently higher SVR rate in Canadian patients compared with American patients is probably attributable to racial and genetic differences (17). In particular, the proportion of black patients enrolled in our study was low (2%) compared with large trials conducted in the United States (9% to 19%) (15,16). Our trial also included a high proportion of genotype 2/3 patients. A novel feature of our study was the involvement of a large number of community-based (ie, nonacademic) treatment centres. This enabled us to compare outcomes obtained at large tertiary specialist centres with less specialized settings. We observed a difference in the overall SVR rates in patients enrolled at academic and community treatment centres (59% versus 48%), which appeared to be attributable almost exclusively to a significant difference in response rates achieved among patients with HCV genotype 1 (52% in academic versus 32% in community centres). These data conflict with a recent report by Jou et al (18) who described no significant differences in virological response rates, adherence, AEs, and dosage adjustments or treatment discontinuations between patients with genotype 1 treated at academic and community centres in the context of the Individualized Dosing Efficacy vs. Flat Dosing to Assess Optimal Pegylated Interferon Therapy (IDEAL) trial. The reasons for this discrepancy are not immediately apparent. In our study, patient characteristics, including age, body mass index, alanine aminotransferase level, prevalence of advanced fibrosis and racial composition, were similar between the two treatment settings. In contrast, patients enrolled at community centres had a significantly lower HCV RNA level, which would be expected to lead to higher rates of SVR in community centres. Therefore, one must consider alternative contributory factors. Importantly, all community practices employed nurses experienced in the management of HCV. Moreover, rates of dosage modifications and treatment discontinuations were similar across treatment settings despite a tendency toward a greater frequency of significant cytopenias among patients treated at community sites. Because approximately two-thirds of the patients enrolled in the study were treated in community centres, further study is necessary to explain the observed differences in treatment response. Potential unmeasured confounding factors include differences in adherence to therapy, socioeconomic status and genetic factors associated with antiviral responsiveness (ie, IL-28B polymorphisms [17]).
Our study confirms that HRQoL is significantly improved in HCV patients who achieve an SVR. Importantly, our study design overcame a frequent criticism of previous studies (19–25). Specifically, SF-36 questionnaires were completed before patients were informed of their virological results at each study visit to eliminate the possibility that their responses would be influenced by positive or negative results, or the attitudes of their caregivers. In addition, we conducted a post hoc analysis that included only patients with an end-of-treatment response to exclude the possibility that responses at the end of follow-up would be influenced by knowledge of the virological status at the end of treatment (ie, ‘artificially’ low HRQoL among end-of-treatment nonresponders compared with relapsers) (Figure 3D). This analysis was consistent with the overall HRQoL analysis in showing that physical component scores were significantly improved in patients with an SVR. The finding that significant improvements in HRQoL were restricted to HCV genotype 1-infected patients is unique. HRQoL scores at the end of follow-up did not differ significantly in genotype 2/3 patients with and without an SVR. The reason for this difference is unclear. Although some biological effects of HCV infection differ according to genotype (eg, a greater prevalence of steatosis in patients with genotype 3), there is no evidence to suggest that infection with genotypes 2/3 gives rise to milder symptoms including fatigue or cognitive impairment. Based on our findings, future studies of HRQoL among patients with HCV should report genotype-specific results.
The available evidence demonstrates that individuals with chronic HCV infection have higher rates of absenteeism, lower productivity levels and higher health care costs than individuals without HCV (3). The causes are multifactorial, but include the effects of the underlying liver disease, comorbidities (eg, depression, diabetes mellitus) and substance abuse issues. In our study, work productivity deteriorated during treatment and returned to baseline levels during follow-up. Unlike the HRQoL analysis, there were no statistically significant differences between patients with and without an SVR in terms of work-place productivity. This is in contrast to several previous studies that have shown significant improvements in work productivity in patients who responded to therapy (19,21,25). These differences may be attributable to our study design. For example, the questionnaire used in our study or the time period over which patients were asked to reflect on their productivity may have been too insensitive to identify differences between patients with and without an SVR. Whereas other studies have asked patients to reflect on productivity over the previous one (21) or three months (25), we used a 60-day time window. In addition, our questions probably lacked specificity regarding improvements in productivity. For example, some patients reported missing work or being underproductive on every day during the previous 60 days at baseline and at the end of follow-up.
Finally, our data provides valuable information regarding health resource use among Canadian patients with HCV before, during and after a course of interferon-based therapy. In general, treatment appeared to have a minimal impact on medical resource use with the exception of telephone consultations and visits to a nurse, which peaked before the week 12 visit. Of note, patients treated in academic centres were more likely than those treated in community settings to consult a nurse by telephone before baseline and the end of treatment. One might speculate that greater patient support from nursing staff at academic centres may have contributed to the observed differences in SVR rates across treatment settings by promoting adherence, although confirmatory data are lacking. Moreover, we did not record the reasons for visits to a nurse (or other provider) in our study. Nevertheless, our data clearly emphasize the importance of nursing care in the management of these complex patients during this difficult therapeutic regimen. The low rates of hospital-based care and specialist hepatology service use suggest that treatment of chronic HCV places little additional burden on the health care system (above and beyond drug costs and testing) and that hepatitis nurses are a worthwhile investment.
The present study had several important limitations. First, the observational design limited our ability to draw firm conclusions. Another was the the lack of biopsy data in the majority of patients, and the incomplete data regarding fibrosis stage in patients who underwent biopsy. The magnitude of the statistically significant differences in HRQoL was small and, therefore, may be of questionable clinical significance. We were also unable to perform a multivariate analysis to analyze predictors of SVR in patients treated at academic and community centres.
CONCLUSION.
The APPROACH study demonstrated that SVR rates achieved with the combination of peginterferon alfa-2a and ribavirin are significantly higher among patients with HCV genotype 1 who were treated at academic compared with community centres. Because the reasons for this discrepancy are unclear, additional investigations are necessary. As expected, work productivity and HRQoL declined during treatment, most likely due to treatment-related side effects, but returned to baseline levels after 24 weeks of untreated follow-up. Finally, viral eradication is associated with significant improvements in physical and mental well-being in patients with HCV genotype 1 infection. These findings have important implications regarding the merits of anti-HCV therapy and may prove useful for future cost-effectiveness analyses.
Acknowledgments
The APPROACH investigators: Frank Anderson, Rahman Bacchus, Robert Bailey, Andrew Bellini, Marc Bradette, Brian Chai, Danny Chen, Brian Conway, Curtis Cooper, Jeff Daiter, Christian Dallaire, William Depew, Lucie Deshaies, Magdy Elkashab, John Farley, Cameron Ghent, Susan Greenbloom, Paul Harris, Douglas Hemphil, Dennis Kunimoto, Pierre Laflamme, Richard Lalonde, Ken Lee, Paul Marotta, Krishna Menon, Earl Morgan, Roger Mousseau, Rob Myers, Michael Oravec, Pierre Pare, Gilles Pinette, Henryk Pluta, Marcia Prest, Alnoor Ramji, Barbara Romanowski, Annie Rousseau, S Sabbah, Linda Scully, Morris Sherman, Michael Silverman, Jean Tchervenkov, Kim Tilbe, Davinder Tripathi, Helga Witt-Sullivan and Eric Yoshida.
Footnotes
FUNDING: This study was funded by Roche Canada (Mississauga, Ontario). Dr Myers is supported by a Clinical Investigator Award from the Alberta Heritage Foundation for Medical Research (now Alberta Innovates – Health Solutions) and New Investigator Award from the Canadian Institutes of Health Research.
CONTRIBUTIONS: The statistical analysis was performed by Dr Robert Balshaw, with the assistance of Nadia Lesnikova of Syreon Corporation (Vancouver, British Columbia). Blair Jarvis prepared the tables and figures, and provided writing assistance.
REFERENCES
- 1.Krajden M, Kuo M, Zagorski B, Alvarez M, Yu A, Krahn M. Health care costs associated with hepatitis C: A longitudinal cohort study. Can J Gastroenterol. 2010;24:717–26. doi: 10.1155/2010/569692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers RP. Pay now or pay (more) later: Tracking the costs of hepatitis C infection. Can J Gastroenterol. 2010;24:715–6. doi: 10.1155/2010/526295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su J, Brook RA, Kleinman NL, Corey-Lisle P. The impact of hepatitis C virus infection on work absence, productivity, and healthcare benefit costs. Hepatology. 2010;52:436–42. doi: 10.1002/hep.23726. [DOI] [PubMed] [Google Scholar]
- 4.Sherman M, Shafran S, Burak K, et al. Management of chronic hepatitis C: Consensus guidelines. Can J Gastroenterol. 2007;21(Suppl C):25C–34C. [PMC free article] [PubMed] [Google Scholar]
- 5.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: A 5-year follow-up of 150 patients. Hepatology. 2009;49:729–38. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–601. doi: 10.1053/j.gastro.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Veldt BJ, Saracco G, Boyer N, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004;53:1504–8. doi: 10.1136/gut.2003.038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou S, Tepper M, El SS. Prediction of hepatitis C burden in Canada. Can J Gastroenterol. 2000;14:575–80. doi: 10.1155/2000/642707. [DOI] [PubMed] [Google Scholar]
- 10.Evaluation of the Hepatitis C prevention, support and research program 1999/2000 – 2005/2006. Hepatitis C Prevention, Support and Research Program, Community Acquired Infections Division, Centre for Communicable Diseases and Infection Control, Infectious Disease and Emergency Preparedness Branch, Public Health Agency of Canada. < www.phac-aspc.gc.ca/publicat/2008/er-re-hepc/er-re-hepc1-eng.php#ref> (Accessed on September 21, 2010). < wwwphac-aspcgcca/publicat/2008/er-re-hepc/er-re-hepc1-engphp#ref2008> (Accessed on September 21, 2010).
- 11.Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: A global overview. Clin Liver Dis. 2010;14:1–21. vii. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Myers RP, Liu M, Shaheen AA. The burden of hepatitis C virus infection is growing: A Canadian population-based study of hospitalizations from 1994 to 2004. Can J Gastroenterol. 2008;22:381–7. doi: 10.1155/2008/173153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 14.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson IM, Brown RS, Jr, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: A randomized trial. Hepatology. 2007;46:971–81. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 16.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–93. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 17.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 18.Jou J, Sulkowski MS, Reddy R, et al. Analysis of site performance in academic and community-based centers in the IDEAL study [abstract 822] Hepatology. 2010;52(Suppl):716A. [Google Scholar]
- 19.Davis GL, Balart LA, Schiff ER, et al. Assessing health-related quality of life in chronic hepatitis C using the Sickness Impact Profile. Clin Ther. 1994;16:334–43. [PubMed] [Google Scholar]
- 20.Bonkovsky HL, Woolley JM. Reduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapy. The Consensus Interferon Study Group. Hepatology. 1999;29:264–70. doi: 10.1002/hep.510290124. [DOI] [PubMed] [Google Scholar]
- 21.McHutchison JG, Ware JE, Jr, Bayliss MS, et al. The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J Hepatol. 2001;34:140–7. doi: 10.1016/s0168-8278(00)00026-x. [DOI] [PubMed] [Google Scholar]
- 22.Rasenack J, Zeuzem S, Feinman SV, et al. Peginterferon alpha-2a (40kD) [Pegasys] improves HR-QOL outcomes compared with unmodified interferon alpha-2a [Roferon-A]: In patients with chronic hepatitis C. Pharmacoeconomics. 2003;21:341–9. doi: 10.2165/00019053-200321050-00005. [DOI] [PubMed] [Google Scholar]
- 23.Hassanein T, Cooksley G, Sulkowski M, et al. The impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol. 2004;40:675–81. doi: 10.1016/j.jhep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Bonkovsky HL, Snow KK, Malet PF, et al. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46:420–31. doi: 10.1016/j.jhep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John-Baptiste AA, Tomlinson G, Hsu PC, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol. 2009;104:2439–48. doi: 10.1038/ajg.2009.346. [DOI] [PubMed] [Google Scholar]