Abstract
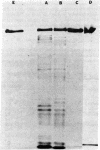
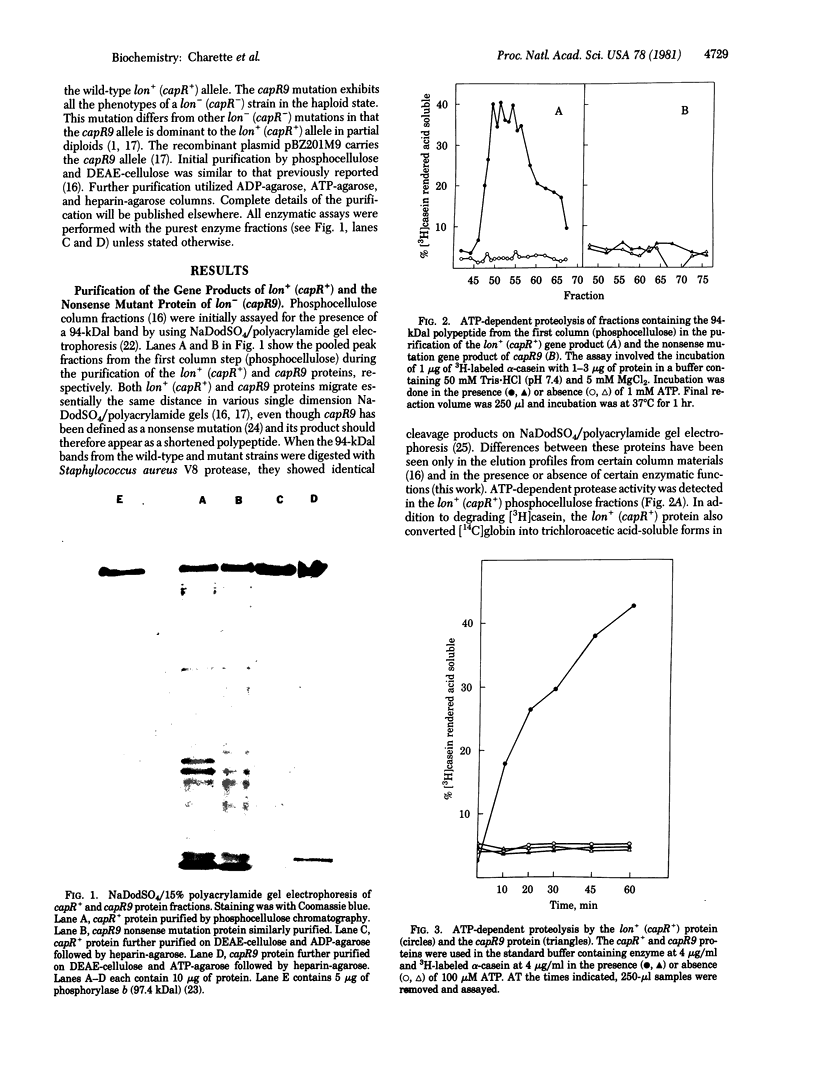
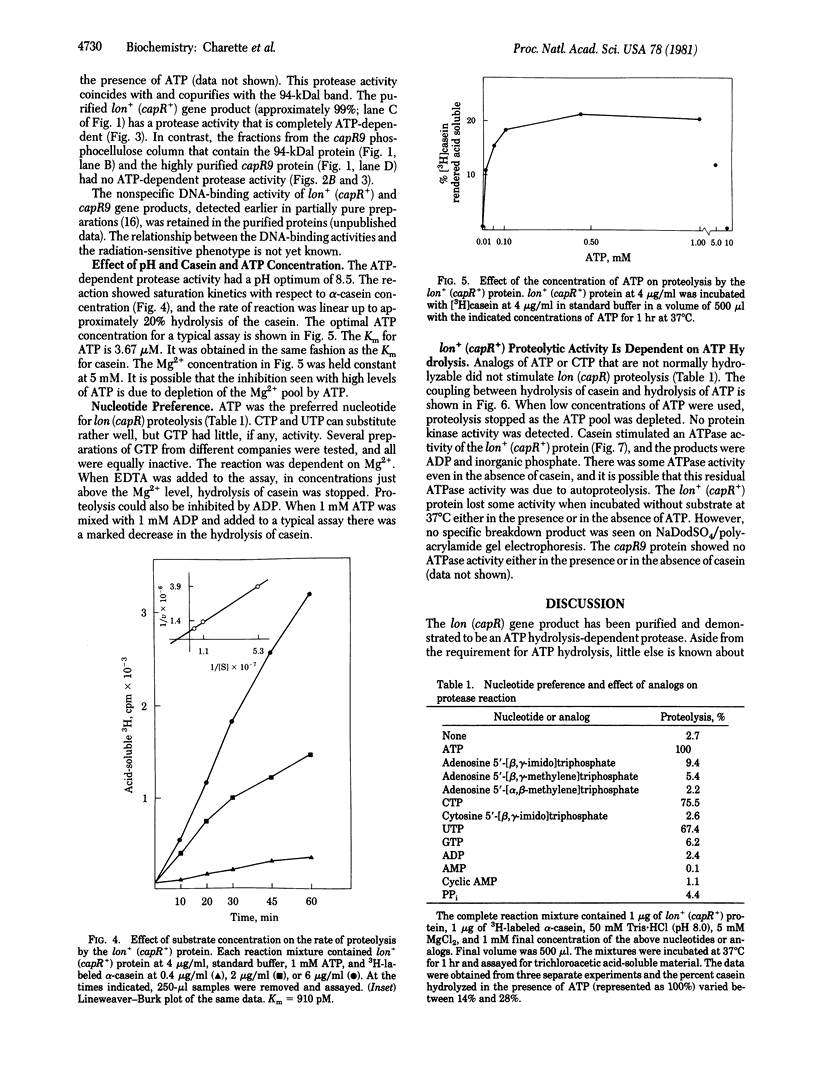
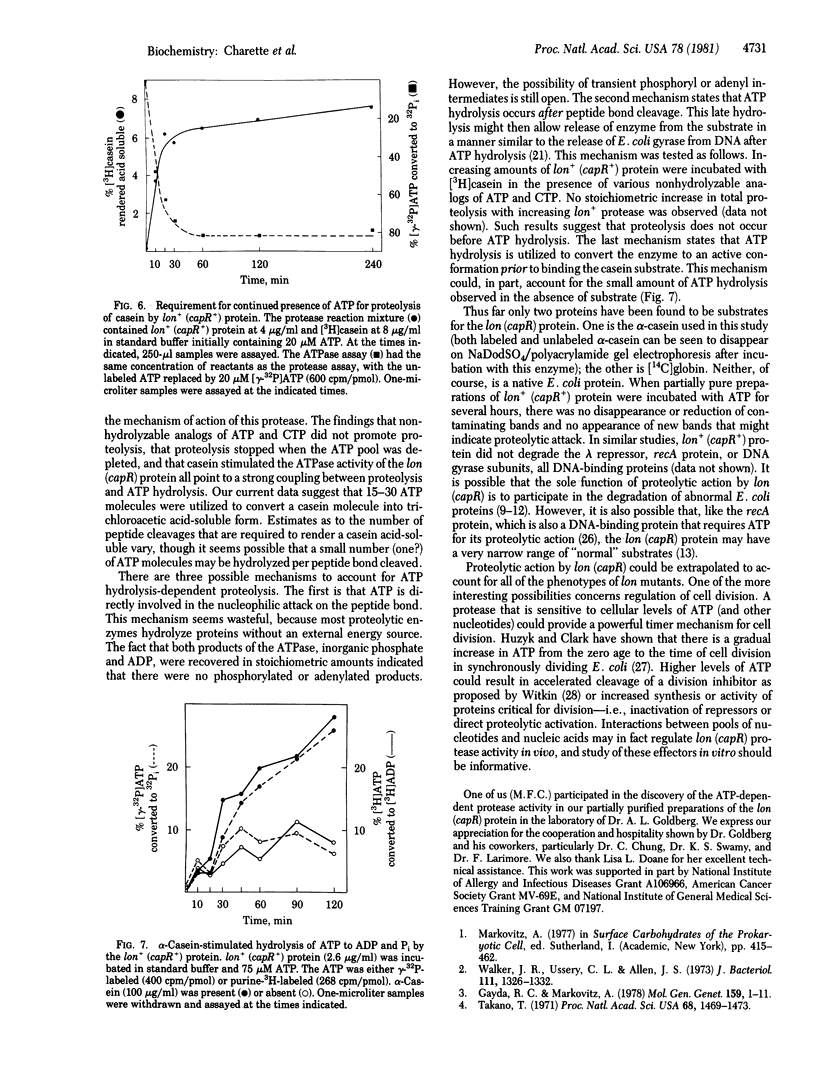
Mutations in the lon (capR) gene result in multiple phenotypes, one of which is the failure to degrade abnormal and normal proteins (Deg-). Previous work with partially purified preparations showed that the lon (capR) gene product is a 94,000-dalton polypeptide with an affinity for nucleic acids. The lon (capR) protein has now been highly purified and is demonstrated to have an ATP-dependent protease activity. The enzyme hydrolyzed 3H-labeled alpha-casein into trichloroacetic acid-soluble forms in Tris buffer containing Mg2+ and ATP. The reaction has a pH optimum of 8.5 and ATP was the preferred nucleotide. CTP and UTP could substitute for ATP (75% and 67%, respectively) but GTP, ADP, AMP, cyclic AMP, and PPi could not. Proteolysis by the lon (capR) protein required ATP hydrolysis. Nonhydrolyzable analogs of ATP and CTP did not promote casein cleavage. When low concentrations of ATP were used, proteolysis stopped as the ATP pool was depleted. Casein stimulated lon (capR) ATPase activity, and the products were ADP and inorganic phosphate in equimolar amounts. No protein kinase activity was detected. The DNA-binding activity, present in partially pure preparations, was retained in the purified protein. The gene product purified from a lon nonsense mutant that exhibits the Deg- phenotype (capR9), lacked both the ATP-dependent protease and ATPase activities, though it retained DNA-binding activity. Absence of an ATP-dependent protease activity could account for many of the pleiotropic effects observed in lon mutants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Gayda R. C., Avni H., Berg P. E., Markovitz A. Outer membrane protein a and other polypeptides regulate capsular polysaccharide synthesis in E. coli K-12. Mol Gen Genet. 1979 Oct 1;175(3):325–332. doi: 10.1007/BF00397232. [DOI] [PubMed] [Google Scholar]
- Gayda R. C., Markovitz A. Altered bacteriophage lambda expression in cell division mutants capR(lon) of Escherichia coli K-12. Mol Gen Genet. 1978 Feb 7;159(1):1–11. doi: 10.1007/BF00401741. [DOI] [PubMed] [Google Scholar]
- Gayda R. C., Markovitz A. Cloned DNA fragment specifying major outer membrane protein a in Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):369–380. doi: 10.1128/jb.136.1.369-380.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda R. C., Yamamoto L. T., Markovitz A. Second-site mutations in capR (lon) strains of Escherichia coli K-12 that prevent radiation sensitivity and allow bacteriophage lambda to lysogenize. J Bacteriol. 1976 Sep;127(3):1208–1216. doi: 10.1128/jb.127.3.1208-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzyk L., Clark D. J. Nucleoside triphosphate pools in synchronous cultures of Escherichia coli. J Bacteriol. 1971 Oct;108(1):74–81. doi: 10.1128/jb.108.1.74-81.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Goldberg A. L. Intermediate steps in the degradation of a specific abnormal protein in Escherichia coli. J Biol Chem. 1977 Dec 10;252(23):8350–8357. [PubMed] [Google Scholar]
- Markovitz A., Baker B. Suppression of radiation sensitivity and capsular polysaccharide synthesis in Escherichia coli K-12 by ochre suppressors. J Bacteriol. 1967 Aug;94(2):388–395. doi: 10.1128/jb.94.2.388-395.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker J. M., Markovitz A. Identification of the gene lon (capR) product as a 94-kilodalton polypeptide by cloning and deletion analysis. J Bacteriol. 1981 Jul;147(1):46–56. doi: 10.1128/jb.147.1.46-56.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shineberg B., Zipser D. The ion gene and degradation of beta-galactosidase nonsense fragments. J Bacteriol. 1973 Dec;116(3):1469–1471. doi: 10.1128/jb.116.3.1469-1471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T. Bacterial mutants defective in plasmid formation: requirement for the lon + allele. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1469–1473. doi: 10.1073/pnas.68.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Koide A., Hermann J., Ericsson L. H., Kumar S., Wade R. D., Walsh K. A., Neurath H., Fischer E. H. Complete amino acid sequence of rabbit muscle glycogen phosphorylase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4762–4766. doi: 10.1073/pnas.74.11.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R. W., Goldberg A. L. ATP-stimulated endoprotease is associated with the cell membrane of E. coli. Nature. 1981 Apr 2;290(5805):419–421. doi: 10.1038/290419a0. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Smith J. A. Cell division of the Escherichia coli lon- mutant. Mol Gen Genet. 1970;108(3):249–257. doi: 10.1007/BF00283355. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Ussery C. L., Allen J. S. Bacterial cell division regulation: lysogenization of conditional cell division lon - mutants of Escherichia coli by bacteriophage. J Bacteriol. 1973 Mar;113(3):1326–1332. doi: 10.1128/jb.113.3.1326-1332.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnbauer B. A., Foley E. C., Henderson G. W., Markovitz A. Identification and purification of the Lon+ (capR+) gene product, a DNA-binding protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2043–2047. doi: 10.1073/pnas.78.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnbauer B. A., Markovitz A. Cloning of gene lon (capR) of Escherichia coli K-12 and identification of polypeptides specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980 Aug;143(2):852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]