Abstract
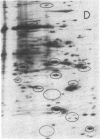
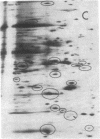
We have attempted to ascertain the proportion of the rat hepatic genome that is under the selective influence of thyroid hormones and to describe the response patterns of individual mRNA sequences in the transition between hypothyroidism and euthyroidism and between euthyroidism and hyperthyroidism. Poly(A)+RNA was extracted from livers of thyroidectomized, intact, euthyroid rats and of thyroidectomized rats rendered euthyroid and hyperthyroid with daily doses of triiodothyronine. The extracted RNA was translated in a reticulocyte lysate system in the presence of [35S]methionine, and the products were analyzed by two-dimensional gel electrophoresis. Triiodothyronine attenuates as well as augments the expression of certain genes at a pretranslational level. This could represent either a direct or an indirect action of the hormone. Triiodothyronine influences approximately 8% of the 231 mRNA sequences visualized, stimulating activity in 11 and inhibiting activity in 7 sequences. Translational activity of at least one mRNA sequence decreased in both thyroidectomized and hyperthyroid animals, compared to euthyroid levels. The relationship of mRNA response to receptor occupancy varied with examples of linear and amplified responses and responses that were maximal at less than full nuclear occupancy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong E. G., Feigelson M. Effects of hypophysectomy and triiodothyronine on de novo biosynthesis, catalytic activity, and estrogen induction of rat liver histidase. J Biol Chem. 1980 Aug 10;255(15):7199–7203. [PubMed] [Google Scholar]
- Coulombe P., Schwartz H. L., Oppenheimer J. H. Relationship between the accumulation of pituitary growth hormone and nuclear occupancy by triiodothyronine in the rat. J Clin Invest. 1978 Nov;62(5):1020–1028. doi: 10.1172/JCI109206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Lingrel J. B. Hemoglobin messenger ribonucleic acid. Distribution of the 9S ribonucleic acid in polysomes of different sizes. Biochemistry. 1969 Mar;8(3):829–831. doi: 10.1021/bi00831a010. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A., Eberhardt N. L. Hormonal domains of response: actions of glucocorticoid and thyroid hormones in regulating pleiotropic responses in cultured cells. Recent Prog Horm Res. 1980;36:195–239. doi: 10.1016/b978-0-12-571136-4.50012-7. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Kurtz D. T., Feigelson P. Multihormonal induction of hepatic alpha2u-globulin mRNA as measured by hybridization to complementary DNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4791–4795. doi: 10.1073/pnas.74.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mariash C. N., Kaiser F. E., Schwartz H. L., Towle H. C., Oppenheimer J. H. Synergism of thyroid hormone and high carbohydrate diet in the induction of lipogenic enzymes in the rat. Mechanisms and implications. J Clin Invest. 1980 May;65(5):1126–1134. doi: 10.1172/JCI109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Coulombe P., Schwartz H. L., Gutfeld N. W. Nonlinear (amplified) relationship between nuclear occupancy by triiodothyronine and the appearance rate of hepatic alpha-glycerophosphate dehydrogenase and malic enzyme in the rat. J Clin Invest. 1978 Apr;61(4):987–997. doi: 10.1172/JCI109024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Shapiro H. C., Schwartz H. L., Surks M. I. Dissociation between thyroxine metabolism and hormonal action in phenobarbital-treated rats. Endocrinology. 1971 Jan;88(1):115–119. doi: 10.1210/endo-88-1-115. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Dowbenko D. J. Role of growth hormone in the multihormonal regulation of messenger RNA for alpha2u globulin in the liver of hypophysectomized rats. Biochemistry. 1977 Aug 23;16(17):3918–3922. doi: 10.1021/bi00636a030. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Dillmann W. H., Oppenheimer J. H. Messenger RNA content and complexity of euthyroid and hypothyroid rat liver. J Biol Chem. 1979 Apr 10;254(7):2250–2257. [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Oppenheimer J. H. Changes in the hepatic levels of messenger ribonucleic acid for malic enzyme during induction by thyroid hormone or diet. Biochemistry. 1980 Feb 5;19(3):579–585. doi: 10.1021/bi00544a029. [DOI] [PubMed] [Google Scholar]
- Tsai J. S., Samuels H. H. Thyroid hormone action: stimulation of growth hormone and inhibition of prolactin secretion in cultured GH1 cells. Biochem Biophys Res Commun. 1974 Jul 10;59(1):420–428. doi: 10.1016/s0006-291x(74)80223-8. [DOI] [PubMed] [Google Scholar]