Abstract
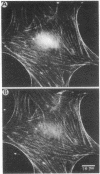
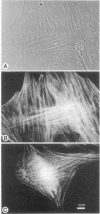
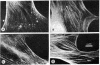
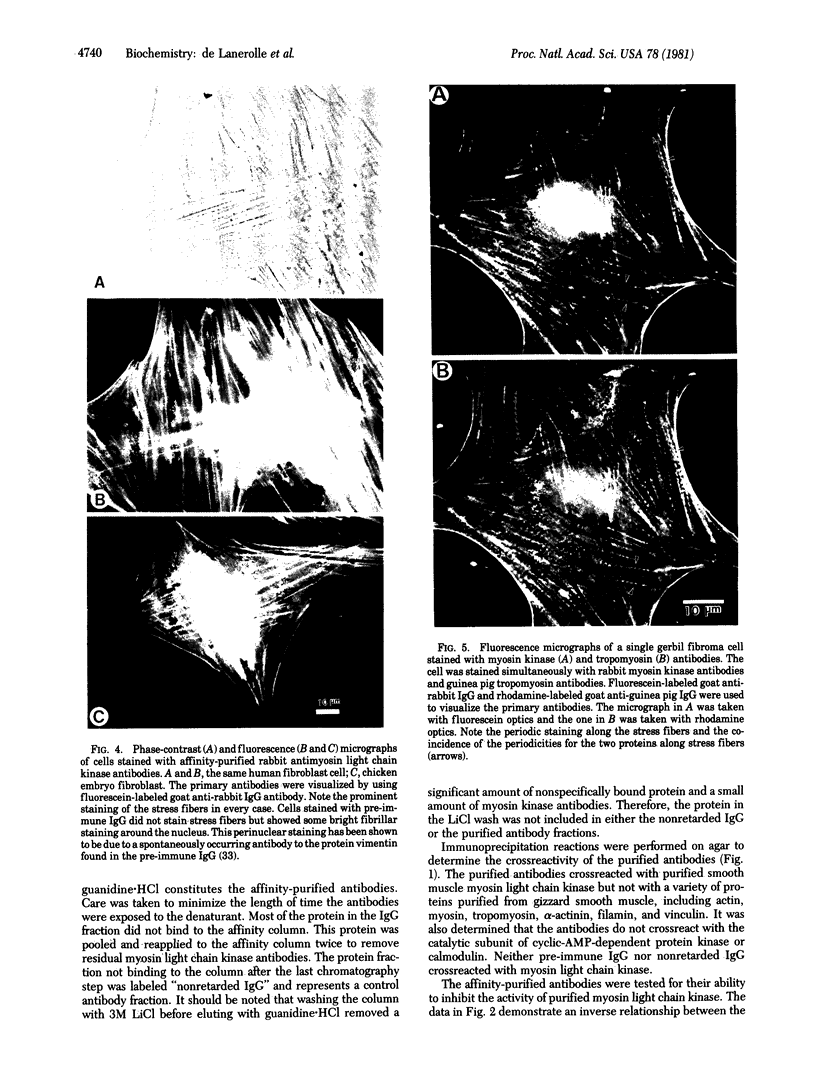
Antibodies to myosin light chain kinase, purified from turkey gizzard smooth muscle, were developed in rabbits and purified by affinity chromatography on a myosin light chain kinase-Sepharose 4B column. The purified antibodies crossreact with purified smooth muscle myosin light chain kinase but not with a variety of contractile or cytoskeletal proteins. The antibodies inhibit the catalytic activity of smooth muscle myosin light chain kinase and there is an inverse relationship between the kinase activity and the amount of antibody present in an assay. Half-maximal inhibition of myosin kinase activity occurs at an antibody/myosin kinase molar ratio of 10:1. The affinity-purified antibodies to smooth muscle myosin kinase were used to study the location of myosin kinase in a variety of nonmuscle cells. Immunofluorescence studies indicate that myosin light chain kinase is localized on microfilament bundles (stress fibers) in cultured fibroblasts. The stress fiber staining pattern is abolished when the antibodies are incubated with purified smooth muscle myosin light chain kinase prior to staining cells, while the staining pattern is unaffected when the antibodies are incubated with actin, myosin, alpha-actinin, or tropomyosin prior to staining. Moreover, the stress fiber staining pattern is periodic in well-spread gerbil fibroma cells and experiments have demonstrated that myosin light chain kinase appears to have the same periodic distribution as myosin but an antiperiodic distribution relative to alpha-actinin. These data indicate that myosin light chain kinase and its substrate, myosin, are in close proximity and are consistent with the hypothesis that myosin light chain kinase regulates actin-myosin interactions in nonmuscle cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975 Aug 14;256(5518):597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Barron J. T., Bárány M., Bárány K., Storti R. V. Reversible phosphorylation and dephosphorylation of the 20,000-dalton light chain of myosin during the contraction-relaxation-contraction cycle of arterial smooth muscle. J Biol Chem. 1980 Jul 10;255(13):6238–6244. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska R., Aromatorio D., Sherry J. M., Hartshorne D. J. Composition of the myosin light chain kinase from chicken gizzard. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1263–1272. doi: 10.1016/0006-291x(77)91429-2. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Hartshorne D. J. A Ca2+-and modulator-dependent myosin light chain kinase from non-muscle cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1352–1359. doi: 10.1016/0006-291x(78)91152-x. [DOI] [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Holmsen H. Myosin phosphorylation in intact platelets. J Biol Chem. 1981 Jul 25;256(14):7510–7514. [PubMed] [Google Scholar]
- Feramisco J. R., Blose S. H. Distribution of fluorescently labeled alpha-actinin in living and fixed fibroblasts. J Cell Biol. 1980 Aug;86(2):608–615. doi: 10.1083/jcb.86.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon W. E., 3rd Immunofluorescent and ultrastructural studies of "sarcomeric" units in stress fibers of cultured non-muscle cells. Exp Cell Res. 1978 Dec;117(2):253–260. doi: 10.1016/0014-4827(78)90138-6. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S., Klee C. B. Interaction of calmodulin with myosin light chain kinase and cAMP-dependent protein kinase in bovine brain. J Biol Chem. 1981 Aug 10;256(15):8183–8189. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H., Watanabe S. Chicken gizzard heavy meromyosin that retains the two light-chain components, including a phosphorylatable one. J Biochem. 1979 Feb;85(2):457–472. doi: 10.1093/oxfordjournals.jbchem.a132352. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey J. M., Taylor K. A., Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature. 1980 Sep 18;287(5779):233–235. doi: 10.1038/287233a0. [DOI] [PubMed] [Google Scholar]
- Scordilis S. P., Adelstein R. S. A comparative study of the myosin light chain kinases from myoblast and muscle sources. Studies on the kinases from proliferative rat myoblasts in culture, rat thigh muscle, and rabbit skeletal muscle. J Biol Chem. 1978 Dec 25;253(24):9041–9048. [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Small J. V., Sobieszek A. Ca-regulation of mammalian smooth muscle actomyosin via a kinase-phosphatase-dependent phosphorylation and dephosphorylation of the 20 000-Mr light chain of myosin. Eur J Biochem. 1977 Jun 15;76(2):521–530. doi: 10.1111/j.1432-1033.1977.tb11622.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O. I., Stossel T. P. Actin-binding protein amplifies actomyosin contraction, and gelsolin confers calcium control on the direction of contraction. Biochem Biophys Res Commun. 1980 Jan 29;92(2):675–681. doi: 10.1016/0006-291x(80)90386-1. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Onishi H., Takahashi K., Watanabe S. Structure and function of chicken gizzard myosin. J Biochem. 1978 Dec;84(6):1529–1542. doi: 10.1093/oxfordjournals.jbchem.a132278. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Rhodes J. A., Hammond S. A. The contractile basis of ameboid movement. II. Structure and contractility of motile extracts and plasmalemma-ectoplasm ghosts. J Cell Biol. 1976 Jul;70(1):123–143. doi: 10.1083/jcb.70.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J. A., Adelstein R. S. Macrophage myosin. Regulation of actin-activated ATPase, activity by phosphorylation of the 20,000-dalton light chain. J Biol Chem. 1979 Sep 25;254(18):8781–8785. [PubMed] [Google Scholar]
- Yerna M. J., Aksoy M. O., Hartshorne D. J., Goldman R. D. BHK21 myosin: isolation, biochemical characterization and intracellular localization. J Cell Sci. 1978 Jun;31:411–429. doi: 10.1242/jcs.31.1.411. [DOI] [PubMed] [Google Scholar]
- Yerna M. J., Dabrowska R., Hartshorne D. J., Goldman R. D. Calcium-sensitive regulation of actin-myosin interactions in baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):184–188. doi: 10.1073/pnas.76.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Otto J. J., Bryan J. Organization of myosin in a submembranous sheath in well-spread human fibroblasts. Exp Cell Res. 1979 Mar 15;119(2):205–219. doi: 10.1016/0014-4827(79)90349-5. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Stull J. T. Myosin phosphorylation during contraction and relaxation of tracheal smooth muscle. J Biol Chem. 1980 Oct 25;255(20):9993–10000. [PubMed] [Google Scholar]