Abstract
OBJECTIVE:
To conduct a pilot study designed to measure the impact of a healthy lifestyle intervention with or without individualized mentorship on adiposity, metabolic profile, nutrition and physical activity in overweight teens.
METHODS:
A total of 38 overweight adolescents (body mass index above the 85th percentile) 12 to 16 years of age, who were enrolled in a healthy lifestyle intervention program for six months, were randomly assigned to a nonmentored or individualized mentored intervention.
RESULTS:
For the entire cohort (final n=32), there was a nonstatistically significant reduction in mean (± SD) body mass index z score (2.08±0.38 to 2.01±0.47, P=0.07) and waist circumference (98±10 cm to 96±11 cm, P=0.08), and significant improvements in high-density lipoprotein level (1.08±0.24 mmol/L to 1.20±0.26 mmol/L, P<0.001), and low-density lipoprotein/high-density lipoprotein ratio (2.55±0.84 to 2.26±0.87, P<0.001) from baseline to the end of the intervention. Subjects consumed fewer high-calorie foods (3.9±1.9 to 3.0±1.5 servings/day, P=0.01) and snacks (9.7±5.5 to 6.8±4.0 servings/day, P=0.02), made fewer fast food restaurant visits (1.4±1.3 to 0.8±0.9 visits/week, P=0.02), and had less screen time (8.3±3.8 to 6.9±3.6 h/day, P=0.01). In addition, mentorship was found to be a feasible approach to supporting weight management in obese teens. Our study was underpowered to determine treatment effect, but promising modifications to lifestyle were observed despite the absence of statistically significant improvements in outcomes.
CONCLUSIONS:
The healthy lifestyle intervention improved subjects’ lifestyles and lipid profiles, and the addition of mentorship in this context is feasible. A larger study with a longer intervention time is required to determine whether behavioural changes are associated with clinical improvement and to determine the role of mentorship in promoting lifestyle change.
Keywords: Adolescent, Cardiovascular disease, Lifestyle intervention, Obesity
Abstract
OBJECTIF :
Mener une étude pilote conçue pour mesurer les répercussions d’une intervention sur un mode de vie sain accompagnée ou non d’un mentorat individualisé sur l’adiposité, le profil métabolique, la nutrition et l’activité physique chez des adolescents en surpoids.
MÉTHODOLOGIE :
Au total, 38 adolescents en surpoids (indice de masse corporelle supérieur au 85e percentile) de 12 à 16 ans qui ont participé à un programme d’intervention sur un mode de vie sain pendant six mois ont été répartis au hasard entre une intervention sans mentorat et une intervention comportant un mentorat individualisé.
RÉSULTATS :
Toute la cohorte (nombre final=32) a présenté une réduction non statistiquement significative de l’écart réduit de l’indice de masse corporelle (±ÉT) (2,08±0,38 à 2,01±0,47, P=0,07) et du tour de taille (98±10 cm à 96±11 cm, P=0,08) moyens, et une amélioration significative du taux de lipoprotéines de haute densité (1,08±0,24 mmol/L à 1,20±0,26 mmol/L, P<0,001) et du ratio entre le taux de lipoprotéines de basse densité et le taux de lipoprotéines de haute densité (2,55±0,84 à 2,26±0,87, P<0,001) entre le début et la fin de l’intervention. Les sujets ont consommé moins d’aliments très caloriques (3,9±1,9 à 3,0±1,5 portions/jour, P=0,01) et de collations (9,7±5,5 à 6,8±4,0 portions/jour, P=0,02), ont moins fréquenté les restaurants minute (1,4±1,3 à 0,8±0,9 visite/semaine, P=0,02) et ont consommé moins de temps d’écran (8,3±3,8 à 6,9±3,6 h/jour, P=0,01). En outre, le mentorat constituait une démarche faisable pour appuyer la prise en charge du poids chez les adolescents obèses. L’étude ne comportait pas assez de cas pour déterminer l’effet du traitement, mais les chercheurs ont observé des modifications prometteuses au mode de vie malgré l’absence d’améliorations statistiquement significatives des issues.
CONCLUSIONS :
L’intervention sur un mode de vie sain a amélioré le mode de vie et le profil lipidique des sujets, et l’ajout de mentorat est faisable dans ce contexte. Une étude plus vaste comportant une période d’intervention plus longue s’impose pour déterminer si les changements comportementaux s’associent à une amélioration clinique et pour déterminer le rôle du mentorat dans la promotion du changement de mode de vie.
Although genetic factors may influence adiposity, lifestyle factors are the primary cause, with children consuming increasing quantities of highly processed, energy-dense foods and sugary drinks while becoming less active and more sedentary (1,2). Few intervention strategies for the management of overweight and obesity in youth have been reported, and they have had limited success (3,4). Obesity in childhood and adolescence is associated with hypertension, lipid abnormalities, insulin resistance and type 2 diabetes (5). Childhood obesity has been associated with cardiovascular disease in adulthood (6,7), and the prevalence of overweight and obesity is increasing (8). To date, most childhood obesity intervention research has been conducted among preadolescent participants (9). The difficulty of research in the adolescent population has been attributed to lower adherence to treatment, lower likelihood of acceptance of highly controlled regimens, a wider range of strategies for treatment avoidance, inaccurate self-reporting and difficulties in motivating adolescents (10). Several studies have used mentors to prevent and reduce high-risk behaviours in adolescents, including sexual risk behaviours (11–13), substance use and smoking (14,15), and violence (14,16,17), with positive long-term effects. The Healthy Buddies program (18) demonstrated that a student-led program is effective in improving healthy-living knowledge, behaviour and attitudes in a younger population. Our study assessed a six-month combined dietary-behavioural-physical activity intervention aimed at promoting lifestyle improvements for overweight adolescents, and evaluated the feasibility of using individual mentorship for motivation in this context.
PARTICIPANTS AND METHODS
Subject selection
A total of 38 overweight adolescents (body mass index [BMI] above the 85th percentile for age and sex), ranging from 12 to 16 years of age, were enrolled between June 2006 and June 2007. All subjects were recruited from the local community through advertisements in local papers and in paediatrician offices. Eligible adolescents were free of morbidities that might have impaired or contraindicated their safe participation in the study. These included physical/intellectual limitations (eg, developmental delay, psychiatric illness, significant nonobesity-related medical conditions, orthopaedic problems, recent surgery performed or planned), treatment with medications that might interfere with weight or growth control (eg, corticosteroids or thyroid hormone), and psychological illness (eg, major depression or an eating disorder). The study was approved by The Hospital for Sick Children (Toronto, Ontario) Research Ethics Board. Informed consent was obtained from subjects, and authorization was obtained from their parents/legal guardians.
Intervention
Subjects and their family members attended a one-day group educational workshop at the beginning of the study. Following the workshop, 20 of the 38 subjects were randomly chosen to be individually paired with a university student mentor (blocked randomization with random number generator, 1:1 treatment allocation, mentor-mentee pair matched according to sex) and received mentorship throughout the intervention (the ‘mentored group’). Subsequently, all subjects attended scheduled clinical visits at baseline, and at one, two, three and six months after randomization. At their baseline visit, all subjects learned about the study recommendations for nutrition and physical activity, and were given specific instructions and written materials regarding how to self-assess behaviour and environment, and successively implement small changes by setting goals toward measurable and attainable outcomes (19). Visits at baseline, three months and six months included all study measurements and nutrition and activity counselling. Visits at one and two months included only nutrition and activity counselling, and basic anthropometric measurements.
Subjects in the mentored group agreed to meet with their mentor in person for 1 h to 2 h once per week to achieve activity goals, participate in physical activity, and discuss and set nutritional goals. Additionally, they agreed to communicate (either through telephone or e-mail) twice per week for support. Mentors had regular contact with study personnel, and monthly group meetings were held to provide support, troubleshoot challenges, provide insight and feedback, and contribute to program refinement. Mentor volunteers were recruited from the University of Toronto (Ontario) chapter of the Golden Key International Honour Society, which comprises students in the top 15% of their class in various programs. Applicants underwent a semistructured interview to assess their suitability for mentoring. Criteria for mentors included previous experience with youth, a desire to be a role model for healthy lifestyle themselves, and a demonstration of commitment. Successful applicants were screened according to the protocol used by Volunteer Resources at The Hospital for Sick Children. Before the intervention, mentors were required to attend three workshops. The first workshop focused on knowledge aspects of obesity-related health problems, and principles of nutrition and physical activity. The second workshop focused on fostering self-efficacy and motivation for change. The third workshop focused on implementation of the program. Mentors also participated in the educational sessions for their prospectively assigned study subjects.
Anthropometric measurements
Height, weight and waist circumference were measured in lightweight clothing with shoes removed. Height and waist circumference were measured to the nearest 1 cm and weight to the nearest 0.1 kg using a combined standing stadiometer and scale (Scale-Tronix 5002 stand-on scale, Scale-Tronix, USA) and a measuring tape. Waist circumference was measured at just above the iliac crest. Right arm blood pressure was measured using a standard sphygmomanometer and a stethoscope. All data were collected by trained study staff. Age- and sex-specific BMI z scores were calculated using algorithms provided by the United States Centers for Disease Control and Prevention (20).
Nutritional assessment
All subjects were taught how to complete four-day (three weekdays and one weekend day) food records. The food records were reviewed by a nutritionist and scored based on the number of servings consumed for each of the food categories identified in the nutrition guidelines for the intervention. A food frequency questionnaire (used in clinical practice) was completed to obtain additional information about the frequency of consumption of snack foods and fast food.
Physical activity – objective assessment
All subjects wore a uniaxial accelerometer (Actigraph 7164; Actigraph LLC, USA) at the mid-axillary line using an elastic waist strap, except during sleep and water activities, for a total of seven days. The Actigraph accelerometer is a small (5.0 cm × 4.0 cm × 1.5 cm), lightweight (43 g) unit with a time sampling mechanism that senses acceleration in the vertical plane, 0.05 G to 2.00 G in magnitude, with a frequency response of 0.25 Hz to 2.5 Hz. It uses transducers and microprocessors to convert recorded accelerations to digital signals in 60 s sampling intervals (21). This activity monitor provides reliable and valid measurements of physical activity levels during walking, running and free-living activities (22,23). Data from the accelerometer were downloaded to a computer, and the software provided with the accelerometer was used to calculate the number of steps and time spent in moderate to vigorous physical activity based on age-dependent validated criteria (24). All days were analyzed individually. Days when the accelerometer was not worn or was incompletely worn (based either on the activity log provided by the subject or on visual inspection of the activity record) were excluded from the analysis. The main measures of physical activity in the study were average daily number of steps and number of minutes of moderate to vigorous activities.
Physical activity – subjective assessment
All subjects were asked to complete an activity log while wearing the accelerometer; days were divided into 15 min periods and for each period, the subjects were asked to list activities. This activity log enabled determination of the daily number of 15 min periods in which the dominant activity was either watching television, using the computer or playing video games (not including time spent on school work). The average number of hours per day for all seven days was defined as screen time.
Laboratory
Early morning fasting (at least 12 h) blood samples were collected for all subjects. Fasting serum total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glucose, insulin and C-reactive protein (CRP) were measured, and low-density lipoprotein (LDL) cholesterol levels were calculated. Nonalcoholic steatohepatitis and fatty liver were screened for with measurements of aspartate aminotransferase and alanine aminotransferase levels. Insulin resistance was estimated using the homeostasis model assessment-estimated insulin resistance (HOMA-IR) score.
Primary and secondary outcomes
The primary outcome measure of the impact of the healthy lifestyle intervention was a reduction in adiposity as reflected by the change in BMI and BMI z score from baseline to six months. Secondary outcomes included changes in metabolic profile, nutrition and physical activity from baseline to six months.
Data analysis
Data are presented as mean ± SD, medians with minimum and maximum values, and frequencies as appropriate for the entire cohort. Changes in the entire intervention population from baseline to the end of the intervention were determined using paired t tests, and associations between changes in behaviour and changes in anthropometric and biomedical outcomes were modelled through a univariate linear regression model using a change in outcomes as the dependent variable. Differences between groups were assessed using t tests with the Satterthwaite method when necessary, Fisher’s exact test and Kruskal-Wallis ANOVA. Because of the limited sample size, multivariate regression models were not created. All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, USA).
RESULTS
Patient population
Shortly after the healthy lifestyle intervention began, six adolescents withdrew (one started corticosteroid medication, one moved to a different region and four lacked motivation), four were mentored and two were not mentored. Sixteen nonmentored subjects (mean age 14.5±1.4 years; five males and 11 females) and 16 mentored subjects (mean age 14.4±1.5 years; eight males and eight females) completed the study.
Anthropometry and blood pressure
Subject characteristics at baseline and at six months for the nonmentored and mentored groups are summarized in Table 1. Subjects’ physical characteristics were not different between the nonmentored and mentored groups at baseline or at six months. Subjects grew in height over the course of the intervention, while weight and BMI remained stable. There was a statistically nonsignificant reduction in BMI z score for the entire cohort, with no differences between study groups. The mentored group had significantly lower systolic blood pressure than the nonmentored group at six months. There was, however, no difference in diastolic blood pressure in both groups at baseline or at six months.
TABLE 1.
Anthropometric measures, and changes in clinical characteristics and lifestyle
|
Nonmentored (n=16) |
Mentored (n=16) |
Overall |
P1 | P2 | P3 | P4 | P5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | n | 6 months | n | Baseline | n | 6 months | n | Baseline | 6 months | ||||||
| Anthropometric measures | |||||||||||||||
| Weight, kg | 90.1±17.1 | 16 | 90.6±5.5 | 16 | 87.1±18.3 | 16 | 88.7±18.8 | 16 | 88.6±17.7 | 89.7±12.2 | 0.64 | 0.69 | 0.08 | 0.70 | 0.18 |
| Height, cm | 166±11 | 16 | 167±11 | 16 | 165±9 | 16 | 167±9 | 16 | 166±10 | 167±10 | 0.96 | 0.002 | 0.002 | 1.00 | <0.001 |
| Waist, cm | 98±9 | 16 | 96±10 | 15 | 98±11 | 16 | 96±12 | 16 | 98±10 | 96±11 | 1.00 | 0.08 | 0.40 | 1.00 | 0.08 |
| BMI, kg/m2 | 32.8±4.5 | 16 | 32.3±5.5 | 16 | 31.8±5.7 | 16 | 31.9±6.0 | 16 | 32.3±5.1 | 32.1±5.8 | 0.59 | 0.44 | 0.65 | 0.83 | 0.64 |
| BMI z score | 2.16±0.35 | 16 | 2.05±0.51 | 16 | 2.00±0.41 | 16 | 1.97±0.43 | 16 | 2.08±0.38 | 2.01±0.47 | 0.24 | 0.16 | 0.16 | 0.63 | 0.07 |
| Weight z score | 2.30±0.53 | 16 | 2.16±0.72 | 16 | 2.09±0.60 | 16 | 2.05±0.60 | 16 | 2.20±0.57 | 2.11±0.66 | 0.32 | 0.15 | 0.20 | 0.64 | 0.08 |
| Blood pressure, mmHg | |||||||||||||||
| Systolic | 121±12 | 16 | 124±14 | 16 | 117±11 | 16 | 113±12 | 16 | 119±12 | 119±13 | 0.33 | 0.41 | 0.10 | 0.02 | 0.86 |
| Diastolic | 63±12 | 16 | 63±6 | 16 | 62±7 | 16 | 58±9 | 16 | 63±10 | 61±8 | 0.74 | 0.94 | 0.09 | 0.07 | 0.23 |
| Metabolic profile | |||||||||||||||
| HDL, mmol/L | 1.05±0.27 | 16 | 1.18±0.31 | 14 | 1.12±0.21 | 15 | 1.22±0.22 | 16 | 1.09±0.24 | 1.20±0.27 | 0.48 | 0.03 | <0.001 | 0.68 | <0.001 |
| LDL, mmol/L | 2.66±0.61 | 16 | 2.55±0.68 | 14 | 2.57±0.62 | 15 | 2.52±0.55 | 16 | 2.62±0.62 | 2.54±0.62 | 0.68 | 0.07 | 0.60 | 0.89 | 0.13 |
| LDL/HDL ratio | 2.71±0.95 | 16 | 2.37±1.06 | 14 | 2.38±0.68 | 15 | 2.16±0.70 | 16 | 2.54±0.85 | 2.27±0.88 | 0.27 | <0.001 | 0.02 | 0.51 | <0.001 |
| Total cholesterol, mmol/L | 4.09±0.68 | 16 | 4.12±0.70 | 14 | 4.14±0.71 | 15 | 4.21±0.71 | 16 | 4.12±0.70 | 4.17±0.71 | 0.84 | 0.50 | 0.54 | 0.72 | 0.87 |
| Triglycerides, mmol/L | 0.82±0.26 | 16 | 0.86±0.38 | 14 | 0.99±0.31 | 15 | 1.03±0.62 | 16 | 0.91±0.29 | 0.95±0.50 | 0.11 | 0.88 | 0.60 | 0.36 | 0.58 |
| C-reactive protein, mg/L | 3.4±3.1 | 16 | 4.8±5.8 | 14 | 1.8±1.6 | 16 | 1.5±1.0 | 16 | 2.0 (0.3–13) | 1.9 (0.3–22) | 0.07 | 0.26 | 0.18 | 0.03 | 0.98 |
| Fasting glucose, mmol/L | 4.97±0.25 | 16 | 4.91±0.21 | 14 | 4.96±0.30 | 16 | 4.93±0.39 | 16 | 4.97±0.28 | 4.92±0.30 | 0.85 | 0.09 | 0.78 | 0.86 | 0.98 |
| Insulin, pmol/L | 130±51 | 16 | 137±64 | 14 | 102±38 | 16 | 95±38 | 16 | 116±45 | 116±51 | 0.10 | 0.80 | 0.44 | 0.03 | 0.17 |
| HOMA-IR | 4.78±1.95 | 16 | 5.00±2.36 | 14 | 3.8±1.5 | 16 | 3.50±1.50 | 16 | 4.29±1.73 | 4.75±1.93 | 0.12 | 0.89 | 0.47 | 0.04 | 0.25 |
| Nutrition servings, n | |||||||||||||||
| High fat/sugar (/week) | 3.6±0.8 | 14 | 2.2±0.9 | 14 | 4.2±2.7 | 14 | 3.7±1.5 | 14 | 3.9±1.9 | 3.0±1.5 | 0.38 | 0.02 | 0.19 | 0.002 | 0.01 |
| Total snack food (/week) | 10.3±6.9 | 16 | 6.3±4.2 | 14 | 8.9±3.3 | 14 | 7.4±3.8 | 14 | 9.7±5.5 | 6.8±4.0 | 0.48 | 0.06 | 0.07 | 0.44 | 0.02 |
| Fast food visits (/week) | 1.6±1.5 | 16 | 0.8±1.0 | 14 | 1.1±1.1 | 14 | 0.7±0.8 | 14 | 1.4±1.3 | 0.8±0.9 | 0.29 | 0.02 | 0.36 | 0.76 | 0.02 |
| Fruits and vegetables (/day) | 1.9±1.5 | 14 | 1.8±1.7 | 14 | 2.9±2.7 | 14 | 2±2 | 14 | 2.4±2.1 | 1.9±1.8 | 0.21 | 0.79 | 0.63 | 0.76 | 0.57 |
| Whole-grain foods (/day) | 0.8±0.9 | 14 | 2.0±1.4 | 14 | 1.2±0.8 | 14 | 1±1 | 14 | 1.0±1.0 | 1.5±1.0 | 0.21 | 0.04 | 0.73 | 0.03 | 0.07 |
| Activity level and fitness | |||||||||||||||
| MVPA, mins/day | 44±21 | 16 | 39±26 | 11 | 47±24 | 16 | 49±32 | 14 | 46±23 | 44±23 | 0.68 | 0.47 | 0.71 | 0.41 | 0.93 |
| Steps, ×1000/day | 8.0±2.2 | 16 | 7.6±2.9 | 11 | 9.2±3.4 | 16 | 9.1±3.5 | 14 | 8.6±2.8 | 8.3±3.2 | 0.27 | 0.37 | 0.92 | 0.20 | 0.50 |
| Screen time, h/day | 8.6±3.9 | 16 | 7.1±3.8 | 16 | 8.0±3.7 | 16 | 6.6±3.5 | 16 | 0.3±3.8 | 6.9±3.6 | 0.65 | 0.05 | 0.12 | 0.70 | 0.01 |
Data presented as mean ± SD or median (range). P1 – between-group difference at baseline; P2 – nonmentored group change from baseline to six months; P3 – mentored group change from baseline to six months; P4 – between-group difference at six months; P5 – entire cohort change from baseline to six months. BMI Body mass index; HDL High-density lipoprotein; HOMA-IR Homeostasis model assessment-estimated insulin resistance; LDL Low-density lipoprotein; MVPA Moderate to vigorous physical activity
Metabolic profile
At six months, there were significant differences between the non-mentored and mentored groups. The mentored group had lower fasting insulin, lower HOMA-IR, and lower CRP levels (all P<0.05) than the nonmentored group. Comparing between baseline and six months, both groups had similar and significant improvements in HDL and LDL/HDL ratio, which was also reflected in the whole-group data (Table 1). There were no significant changes for the entire cohort in other lipid measures, CRP, glucose, insulin or HOMA-IR.
Nutrition and physical activity
In terms of nutrition, there were no differences between the groups at baseline. Over the course of the study, the nonmentored group demonstrated significant decreases in high fat/sugar and fast food consumption, and a significant increase in whole-grain food consumption, whereas the mentored group had no significant nutritional changes. At six months, the nonmentored group was consuming less high fat/sugar food and more whole grains than the mentored group. As a cohort, the subjects’ consumption of high caloric-density foods and visits to fast food restaurants were significantly reduced following the healthy lifestyle intervention.
Although there were some between-group differences in physical activity and fitness at baseline and at six months, the absolute differences were small and likely not clinically significant. There were no between-group differences in activity and fitness outcomes between baseline and six months. For the cohort, there was a decrease in screen time.
Cardiovascular risk factors associated with lifestyle change
Over the course of the healthy lifestyle intervention, changes in both nutrition and physical activity were associated with improvements in BMI z score and fasting LDL cholesterol level. A decrease of 1 h/day in screen time and an increase of 1 h/day of moderate to vigorous physical activity between baseline and six months were associated with a mean decrease of 0.11±0.05 and 0.23±0.11 in BMI z score (P<0.05), respectively. Each additional serving/day of whole-grain food (compared with baseline) was associated with a close-to-significant decrease in BMI z score of 0.20±0.10 (P=0.06) and a decrease in LDL cholesterol of 0.34±0.18 mmol/L. Each additional serving/day of fruits and vegetables (compared with baseline) was significantly associated with a decrease in LDL cholesterol of 0.32±0.11 mmol/L (P=0.01).
Mentorship
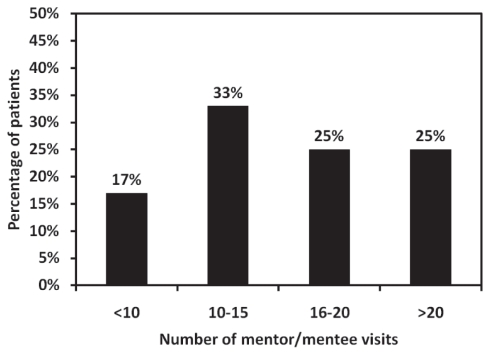
Of 24 expected visits, mentored subjects met with their mentors an average of 14±6 times and for an average time of 105±60 min/visit over the course of the six-month intervention (Figure 1). Mentored subjects reported a positive mentorship experience and used the following descriptors for what they liked most: “support”, “encouragement”, “learning”, “ideas for activity”, “follow-up on goals set” and “companionship”. The most common response was companionship while being physically active. Mentored subjects did not report any negative experiences with their mentors. When asked to rate the helpfulness of having a mentor on a scale of 1 to 10, 13 of 16 subjects reported 7/10 or greater, and eight subjects reported 10/10. All three subjects reporting less than 7/10 had participated in relatively few visits with their mentor (eight or fewer of 24 expected) over the course of the intervention, and the reasons included both lack of effort by the subject and/or inability of the subject to coordinate scheduled meetings with their mentor. When asked to rate how well they got along with their mentor, only one mentored subject reported less than 8/10.
Figure 1).
Distribution of the number of mentor/mentee visits
DISCUSSION
Following our six-month healthy lifestyle intervention, subjects chose to eat fewer energy-dense and low-nutrient foods, and spent less time in sedentary pursuits. Our results support that a combined, patient-centred, dietary-behavioural-physical activity intervention was feasible for improving nutrition and activity habits in overweight adolescents. Additionally, we suggest that mentorship is a viable strategy for motivating change in overweight adolescents, even if behavioural and medical benefits are not immediately evident. To our knowledge, the present study was the first to investigate an obesity intervention for adolescents that included the use of individualized mentorship to promote healthy lifestyle habits.
Research in adolescent youth obesity treatment has progressed in recent years, and few reports of long-term success in adolescent weight management exist (9). Recently, some studies reported short- and long-term success for reducing adiposity and other cardiovascular risk factors in overweight adolescents (25–28). Several general features distinguish these reported interventions from ours. They include high intensity, monitored physical activity, weekly nutrition sessions, regular psychotherapy, parental involvement, and reward systems for participation. Our subject-centred approach may be more appealing to adolescents who are known to be less accepting of highly regimented programs (29).
Interventions resulting in significant improvement in cardiovascular risk factors have used a structured and monitored physical activity component with compulsory predetermined frequency, intensity and duration (30–34). Our study provided recommendations for progressive increases in intensity and duration of physical activity throughout the program, but subjects were not required to attend regular monitored exercise sessions as in other studies. Our subject-centred approach to incremental goal setting for participation in physical activity resulted in significant variability in its duration and intensity depending on subject motivation. This less-regimented activity program may require a greater duration to achieve more substantial improvement in cardiovascular risk factors. Although subjects’ physical activity did not appear to increase significantly, they demonstrated significant enhancements in their lifestyle behaviours. A decrease in screen time may have reduced snacking on high caloric-density foods and increased participation in more active pursuits (35). Other interventions that have specifically targeted a reduction in sedentary pursuits in youth have also observed a reduction in the markers of adiposity (36). The healthy lifestyle intervention also significantly improved the subjects’ metabolic profile. This may, in part, be due to their improved nutrition behaviours as seen in the nonmentored group and the cohort as a whole. Emphasis on lifestyle changes may have more impact than concentrating mainly on increased physical activity in affecting changes on BMI or weight improvements in adolescents over the long term.
Our intervention, which emphasized healthy lifestyle rather than weight loss, was less intense than other interventions for which significant cardiovascular risk factor reduction was reported with respect to activity and diet. Other interventions of similar duration have resulted in greater changes in adiposity markers and other risk factors; this may be explained by their imposed calorie restriction, strict activity demands and/or strict inclusion/exclusion criteria. To be successful, our ‘small changes’ approach is expected to require more than six months. A potential advantage of our approach over more prescriptive interventions is that participants make personalized activity choices and other lifestyle changes in their own community rather than following prescribed exercise routines in a clinical setting. Our approach may increase the likelihood of longer term adherence to a healthier lifestyle.
Parental attendance at the family education day, which commenced our intervention, was mandatory. Subsequently, no provisions for parental education or involvement during clinic visits were made. A recent systematic review of environmental correlates of obesity-related dietary behaviours in youth (37) highlighted the importance of parental modelling of healthy dietary habits. Our subjects also responded favourably to companionship while being physically active, and parents should definitely be encouraged to be involved in their children’s physical activities. Parental support for physical activity is also positively associated with physical activity in adolescent girls (38). Because of the parental influence on children and adolescents’ lifestyle habits, parent involvement should be a key feature of interventions for overweight adolescents (39).
Research examining the outcomes from natural mentoring relationships has found that these relationships, when they occur with persons other than family members, predict a greater likelihood of participation in physical activity (40). Although we have shown that mentorship is a feasible approach to augmenting an intervention for overweight adolescents, the study was not powered to detect differences between the mentored and non-mentored groups. Additionally, a higher number of mentor-mentee contacts throughout the intervention and longer duration of mentorship may have been required to increase the effectiveness of the intervention for promoting positive change in the mentees and to produce clinically significant improvements in cardiovascular risk factors.
Our study had several limitations. First, it was not powered to detect differences between the mentoring groups. The observed differences between the two groups and from baseline to six months, although promising, need to be confirmed in a larger study. Second, the six-month intervention period may not have been long enough to detect clinically important changes. A longer intervention period with a greater number of participants is necessary. Third, the sustainability of the observed lifestyle changes in our study is unknown. Particularly in the adolescent population, improved healthy lifestyle behaviour needs to be maintained into adulthood to have a significant impact on overall cardiovascular health.
CONCLUSIONS
Our pilot study demonstrated the feasibility of using a subject-centred approach to goal setting to improve nutrition and physical activity level, and reduce adiposity in overweight adolescents. The addition of mentorship was well accepted by the subjects and is an interesting area for future research. A longer intervention period with a greater number of subjects is needed to determine whether the behavioural changes we observed will result in significant clinical improvement over time and to determine the exact role of mentorship in a healthy lifestyle intervention for overweight adolescents.
Footnotes
FINANCIAL SUPPORT: This study was supported by the CIBC World Markets Children’s Miracle Foundation Chair in Child Health Research (BWM).
REFERENCES
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: Public-health crisis, common sense cure. Lancet. 2002;360:473–82. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 2.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–35. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 3.Campbell K, Waters E, O’Meara S, Kelly S, Summerbell C. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2002;(2):CD001871. doi: 10.1002/14651858.CD001871. [DOI] [PubMed] [Google Scholar]
- 4.Collins CE, Warren J, Neve M, McCoy P, Stokes BJ. Measuring effectiveness of dietetic interventions in child obesity: A systematic review of randomized trials. Arch Pediatr Adolesc Med. 2006;160:906–22. doi: 10.1001/archpedi.160.9.906. [DOI] [PubMed] [Google Scholar]
- 5.McCrindle BW, Manlhiot C. Elevated atherogenic lipoproteins in childhood: Risk, prevention, and treatment. J Clin Lipidol. 2008;2:138–46. doi: 10.1016/j.jacl.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: The Bogalusa Heart Study. Pediatrics. 2001;108:712–8. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA. 2003;290:2271–6. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 9.Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2003;(3):CD001872. doi: 10.1002/14651858.CD001872. [DOI] [PubMed] [Google Scholar]
- 10.Lobstein T, Baur L, Uauy R. Obesity in children and young people: A crisis in public health. Obes Rev. 2004;5(Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 11.DiClemente RJ, Wingood GM. A randomized controlled trial of an HIV sexual risk-reduction intervention for young African-American women. JAMA. 1995;274:1271–6. [PubMed] [Google Scholar]
- 12.Kirby D, Short L, Collins J, et al. School-based programs to reduce sexual risk behaviors: A review of effectiveness. Public Health Rep. 1994;109:339–60. [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel DM, Aten MJ, Enaharo M. Long-term effects of a middle school- and high school-based human immunodeficiency virus sexual risk prevention intervention. Arch Pediatr Adolesc Med. 2001;155:1117–26. doi: 10.1001/archpedi.155.10.1117. [DOI] [PubMed] [Google Scholar]
- 14.DuBois DL, Holloway BE, Valentine JC, Cooper H. Effectiveness of mentoring programs for youth: A meta-analytic review. Am J Community Psychol. 2002;30:157–97. doi: 10.1023/A:1014628810714. [DOI] [PubMed] [Google Scholar]
- 15.Sipe CL. Mentoring programs for adolescents: A research summary. J Adolesc Health. 2002;31:251–60. doi: 10.1016/s1054-139x(02)00498-6. [DOI] [PubMed] [Google Scholar]
- 16.Moody KA, Childs JC, Sepples SB. Intervening with at-risk youth: Evaluation of the youth empowerment and support program. Pediatr Nurs. 2003;29:263–70. [PubMed] [Google Scholar]
- 17.Roberts H, Liabo K, Lucas P, DuBois D, Sheldon TA. Mentoring to reduce antisocial behaviour in childhood. BMJ. 2004;328:512–4. doi: 10.1136/bmj.328.7438.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stock S, Miranda C, Evans S, et al. Healthy Buddies: A novel, peer-led health promotion program for the prevention of obesity and eating disorders in children in elementary school. Pediatrics. 2007;120:e1059–68. doi: 10.1542/peds.2006-3003. [DOI] [PubMed] [Google Scholar]
- 19.McCrindle BW, Wengle JG. Get a Healthy Weight for Your Child. Toronto: Robert Rose Inc; 2005. [Google Scholar]
- 20.Department of Health and Human Services . CDC Growth Charts for the United-States: Methods and Development. Hyattsville: 2000. [Google Scholar]
- 21.Stickland MK, Petersen SR, Bouffard M. Prediction of maximal aerobic power from the 20-m multi-stage shuttle run test. Can J Appl Physiol. 2003;28:272–82. doi: 10.1139/h03-021. [DOI] [PubMed] [Google Scholar]
- 22.Eisenmann JC, Strath SJ, Shadrick D, Rigsby P, Hirsch N, Jacobson L. Validity of uniaxial accelerometry during activities of daily living in children. Eur J Appl Physiol. 2004;91:259–63. doi: 10.1007/s00421-003-0983-3. [DOI] [PubMed] [Google Scholar]
- 23.Trost SG. Objective measurement of physical activity in youth: Current issues, future directions. Exerc Sport Sci Rev. 2001;29:32–6. doi: 10.1097/00003677-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Johnston CA, Tyler C, McFarlin BK, et al. Weight loss in overweight Mexican American children: A randomized, controlled trial. Pediatrics. 2007;120:e1450–7. doi: 10.1542/peds.2006-3321. [DOI] [PubMed] [Google Scholar]
- 26.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol. 2006;48:1865–70. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Reinehr T, Temmesfeld M, Kersting M, de Sousa G, Toschke AM. Four-year follow-up of children and adolescents participating in an obesity intervention program. Int J Obes (Lond) 2007;31:1074–7. doi: 10.1038/sj.ijo.0803637. [DOI] [PubMed] [Google Scholar]
- 28.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: A randomized controlled trial. JAMA. 2007;297:2697–704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 29.Whitlock EA, O’Connor EP, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management programs in children and adolescents. Evid Rep Technol Assess (Full Rep) 2008:1–308. [PMC free article] [PubMed] [Google Scholar]
- 30.Watts K, Beye P, Siafarikas A, et al. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004;43:1823–7. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Watts K, Beye P, Siafarikas A, et al. Effects of exercise training on vascular function in obese children. J Pediatr. 2004;144:620–5. doi: 10.1016/j.jpeds.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 32.Woo KS, Chook P, Yu CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109:1981–6. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 33.Atlantis E, Barnes EH, Singh MA. Efficacy of exercise for treating overweight in children and adolescents: A systematic review. Int J Obes (Lond) 2006;30:1027–40. doi: 10.1038/sj.ijo.0803286. [DOI] [PubMed] [Google Scholar]
- 34.Connelly JB, Duaso MJ, Butler G. A systematic review of controlled trials of interventions to prevent childhood obesity and overweight: A realistic synthesis of the evidence. Public Health. 2007;121:510–7. doi: 10.1016/j.puhe.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Simon C, Wagner A, DiVita C, et al. Intervention centred on adolescents’ physical activity and sedentary behaviour (ICAPS): Concept and 6-month results. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S96–103. doi: 10.1038/sj.ijo.0802812. [DOI] [PubMed] [Google Scholar]
- 36.DeMattia L, Lemont L, Meurer L. Do interventions to limit sedentary behaviours change behaviour and reduce childhood obesity? A critical review of the literature. Obes Rev. 2007;8:69–81. doi: 10.1111/j.1467-789X.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 37.van der Horst K, Oenema A, Ferreira I, et al. A systematic review of environmental correlates of obesity-related dietary behaviors in youth. Health Educ Res. 2007;22:203–26. doi: 10.1093/her/cyl069. [DOI] [PubMed] [Google Scholar]
- 38.Neumark-Sztainer D, Story M, Hannan PJ, Tharp T, Rex J. Factors associated with changes in physical activity: A cohort study of inactive adolescent girls. Arch Pediatr Adolesc Med. 2003;157:803–10. doi: 10.1001/archpedi.157.8.803. [DOI] [PubMed] [Google Scholar]
- 39.Epstein LH. Family-based behavioural intervention for obese children. Int J Obes Relat Metab Disord. 1996;20(Suppl 1):S14–21. [PubMed] [Google Scholar]
- 40.DuBois DL, Silverthorn N. Characteristics of natural mentoring relationships and adolescent adjustment: Evidence from a national study. J Prim Prev. 2005;26:69–92. doi: 10.1007/s10935-005-1832-4. [DOI] [PubMed] [Google Scholar]