Abstract
Diabetic patients treated with inhaled insulin exhibit reduced fasting plasma glucose levels. In dogs, insulin action in muscle is enhanced for as long as 3 h after insulin inhalation. This study was designed to determine whether this effect lasts for a prolonged duration such that it could explain the effect observed in diabetic patients. Human insulin was administered via inhalation (Exubera; n = 9) or infusion (Humulin R; n = 9) in dogs using an infusion algorithm that yielded matched plasma insulin kinetics between the two groups. Somatostatin was infused to prevent insulin secretion, and glucagon was infused to replace basal plasma levels of the hormone. Glucose was infused into the portal vein at 4 mg/kg/min and into a peripheral vein to maintain the arterial plasma glucose level at 160 mg/dl. Arterial and hepatic sinusoidal insulin and glucose levels were virtually identical in the two groups. Notwithstanding, glucose utilization was greater when insulin was administered by inhalation. At its peak, the peripheral glucose infusion rate was 4 mg/kg/min greater in the inhalation group, and a 50% difference between groups persisted over 8 h. Inhalation of insulin caused a greater increase in nonhepatic glucose uptake in the first 3 h after inhalation; thereafter, net hepatic glucose uptake was greater. Inhalation of insulin was associated with greater than expected (based on insulin levels) glucose disposal. This may explain the reduced fasting glucose concentrations observed in humans after administration of certain inhaled insulin formulations compared with subcutaneous insulin.
In clinical trials comparing inhaled [Exubera; (insulin human rDNA origin) inhalation powder] with subcutaneously administered (Humulin R; Eli Lilly & Co., Indianapolis, IN) insulin, Exubera reduced postprandial glucose levels at least as effectively as Humulin in the first 3 h while maintaining lower glucose concentrations compared with Humulin during the 4th and 5th h after meal consumption (Skyler et al., 2005). It is remarkable that fasting plasma glucose levels also were reduced by as much as 40 mg/dl in patients administered insulin by inhalation compared with subcutaneous insulin, whether as dry powder (Hollander et al., 2004; Quattrin et al., 2004; Skyler et al., 2005; Garg et al., 2006) or liquid (Hermansen et al., 2004) formulations. Thus, insulin delivery to the lungs may help to reduce insulin needs for insulin-dependent patients, especially overnight. In addition, inhaled insulin is also associated with less weight gain compared with subcutaneous insulin, which may directly result from reduced nocturnal insulin requirements (Hollander et al., 2007).
We previously conducted a series of studies in dogs to investigate the specific glucometabolic effects of insulin delivered by inhalation compared with pharmacokinetically matched intravenous (Edgerton et al., 2005, 2006a) or subcutaneous (Cherrington et al., 2004; Edgerton et al., 2006b) administration. Although these experiments confirmed the association of enhanced glucose clearance with the inhalation of insulin, they were limited to 4-h duration, and plasma insulin levels were allowed to fall to near zero after 3 h.
The aim of the present study was to determine the 8-h time course of the increase in nonhepatic glucose uptake (non-HGU) associated with inhaled insulin compared with that observed with intravenous insulin. In addition, we explored a possible causal mechanism of this effect by measuring pulmonary angiotensin-converting enzyme (ACE) activity in response to insulin inhalation.
Materials and Methods
Experiments were conducted at the Lovelace Respiratory Research Institute (Albuquerque, NM) on 18 healthy, conscious, 18-h-fasted female beagle dogs (8–13 kg). Before the study, the dogs were fed a standard chow diet [Teklad 25% Mini Lab Dog Diet (W) 8759; Harlan Teklad, Madison, WI] once a day (∼945 kcal), and water was provided ad libitum. Between 3:00 PM and 4:00 PM the night before the scheduled experiment, each dog was fed one 418-g can of Hills Prescription Diet-Canine p/d (Hill’s Pet Nutrition, Inc., Topeka, KS) (591 kcal). No food was given after this time. The surgical facility met the standards of the American Association for the Accreditation of Laboratory Animal Care, and the protocols were approved by the Lovelace Respiratory Research Institute Institutional Animal Care and Use Committee before the start of the study. All dogs underwent a laparotomy 3 weeks before the experiment to implant infusion catheters into the inferior vena cava (IVC) and jejunal and splenic veins, and sampling catheters were implanted into the hepatic portal vein, hepatic vein, and left femoral artery. Ultrasonic flow probes (Transonic Systems Inc., Ithaca, NY) were placed around the portal and hepatic veins as described elsewhere (Edgerton et al., 2001). Intraportal catheters (splenic and jejunal) were used for the infusion of glucose (50% dextrose; Baxter, McGaw Park, IL). Each animal was used only once.
On the day of exposure, all dogs were anesthetized. The dogs were given 0.2 ml of acepromazine subcutaneously; approximately 15 min later, 5% isoflurane was administered by inhalation using a mask until palpebral and pedal reflexes disappeared. The dogs were then intubated with an endotracheal tube, and anesthesia was maintained with 1 to 2% isoflurane in oxygen. Three intravenous catheters (Terumo Medical Corp., Somerset, NJ) were placed into the cephalic and/or saphenous veins to allow infusion of insulin, somatostatin, and glucagon. Insulin was then administered either by inhalation or intravenous infusion.
Exubera is a dry powder human insulin of recombinant origin specially formulated for intrapulmonary administration. This insulin is packaged in a foil blister pack, with each blister containing 1.0 mg of human insulin. Humulin R, which is a recombinant human insulin, was used for intravenous infusion. It is assumed that the biological activity of insulin in both preparations was identical.
In the inhalation (INH) group, at baseline (t = 0 min), dogs inhaled the content of one blister of insulin using a modified P2.3 device (Nektar Inc., San Carlos, CA), as described previously (Edgerton et al., 2005). No adverse clinical signs related to the inhalation of insulin were observed during the study. To prevent insulin deficiency after the washout of inhaled insulin, intravenous insulin was infused in the INH group between 95 and 485 min (Table 1). In the intravenous (IV) group, after sham inhalation exposure, Humulin R diluted in normal saline (0.9% NaCl; Baxter) with added plasma (3:100 ml) was infused into the IVC, using an algorithm designed to match the arterial plasma insulin kinetic profile of the INH group (Table 2). Thus, during the first several hours of the study, insulin infusion was used in the IV group to match the insulin kinetics of the INH group, after which the same intravenous infusion rate was used in both groups to maintain the plasma insulin levels at ∼18 μU/ml for the duration of the study.
TABLE 1.
Algorithm for intravenous infusion of insulin in the insulin inhalation group (95–485 min)
| Time | Intravenous Infusion |
|---|---|
| mU/kg/min | |
| 0–95 min | N.A. |
| 95–110 min | 0.10 |
| 110–125 min | 0.20 |
| 125–155 min | 0.26 |
| 155–185 min | 0.32 |
| 185–215 min | 0.38 |
| 215–245 min | 0.44 |
| 245–485 min | 0.50 |
N.A., not applicable.
TABLE 2.
Algorithm for intravenous infusion of insulin in the intravenous insulin infusion group (0–485 min)
| Time | Intravenous Infusion |
|---|---|
| mU/kg/min | |
| 0–5 min | 1.6 |
| 5–10 min | 2.0 |
| 10–15 min | 2.2 |
| 15–20 min | 2.0 |
| 20–35 min | 1.8 |
| 35–50 min | 1.4 |
| 50–65 min | 1.0 |
| 65–80 min | 0.8 |
| 80–95 min | 0.7 |
| 95–125 min | 0.6 |
| 125–485 min | 0.5 |
Each dog in the INH group received 1 mg of insulin via inhalation of Exubera. The amount of aerosolized powder actually delivered to the dogs ranged from 50 to 70% of the starting amount. With the method of administration used, it is reasonable to assume that all of this material was delivered to the respiratory tract and, based on the size of the aerosolized insulin particles and the method of exposure used, that approximately half of this amount was delivered to the alveolar region, where most absorption of deposited aerosols occurs (Patton et al., 2004). Therefore, it is estimated that each dog in the INH group received 0.50 to 0.70 mg (∼15 U) of insulin in the total respiratory tract and 0.25 to 0.35 mg (∼7.5 U) in the alveolar region.
Somatostatin (0.8 μg/kg/min), which inhibits endogenous insulin and glucagon secretion, and glucagon (0.5 ng/kg/min), to replace basal levels of endogenous glucagon, were infused from 5 to 485 min. Intraportal glucose infusion (50% dextrose) was administered at a rate of 4 mg/kg/min from 5 to 485 min. Peripheral intravenous glucose infusion was also administered, as needed, to maintain the plasma glucose concentration near 160 mg/dl.
Blood Sampling and Analytical Procedures. Blood samples were collected from the femoral artery and the hepatic portal and hepatic veins. Hematocrit, plasma glucose, glucagon, insulin, C-peptide, and cortisol concentrations were collected as described previously (Edgerton et al., 2001). The Vanderbilt Hormone Assay Core (Vanderbilt University Medical Center, Nashville, TN) assessed plasma insulin, glucagon, and C-peptide, using immunoassay procedures. Blood for measurement of bradykinin (BK) and BK1–5 was drawn into cold anhydrous ethanol. BK was assayed using a BK ELISA kit (S-1135; Bachem California, Torrance, CA), and BK1–5 was determined using a dual-isotope dilution mass spectrometric assay as described previously (Murphey et al., 2001). Blood for measuring angiotensin (AT) I and II was added to a chilled EDTA tube with 100 μl of angiotensin inhibitor (Wake Forest University, Winston-Salem, NC) and determined by radioimmunoassay at the Hypertension and Vascular Disease Center (Wake Forest University School of Medicine, Winston-Salem, NC). All samples were stored at −70°C.
Blood Flow Measurement. Blood flow in the hepatic artery and portal vein and mean arterial blood pressure were measured using ultrasonic flow probes and a Transit-time Perivascular Flow Meter (model T403; Transonic Systems Inc.).
Data Analysis. Net hepatic glucose balance (NHGB) was calculated using the arterial-venous difference method: NHGB = loadout − loadin, where loadout = H × HF and loadin = (A × AF) + (P × PF); H, A, and P are the glucose concentrations in the hepatic vein, femoral artery, and portal vein blood or plasma, respectively; HF, AF, and PF are the blood flows in the hepatic vein, hepatic artery, and portal vein, respectively, as determined by the ultrasonic flow probes. According to this model, a positive value represents a net output by the liver, whereas a negative value represents a net uptake by the liver. For balance calculations, glucose concentrations were converted from plasma to blood values by using previously published correction factors (Edgerton et al., 2005). NonHGU was calculated as the glucose infusion rate plus NHGB, with changes in the glucose mass accounted for when deviations from steady state were present (Galassetti et al., 1999; Donmoyer et al., 2000; Moore et al., 2000). The approximate plasma insulin and glucagon concentrations in the hepatic sinusoidal bed were calculated using the formula [A] × %AF + [P] × %PF, where [A]and [P]are arterial and portal vein hormone concentrations, respectively, and %AF and %PF are the percentage arterial and portal contributions to the total hepatic blood flow, respectively. Area under the curve (AUC) was calculated by summing average parameter values at each minute during a specified time period. Because there was an interval of at least 5 min between samples, values were estimated by taking the average value at each of the two nearest time points and multiplying by the number of minutes between them.
Statistical Analysis. Statistical analyses were performed using SigmaStat (SPSS Inc., Chicago, IL) procedures. Data are presented as mean ± S.E.M. Time course data were analyzed with repeated-measures two-way analysis of variance, and univariate F tests were used for post hoc group comparisons. One-way analysis of variance was applied to group comparisons of single time points and AUC data. Statistical significance was established at p < 0.05.
Results
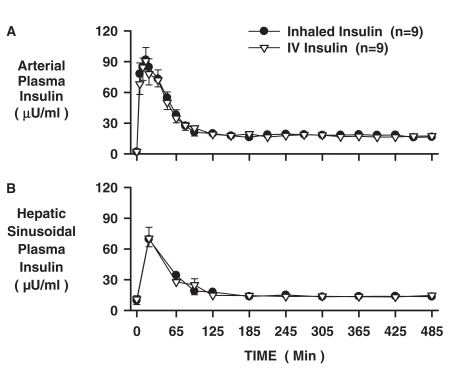
Arterial plasma insulin levels peaked in 15 min at 92 ± 10 and 91 ± 13 μU/ml in the INH and IV groups, respectively, and reached a plateau of 18 ± 1 μU/ml by 2 h (Fig. 1A). Hepatic sinusoidal plasma insulin levels peaked at 20 min at 69 ± 7 and 70 ± 11 μU/ml in the INH and IV groups, respectively (Fig. 1B) and reached a plateau of 14 ± 1 μU/ml by 2 h. The total 485-min AUC for arterial insulin were 12,098 ± 927 and 11,790 ± 1010 μU/ml, in the INH and IV groups, respectively. Thus, the insulin concentrations and kinetic profiles were accurately matched between groups.
Fig. 1.
Arterial and hepatic sinusoidal plasma insulin levels for the INH and IV insulin groups (n = 9 each; mean ± S.E.M).
Arterial C-peptide levels dropped rapidly (<30 min) in both groups to concentrations near the level of detection of the assay (0.05 ng/ml) after initiation of somatostatin infusion, indicating that endogenous insulin secretion was quickly and effectively suppressed (Table 3). The arterial glucagon levels were close to basal and remained equivalent in the two groups throughout the experiment (Table 3). Arterial plasma cortisol levels, an index of stress, were also not different between groups (Table 3).
TABLE 3.
Arterial plasma C-peptide, glucagon, and cortisol levels before treatment (0 min) and during the experimental period (65–485 min)
Mean ± S.E.M.; n = 9 per group.
| 0 | 65 | 125 | 185 | 245 | 305 | 365 | 425 | 485 | |
|---|---|---|---|---|---|---|---|---|---|
| Arterial plasma C-peptide (ng/ml) | |||||||||
| Inhalation | 0.21 ± 0.03 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.03 | 0.06 ± 0.04 |
| Intravenous infusion | 0.26 ± 0.07 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.08 ± 0.04 |
| Arterial plasma glucagon (pg/ml) | |||||||||
| Inhalation | 39 ± 4 | 37 ± 3 | 35 ± 2 | 35 ± 1 | 37 ± 2 | 36 ± 2 | 33 ± 2 | 33 ± 2 | 29 ± 2 |
| Intravenous infusion | 42 ± 3 | 51 ± 2 | 41 ± 3 | 37 ± 5 | 41 ± 3 | 38 ± 2 | 38 ± 3 | 33 ± 3 | 37 ± 3 |
| Arterial plasma cortisol (μg/ml) | |||||||||
| Inhalation | 5.5 ± 0.9 | 6.7 ± 1.7 | 5.1 ± 1.4 | 3.4 ± 0.4 | 2.4 ± 0.2 | 2.5 ± 0.5 | 3.5 ± 0.5 | 3.9 ± 1.0 | 3.6 ± 0.4 |
| Intravenous infusion | 10.7 ± 1.9 | 7.8 ± 1.1 | 4.0 ± 0.6 | 3.9 ± 0.6 | 3.1 ± 0.5 | 3.3 ± 0.5 | 4.0 ± 0.9 | 3.5 ± 0.6 | 5.4 ± 1.1 |
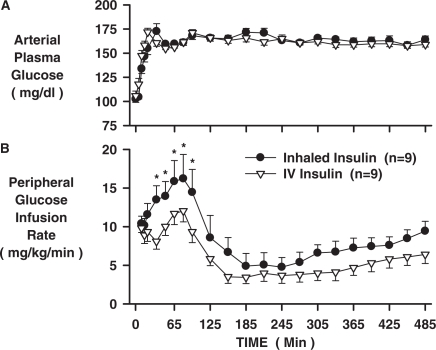
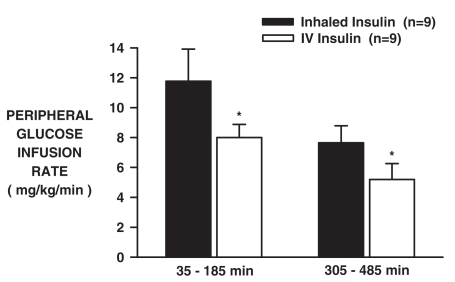
The arterial plasma glucose levels increased from basal to 164 ± 2 and 162 ± 2 mg/dl in the INH and IV groups, respectively (Fig. 2A), as a result of portal (4 mg/kg/min) and peripheral (variable rate) glucose infusion. Despite matched plasma insulin and glucose levels, the peripheral glucose infusion rate (peGIR) was greater after inhalation, peaking at 16.2 ± 3.1 versus 12.0 ± 1.4 mg/kg/min at 80 min (Fig. 2B; p < 0.05). Between 35 and 185 min, the peGIR AUC were 1837 ± 352 versus 1241 ± 144 mg/kg in the INH and IV groups, respectively. During the last 3 h (305–485 min), the peGIR averaged 7.7 ± 1.1 versus 5.2 ± 1.1 (Fig. 2B; p < 0.05), and the peGIR AUC were 1547 ± 240 versus 1054 ± 219 mg/kg during that period in the INH and IV groups, respectively. Thus, the peGIR was greater in the INH compared with the IV group by 48% (3.8 mg/kg/min; p < 0.05) between 35 and 185 min and by 47% (2.5 mg/kg/min; p < 0.05) between 305 and 485 min (Fig. 3).
Fig. 2.
Arterial plasma glucose levels and peripheral glucose infusion rates for the INH and IV insulin groups (n = 9 each; mean ± S.E.M; *, p < 0.05, INH versus IV).
Fig. 3.
Mean peripheral glucose infusion rates (35–95 and 305–485 min) for the INH and IV insulin groups (n = 9 each; mean ± S.E.M; *, p < 0.05, INH versus IV).
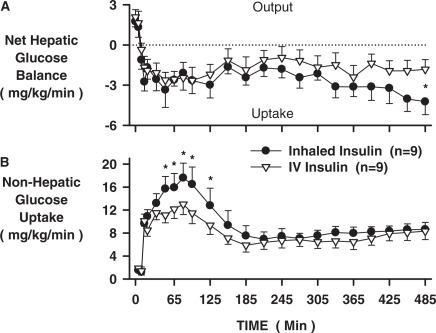
Between 35 and 185 min, net HGU was similar in the two groups (2.5 ± 0.5 versus 2.2 ± 0.6 mg/kg/min; Fig. 4A), whereas nonHGU was greater (13.6 ± 2.2 versus 10.2 ± 1.3 mg/kg/min; Fig. 4B; p < 0.05) in the INH versus IV insulin groups, respectively. Over time, however, a difference in liver glucose uptake began to predominate such that during the last 90 min, net HGU was 3.6 ± 0.9 versus 1.8 ± 0.7 mg/kg/min (p < 0.05), whereas nonHGU was 8.4 ± 0.9 versus 7.9 ± 1.3 mg/kg/min in the INH versus IV groups. Hepatic glucose fractional extraction was twice as great during the last 90 min in the INH versus the IV group (0.08 ± 0.02 versus 0.04 ± 0.02 mg/kg/min, respectively; p < 0.05).
Fig. 4.
Net hepatic glucose balance and nonHGU glucose uptake for the INH and IV insulin groups (n = 9 each; mean ± S.E.M; *, p < 0.05 INH versus IV).
To determine whether inhalation of insulin affected ACE activity, arterial blood BK, BK1–5, ATI, and ATII were measured (Table 4). ACE activity leads to degradation of BK into BK1–5 and ATI into ATII; therefore, the ratios provide indices of ACE activity. There were no differences in the arterial levels or ratios of BK1–5 to BK or ATII to ATI. Furthermore, mean arterial pressure, which is affected by the ACE system, did not differ between groups throughout the study (data not shown).
TABLE 4.
Arterial blood angiotensin I, angiotensin II, bradykinin, and bradykinin 1–5 levels and ratios of angiotensin II/I and bradykinin 1–5 to bradykinin before treatment (0 min) and during the experimental period (65–485 min)
Mean ± S.E.M.; n = 9 per group.
| 0 | 65 | 125 | 185 | 245 | 305 | 365 | 425 | 485 | |
|---|---|---|---|---|---|---|---|---|---|
| Arterial blood angiotensin I (pg/ml) | |||||||||
| Inhalation | 269 ± 89 | 94 ± 26 | 75 ± 13 | 66 ± 9 | 82 ± 15 | 81 ± 14 | 84 ± 9 | 100 ± 23 | 100 ± 14 |
| Intravenous infusion | 416 ± 120 | 193 ± 58 | 133 ± 37 | 120 ± 47 | 123 ± 41 | 182 ± 76 | 133 ± 36 | 142 ± 34 | 143 ± 37 |
| Arterial blood angiotensin II (pg/ml) | |||||||||
| Inhalation | 38 ± 14 | 9 ± 2 | 9 ± 2 | 8 ± 1 | 9 ± 3 | 9 ± 1 | 10 ± 2 | 9 ± 1 | 9 ± 1 |
| Intravenous infusion | 72 ± 24 | 14 ± 3 | 10 ± 2 | 10 ± 2 | 8 ± 2 | 9 ± 2 | 9 ± 2 | 8 ± 1 | 8 ± 2 |
| Arterial blood bradykinin (fmol/ml) | |||||||||
| Inhalation | 11 ± 2 | 10 ± 2 | 10 ± 1 | 11 ± 2 | 12 ± 2 | 11 ± 2 | 9 ± 1 | 9 ± 2 | 10 ± 2 |
| Intravenous infusion | 7 ± 1 | 6 ± 1 | 7 ± 2 | 7 ± 1 | 7 ± 1 | 8 ± 1 | 8 ± 1 | 9 ± 1 | 9 ± 1 |
| Arterial blood bradykinin 1–5 (fmol/ml) | |||||||||
| Inhalation | 13 ± 2 | 12 ± 2 | 14 ± 3 | 15 ± 4 | 17 ± 4 | 16 ± 4 | 17 ± 3 | 17 ± 3 | 16 ± 4 |
| Intravenous infusion | 14 ± 5 | 9 ± 3 | 8 ± 3 | 10 ± 3 | 11 ± 3 | 14 ± 3 | 14 ± 3 | 17 ± 3 | 21 ± 3 |
| Angiotensin II/I ratio | |||||||||
| Inhalation | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Intravenous infusion | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Bradykinin 1–5/bradykinin ratio | |||||||||
| Inhalation | 1.8 ± 0.6 | 1.6 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.5 | 1.8 ± 0.5 | 1.9 ± 0.5 | 2.0 ± 0.4 | 3.0 ± 1.1 | 2.1 ± 0.5 |
| Intravenous infusion | 2.2 ± 0.8 | 1.4 ± 0.4 | 1.7 ± 0.7 | 1.4 ± 0.3 | 1.6 ± 0.4 | 1.7 ± 0.5 | 2.0 ± 0.5 | 2.3 ± 0.6 | 2.7 ± 0.7 |
Discussion
The primary purpose of this study was to determine whether the increase in nonHGU associated with inhalation of insulin is prolonged beyond 4 h. To accomplish this, the arterial plasma insulin kinetics were closely matched in two groups of dogs in which insulin initially entered the blood via pulmonary absorption or via the IVC. Later in the study, insulin was infused in both groups to prevent insulin deficiency. Plasma glucose levels were clamped, and indices of peripheral and hepatic glucose turnover were assessed for 8 h. Inhalation of insulin was associated with increased glucose disposal in the dog, even after 8 h, despite the fact that by that time, all of the circulating insulin was derived from insulin infusion.
As in previous studies (Edgerton et al., 2005, 2006a,b), the effect of insulin inhalation on enhanced peripheral nonHGU lasted approximately 3 h. This occurred despite virtual identity in arterial insulin and glucose levels between the inhaled and infused insulin groups. Thereafter, nonHGU was similar in the two groups. However, throughout the 8 h of observation, net HGU progressively increased in the inhaled insulin group compared with the insulin infusion group. Thus, despite matched glucose and insulin loads at the liver, during the last 3 h of the study, the majority of the increase in glucose disposal resulting from inhalation of insulin was accounted for by the liver. This effect was manifest despite the fact that circulating insulin derived from lungs would have been cleared from the circulation within 3 to 4 h after inhalation (Edgerton et al., 2005, 2006a,b).
In light of these results, one can speculate that the progressive increase of HGU may reflect specific inhaled insulin-dependent transcriptional and translational effects on enzymes that regulate HGU. In addition, reciprocity between liver and muscle occurs, so that as liver glucose uptake is enhanced, there is a reciprocal decrease in nonHGU such that there is little impact on whole-body glucose disposal (Adkins et al., 1987; Galassetti et al., 1998; Moore et al., 2000).
In previous studies, circulating insulin levels fell below basal ∼3 h after inhalation in the presence of somatostatin (Edgerton et al., 2005, 2006). In the present study, we chose to use late infusion of insulin in both groups to prevent insulin deficiency so as to avoid the substantial hyperglycemia that would have occurred over the 8-h duration of the study. In addition, this study design more closely emulates the situation of human patients who do not usually experience complete insulinopenia or marked hyperglycemia overnight. In addition, the glucose-lowering effect may be dependent on some minimal insulin presence; for example, if enhancing insulin-stimulated glucose transport or slowing deactivation of the insulin receptor is involved.
The Tmax after Exubera inhalation is more rapid in the canine compared with the human (15 versus 50–60 min) (Becker et al., 2006; Fountaine et al., 2008). However, comparing the human data with another, faster acting inhaled insulin (Technosphere; Tmax, 15 min) (Rave et al., 2007), the effect on fasting glucose was comparable (personal communication). Therefore, it is not likely that the difference in pharmacokinetics between species is a confounding factor.
Possible mechanisms of increased nonHGU associated with insulin inhalation have been postulated, including a nitric oxide (NO)-mediated event (Edgerton et al., 2006a) and the blockade of the renin-angiotensin system through inhibition of ACE, which has been shown clinically to increase insulin sensitivity (Henriksen and Jacob, 2003a,b; Damas et al., 2004; Jandeleit-Dahm et al., 2005). In fact, in addition to its stimulatory effects on NO synthase, insulin decreases ACE activity, including activity in the lung (Krulewitz et al., 1984; Erman et al., 1998; Sharifi et al., 2004), thus increasing kinin levels and decreasing ATII. Binding of BK to the B2 receptor directly increases NO levels, enhances insulin signaling (via insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity), increases glucose transporter-4 glucose transporter translocation in skeletal muscle, and has been shown to be a potent insulin-dependent enhancer of glucose uptake in muscle and fat (Henriksen and Jacob, 2003a; Damas et al., 2004). Studies also suggest that reduced binding of ATII to the AT1 receptor may also increase insulin sensitivity (Henriksen et al., 2001). Although the present study was not designed to specifically test the hypothesis that inhalation of insulin increases glucose utilization by an ACE-dependent effect, ACE activity and arterial blood concentrations of BK, BK1 to 5, ATI, and ATII were measured. No differential effects of treatment were observed, however, suggesting that the ACE system may not be the mediator of the specific glucometabolic effects of inhaled insulin.
It can be hypothesized that decreased fasting glucose levels after insulin inhalation are due to a depot effect stemming from slowly absorbed inhaled insulin. However, this study and previous studies in humans (Heise et al., 2005) and dog (Cherrington et al., 2004; Edgerton et al., 2005, 2006b) indicate that a slow release of insulin from a putative intrapulmonary depot did not occur despite evidence for intrapulmonary accumulation of insulin (Brain et al., 2008). Given the protracted nature of the effect on glucose disposal, however, we believe that inhaled insulin does generate a long-lived signal in the lungs. The formation of a circulating insulin-sensitizing factor is one possibility, but other mechanisms of action should be considered; for example, stimulation of neuronal pathways.
The future of insulin inhalation is unclear after Exubera’s withdrawal from the market in 2007, and the observation of more frequent lung cancer cases in clinical trials in 2008 may add to the uncertainty.1 Despite these concerns, however, identification of the mechanism involved in the glucose-lowering effect may prove useful in the treatment of diabetes. Further studies will be required to identify secondary molecular mechanisms and to clarify whether these can be utilized for therapeutic advantage by means other than inhalation of insulin.
In summary, greater glucose disposal was observed for as long as 8 h after insulin inhalation when circulating insulin and glucose concentrations were matched between inhaled and infused insulin groups. Although this was initially due to a greater nonHGU, in time, increased net HGU primarily accounted for the difference. This finding may explain why inhalation of insulin before an evening meal is often associated with lower fasting plasma glucose levels the next morning.
ABBREVIATIONS:
- nonHGU
nonhepatic glucose uptake
- ACE
angiotensin-converting enzyme
- IVC
inferior vena cava
- INH
inhalation group
- IV
intravenous group
- BK
bradykinin
- AT
angiotensin
- NHGB
net hepatic glucose balance
- AUC
area(s) under the curve
- peGIR
peripheral glucose infusion rate
- NO
nitric oxide
Footnotes
This work was supported by Pfizer Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
On October 18, 2007, Pfizer Inc., announced that it would cease marketing Exubera because it did not meet the needs or financial expectations of customers. On April 9, 2008, Pfizer Inc., announced that it was updating the Exubera Product Insert to include the following statement: “In studies of Exubera in people with diabetes, lung cancer occurred in a few more people who were taking Exubera than in people who were taking other diabetes medicines. All of the people in these studies who developed lung cancer used to smoke cigarettes. There were too few cases to know if the lung cancer was related to Exubera.”
References
- Adkins BA, Myers SR, Hendrick GK, Stevenson RW, Williams PE, Cherrington AD. Importance of the route of intravenous glucose delivery to hepatic glucose balance in the conscious dog. J Clin Invest. 1987;79:557–565. doi: 10.1172/JCI112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RH, Sha S, Frick AD, Fountaine RJ. The effect of smoking cessation and subsequent resumption on absorption of inhaled insulin. Diabetes Care. 2006;29:277–282. doi: 10.2337/diacare.29.02.06.dc05-1913. [DOI] [PubMed] [Google Scholar]
- Brain JD, Finch GL, Riese RJ, Schwartz PF, Teeter JG. Trough insulin levels in bronchoalveolar lavage following inhalation of human insulin (Exubera) in patients with diabetes mellitus. Am J Respir Crit Care Med. 2008;177:A616. doi: 10.1089/dia.2011.0148. [DOI] [PubMed] [Google Scholar]
- Cherrington AD, Neal DW, Edgerton DS, Glass D, Bowen L, Hobbs CH, Leach C, Rosskamp R, Strack TR. Inhalation of insulin in dogs: assessment of insulin levels and comparison to subcutaneous injection. Diabetes. 2004;53:877–881. doi: 10.2337/diabetes.53.4.877. [DOI] [PubMed] [Google Scholar]
- Damas J, Garbacki N, Lefèbvre PJ. The kallikrein-kinin system, angiotensin converting enzyme inhibitors and insulin sensitivity. Diabetes Metab Res Rev. 2004;20:288–297. doi: 10.1002/dmrr.489. [DOI] [PubMed] [Google Scholar]
- Donmoyer CM, Chen SS, Hande SA, Lacy DB, Ejiofor J, McGuinness OP. Hyperinsulinemia compensates for infection-induced impairment in net hepatic glucose uptake during TPN. Am J Physiol Endocrinol Metab. 2000;279:E235–E243. doi: 10.1152/ajpendo.2000.279.2.E235. [DOI] [PubMed] [Google Scholar]
- Edgerton DS, Cardin S, Emshwiller M, Neal D, Chandramouli V, Schumann WC, Landau BR, Rossetti L, Cherrington AD. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes. 2001;50:1872–1882. doi: 10.2337/diabetes.50.8.1872. [DOI] [PubMed] [Google Scholar]
- Edgerton DS, Cherrington AD, Williams P, Neal DW, Scott M, Bowen L, Wilson W, Hobbs CH, Leach C, Kuo MC, et al. Inhalation of human insulin (Exubera) augments the efficiency of muscle glucose uptake in vivo. Diabetes. 2006a;55:3604–3610. doi: 10.2337/db06-0718. [DOI] [PubMed] [Google Scholar]
- Edgerton DS, Neal DW, Scott M, Bowen L, Wilson W, Hobbs CH, Leach C, Sivakumaran S, Strack TR, Cherrington AD. Inhalation of insulin (Exubera) is associated with augmented disposal of portally infused glucose in dogs. Diabetes. 2005;54:1164–1170. doi: 10.2337/diabetes.54.4.1164. [DOI] [PubMed] [Google Scholar]
- Edgerton DS, Stettler KM, Neal DW, Scott M, Bowen L, Wilson W, Hobbs CH, Leach C, Strack TR, Cherrington AD. Inhalation of human insulin is associated with improved insulin action compared with subcutaneous injection and endogenous secretion in dogs. J Pharmacol Exp Ther. 2006b;319:1258–1264. doi: 10.1124/jpet.106.108373. [DOI] [PubMed] [Google Scholar]
- Erman A, Chen-Gal B, David I, Giler S, Boner G, van Dijk DJ. Insulin treatment reduces the increased serum and lung angiotensin converting enzyme activity in streptozotocin-induced diabetic rats. Scand J Clin Lab Invest. 1998;58:81–87. doi: 10.1080/00365519850186869. [DOI] [PubMed] [Google Scholar]
- Fountaine R, Milton A, Checchio T, Wei G, Stolar M, Teeter J, Jaeger R, Fryburg D. Acute passive cigarette smoke exposure and inhaled human insulin (Exubera) pharmacokinetics. Br J Clin Pharmacol. 2008;65:864–870. doi: 10.1111/j.1365-2125.2008.03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galassetti P, Koyama Y, Coker RH, Lacy DB, Cherrington AD, Wasserman DH. Role of a negative arterial-portal venous glucose gradient in the postexercise state. Am J Physiol. 1999;277:E1038–E1045. doi: 10.1152/ajpendo.1999.277.6.E1038. [DOI] [PubMed] [Google Scholar]
- Galassetti P, Shiota M, Zinker BA, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient decreases skeletal muscle glucose uptake. Am J Physiol. 1998;275:E101–E111. doi: 10.1152/ajpendo.1998.275.1.E101. [DOI] [PubMed] [Google Scholar]
- Garg S, Rosenstock J, Silverman BL, Sun B, Konkoy CS, de la Peña A, Muchmore DB. Efficacy and safety of preprandial human insulin inhalation powder versus injectable insulin in patients with type 1 diabetes. Diabetologia. 2006;49:891–899. doi: 10.1007/s00125-006-0161-3. [DOI] [PubMed] [Google Scholar]
- Heise T, Bott S, Tusek C, Stephan JA, Kawabata T, Finco-Kent D, Liu C, Krasner A. The effect of insulin antibodies on the metabolic action of inhaled and subcutaneous insulin. Diabetes Care. 2005;28:2161–2169. doi: 10.2337/diacare.28.9.2161. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S. Angiotensin converting enzyme inhibitors and modulation of skeletal muscle insulin resistance. Diabetes Obes Metab. 2003a;5:214–222. doi: 10.1046/j.1463-1326.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol. 2003b;196:171–179. doi: 10.1002/jcp.10294. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension. 2001;38:884–890. doi: 10.1161/hy1101.092970. [DOI] [PubMed] [Google Scholar]
- Hermansen K, Rönnemaa T, Petersen AH, Bellaire S, Adamson U. Intensive therapy with inhaled insulin via the AERx insulin diabetes management system: a 12-week proof-of-concept trial in patients with type 2 diabetes. Diabetes Care. 2004;27:162–167. doi: 10.2337/diacare.27.1.162. [DOI] [PubMed] [Google Scholar]
- Hollander PA, Blonde L, Rowe R, Mehta AE, Milburn JL, Hershon KS, Chiasson JL, Levin SR. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care. 2004;27:2356–2362. doi: 10.2337/diacare.27.10.2356. [DOI] [PubMed] [Google Scholar]
- Hollander PA, Krasner A, Klioze S, Schwartz P, Duggan W. Body weight changes associated with insulin therapy: a retrospective pooled analysis of inhaled human insulin (Exubera) versus subcutaneous insulin in five controlled phase III trials. Diabetes Care. 2007;30:2508–2510. doi: 10.2337/dc06-2083. [DOI] [PubMed] [Google Scholar]
- Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens. 2005;23:463–473. doi: 10.1097/01.hjh.0000160198.05416.72. [DOI] [PubMed] [Google Scholar]
- Krulewitz AH, Baur WE, Fanburg BL. Hormonal influence on endothelial cell angiotensin-converting enzyme activity. Am J Physiol. 1984;247:C163–C168. doi: 10.1152/ajpcell.1984.247.3.C163. [DOI] [PubMed] [Google Scholar]
- Moore MC, Hsieh PS, Neal DW, Cherrington AD. Nonhepatic response to portal glucose delivery in conscious dogs. Am J Physiol Endocrinol Metab. 2000;279:E1271–E1277. doi: 10.1152/ajpendo.2000.279.6.E1271. [DOI] [PubMed] [Google Scholar]
- Moses RG, Bartley P, Lunt H, O’Brien RC, Donnelly T, Gall M-A, Vesterager A, Wollmer P, Roberts A. Safety and efficacy of inhaled insulin (AERx®iDMS) compared with subcutaneous insulin therapy in patients with type 1 diabetes one-year data from a randomized, parallel group trial. Diabet Med. 2009 doi: 10.1111/j.1464-5491.2008.02654.x. [DOI] [PubMed] [Google Scholar]
- Murphey LJ, Hachey DL, Vaughan DE, Brown NJ, Morrow JD. Quantification of BK1–5, the stable bradykinin plasma metabolite in humans, by a highly accurate liquid-chromatographic tandem mass spectrometric assay. Anal Biochem. 2001;292:87–93. doi: 10.1006/abio.2001.5073. [DOI] [PubMed] [Google Scholar]
- Patton JS, Bukar JG, Eldon MA. Clinical pharmacokinetics and pharmacodynamics of inhaled insulin. Clin Pharmacokinet. 2004;43:781–801. doi: 10.2165/00003088-200443120-00002. [DOI] [PubMed] [Google Scholar]
- Quattrin T, Bélanger A, Bohannon NJ, Schwartz SL. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care. 2004;27:2622–2627. doi: 10.2337/diacare.27.11.2622. [DOI] [PubMed] [Google Scholar]
- Rave K, Heise T, Pfützner A, Boss AH. Coverage of postprandial blood glucose excursions with inhaled technosphere insulin in comparison to subcutaneously injected regular human insulin in subjects with type 2 diabetes. Diabetes Care. 2007;30:2307–2308. doi: 10.2337/dc07-0478. [DOI] [PubMed] [Google Scholar]
- Sharifi AM, Mousavi SH, Larijani B. Study of interaction between nitric oxide and ACE activity in STZ-induced diabetic rats: role of insulin. Pharmacol Res. 2004;50:261–266. doi: 10.1016/j.phrs.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Weinstock RS, Raskin P, Yale JF, Barrett E, Gerich JE, Gerstein HC. Use of inhaled insulin in a basal/bolus insulin regimen in type 1 diabetic subjects: a 6-month, randomized, comparative trial. Diabetes Care. 2005;28:1630–1635. doi: 10.2337/diacare.28.7.1630. [DOI] [PubMed] [Google Scholar]