Abstract
Angiotensin II (Ang II) regulates adrenal steroidogenesis and adrenal cortical arterial tone. Vascular metabolism could decrease Ang II concentrations and produce metabolites with vascular activity. Our goal was to study adrenal artery Ang II metabolism and to characterize metabolite vascular activity. Bovine adrenal cortical arteries were incubated with Ang II (100 nmol/L) for 10 and 30 min. Metabolites were analyzed by mass spectrometry. Ang (1-7), Ang III and Ang IV concentrations were 146±21, 173±42 and 58±11 pg/mg at 10 min and 845±163, 70±14 and 31±3 pg/mg at 30 min, respectively. Concentration-related relaxations of U46619-preconstricted cortical arteries to Ang II (maximum relaxation=29±3%, EC50=3.4 pmol/L) were eliminated by endothelium removal and inhibited by the NO synthase inhibitor, nitro-l-arginine (L-NA, 30 μmol/L, maximum relaxation=14±7%). Ang II relaxations were enhanced by the angiotensin type-1 receptor antagonist, losartan (1 μmol/L, maximum relaxation=41±3%, EC50=11 pmol/L). Losartan-enhanced Ang II relaxations were inhibited by L-NA (maximum relaxation=18±5%) and the angiotensin type-2 receptor antagonist PD123319 (10 μmol/L, maximum relaxation=27±5%). Ang (1-7) and Ang III caused concentration-related relaxations with less potency (EC50= 43 and 24 nmol/L, respectively) but similar efficacy (maximum relaxations=39±3%, 48±5%, respectively) as losartan-enhanced Ang II relaxations. Ang (1-7) relaxations were inhibited by L-NA (maximum relaxation=16±4%) and the Ang (1-7) receptor antagonist, 7D-Ala-Ang (1-7) (1 μmol/L, maximum relaxation=10±3%) and eliminated by endothelium removal. Thus, Ang II metabolism by adrenal cortical arteries to metabolites with decreased vascular activity represents an inactivation pathway possibly decreasing Ang II presentation to adrenal steroidogenic cells and limits Ang II vascular effects.
Keywords: Angiotensin (1-7), angiotensin III, angiotensin IV, mass spectrometry
Introduction
Angiotensin II (Ang II) is a major regulator of vascular resistance, adrenal steroid hormone release, electrolyte and blood volume and systemic blood pressure (see reviews 1–5). Ang II is produced by the conversion of Ang I to Ang II by angiotensin converting enzyme (ACE). Numerous cardiovascular diseases including hypertension and heart failure have been linked to increased activity of the renin-angiotensin system (4,5). Within the past decade increasing attention has focused on the role of the angiotensin heptapeptide metabolite, angiotensin 1-7 (Ang (1-7)). Ang (1-7) is produced primarily through the action of the ACE homologue, ACE2 (5–9). ACE2 removes the carboxy-terminal phenylalanine of Ang II to produce Ang (1-7). Other enzymes may convert Ang II to Ang (1-7) including neprilysin (9), but their role in the physiological production in Ang (1-7) has not been clearly defined.
Ang (1-7) has numerous biological effects including vasorelaxation at low concentrations, vasoconstriction at high concentrations, blockade of Ang II constriction, inhibition of ACE, alteration of cardiac output, ischemic cardiac protection and anti-fibrotic and proliferative effects (see reviews 2,5,7,10,11). Thus, Ang (1-7) displays a myriad of cardiovascular protective properties. The biological effects of the Ang II metabolites Ang III and Ang IV, have not been as thoroughly examined. However, Ang III shares many biological activities as Ang II including vasodilation (11,12). Similarly, Ang IV has been shown to cause vascular dilation (11) and increases cell proliferation (13).
Adrenal blood flow is closely coupled to steroidogenesis. Increases in adrenal flow alone can stimulate steroid hormone release (14). Increased vascular resistance of adrenal cortical arteries restricts blood flow and decreases adrenal hormone release. In isolated bovine small cortical arteries, Ang II causes a biphasic response on vascular diameter. At low concentrations, Ang II causes dilations which reverses to constriction at higher concentrations (> 10 nmol/L) (15). Changes in circulating Ang II concentrations or local metabolism of Ang II could alter cortical arterial tone and cortical blood flow to alter adrenal hormone release. Additionally, cortical metabolism of Ang II could limit Ang II presentation to adrenal steroidogenic cells.
Previously, we demonstrated that adrenal zona glomerulosa cells and adrenal vascular endothelial cells metabolize Ang II to several smaller peptides including Ang III, Ang IV and Ang (1-7) (16,17). While Ang III is equipotent to Ang II in stimulating steroidogenesis (18,19), Ang IV and Ang (1-7) are inactive (16). Ang II metabolism by intact adrenal arteries has not been evaluated and thus, the consequence of this metabolism on adrenal arterial vascular tone has not characterized.
Methods
Isometric tension recording
Fresh bovine adrenal glands were obtained from a local abattoir. Small cortical arteries closely attached to the adrenal surface (200–300 μm) were dissected in HEPES buffer (in mmol/L: HEPES 10, NaCl 150, KCl 5, CaCl2 2, MgCl21, glucose 6, pH 7.4), cleaned of connective tissue and mounted in a 4-chamber wire myograph (model 610M, Danish Myo Technology A/S) as previously described (20,21). Arteries were maintained at 37°C in physiological saline solution (PSS) (in mmol/L: NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4 1.17, NaHCO3 24, KH2PO4 1.18, EDTA 0.026, and glucose 5.5), gassed with 95% O2/5% CO2. Arteries were stretched to a resting tension of 1 millinewton (mN) and stimulated 2–3 times with KCl (60 mmol/L) plus the thromboxane mimetic, U46619 (100 nmol/L) for 10 min at 10 min intervals. Arteries were contracted with submaximal concentrations of U46619 (50–300 nmol/L) to 50–75% of their maximum KCl and U46619 challenge. Where indicated, the endothelium was removed by gently rubbing the arterial intimal surface with a human hair. The endothelium was considered intact if acetylcholine (1 μmol/L) caused >50% relaxation and effectively removed if acetylcholine induced <10% relaxation. Cumulative concentration responses to Ang II (0.1 pmol/L–10 nmol/L), Ang III (10 pmol/L–10 nmol/L) and Ang (1-7) (10 pmol/L–10 nmol/L) were performed. Responses were repeated in arterial segments pretreated with the AT1 receptor antagonist, losartan (1 μmol/L), the AT2 receptor antagonist, PD123319 (1–10 μmol/L), the Ang (1-7) receptor antagonist, [7-D-Ala]-Ang (1-7) (Ala7-Ang (1-7), 1 μmol/L) or the nitric oxide (NO) synthase inhibitor nitro-L-arginine (L-NA, 30 μmol/L) either separately or with losartan. Results are expressed as % relaxation of the U46619-treatedarteries with 100% representing basal tension.
Angiotensin incubations and extractions
Adrenal cortical arteries (35 ± 3 mg tissue, wet weight/incubation) were incubated in 2 ml HEPES buffer at 37°C with Ang II (100 nmol/L) for 10 and 30 min. The media was removed and an internal standard (13C515N1-Ang IV, 2.0 ng) was added. Samples were extracted as previously described (17). Briefly, formic acid was added (final concentration of 0.5%) and the samples were applied to Waters C18 SPE cartridges that had been preconditioned with 5 ml of ethanol and 15 ml deionized water, respectively. The cartridges were washed with deionized water (3 ml) and the columns were dried by vacuum. Angiotensin peptides were eluted with 7 ml methanol containing 5% formic acid and dried under a stream of nitrogen gas. For liquid chromatography-mass spectrometry (LC-MS) and LC-MS/MS analysis preparation, samples were redissolved in 30 μl of 16% acetonitrile in water containing 0.1% formic acid.
LC-MS and LC-MS/MS analyses
Analyses were performed using a Waters-Micromass Quattro micro™ API electrospray triple quadrupole mass spectrometric system coupled with a Waters 2695 high performance liquid chromatograph as previously described (17). The mass spectrometer is equipped with a Z-spray dual orthogonal ionization source and is controlled by MassLynx 4.0 software (Waters Corporation, Milford, MA, USA). Samples were separated on a reverse-phase C18 column (Jupiter 2.0 × 250 mm, Phenomenex) using water-acetonitrile with 0.1% formic acid as a mobile phase at a flow rate of 0.2 ml/min at ambient room temperature. The mobile phase of 16% acetonitrile in water linearly increased to 38% acetonitrile over 10 min, then to 100% acetonitrile over the final 15 min. Positive ion electrospray ionization (ESI) mass spectrometric conditions were as follows: capillary voltage, 3.5 kV; cone voltage, 15 ~18 V; desolvation temperature, 300°C; desolvation gas flow, 1000 l/h; source temperature, 120°C. LC-MS analysis was performed in positive electrospray ionization mode in the single ion recording (SIR) mode. m/z of 300.7, 311.8, 350.0, 388.8 and 391.3 were used for Ang (1-7), Ang III, Ang II, Ang IV and the internal standard, respectively.
LC-MS/MS was performed with multiple reaction monitoring (MRM) of transitions: m/z 300.6-->109.6 (Ang (1-7)), m/z 311.3→228.4 (Ang III), m/z 349.6→255.2 (Ang II), m/z 388.8→263.4 (Ang IV), and m/z 391.3→269.2 (internal standard). Argon gas was used as collision gas and the collision voltages were set at 27–30 eV. Standard calibration curves were constructed over the range of 5 pg to 625 pg for LC-MS and 50 pg to 1250 pg for LC-MS/MS per injection. Sample angiotensin peptide concentrations were determined by comparing ratios of the peak areas to the standard calibrations.
Drugs and chemicals
Ang (1-7), nitro-L-arginine (L-NA), PD123319 and indomethacin were purchased from Sigma (St. Louis, MO). U46619 was obtained from Cayman Chemical Company (Ann Arbor, MI). Ang II, Ang III, Ang IV and Ala7-Ang (1-7) were purchased from BACHEM (King of Prussia, PA, USA). Losartan was a kind gift from Merck (Whitehouse Station, NJ, USA). The 13C515N1-Ang IV internal standard was synthesized by the Protein Nucleic Acid Facility of the Medical College of Wisconsin. All solvents were HPLC grade and purchased from Burdick and Jackson.
Data analysis
Data are presented as mean ± SEM. Significant differences between mean values were evaluated by Student t test or ANOVA followed by the Student-Newman-Keuls multiple comparison test. EC50 values were calculated using GraphPad Prism analysis software (GraphPad Software, San Diego, CA). A value of P<0.05 was considered statistically significant.
Results
Role of the endothelium in Ang II relaxations
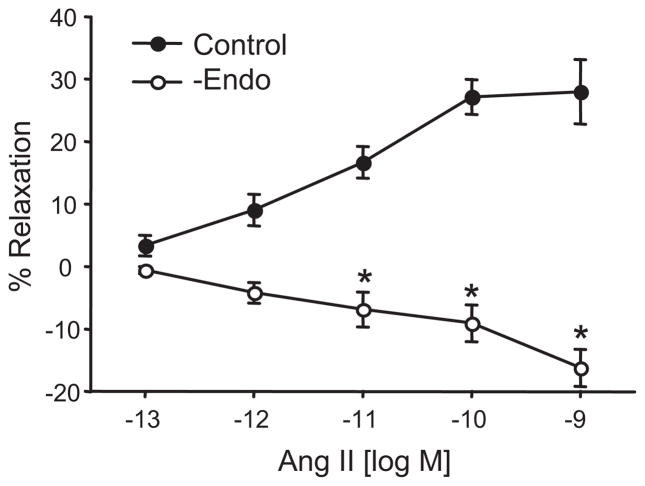
Small adrenal cortical arteries were contracted with the thromboxane mimetic, U46619 and responses to increasing Ang II concentrations were determined (Figure 1). Ang II caused concentration-dependent relaxations (maximum relaxation = 28±5% at 10 nmol/L). The relaxations were eliminated by endothelium removal. Thus, Ang II causes vascular relaxation through endothelium-dependent mechanisms. Relaxations to Ang II could occur through endothelial cell Ang II metabolism to vasoactive peptides and/or through direct stimulation of endothelial cell relaxing factor release.
Figure 1.
Effect of the endothelium on Ang II-induced relaxations of isolated bovine adrenal cortical arteries. Relaxations were performed under control conditions or after endothelial removal. n = 4–13. * p< 0.05 vs. control.
Identification and quantification of Ang II metabolites by adrenal cortical arteries
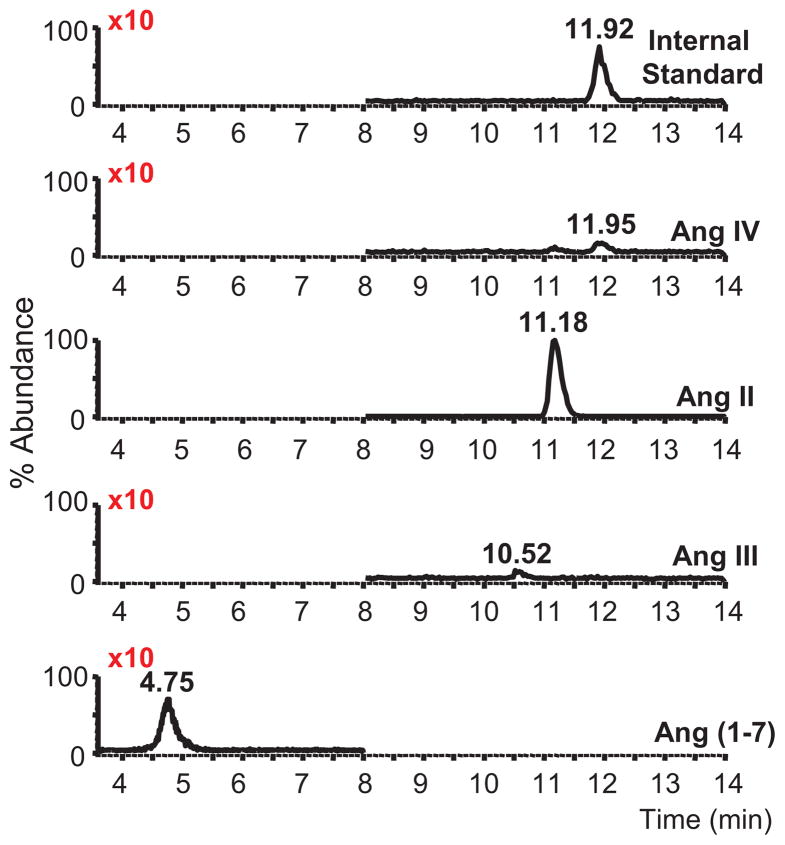
To clarify the role of metabolites in Ang II relaxations, we examined Ang II metabolism by freshly isolated adrenal cortical arteries. Cortical arteries were incubated with Ang II (100 nmol/L) for 30 min. The buffer was removed, extracted and analyzed for angiotensin peptides by LC-MS/MS. The LC-MS/MS chromatogram in Figure 2 shows recovery of the internal standard and the presence of Ang IV, Ang II, Ang III, and Ang (1-7). Abundance of the internal standard, Ang IV, Ang III, and Ang (1-7) were amplified 10X for the demonstration of the relative production of the peptides. The predominant metabolite was Ang (1-7). LC-MS scan mode analysis of the Ang III and Ang (1-7) sample peptides showed strong (M + 3H)3+ ions at m/z 300.7 and 350.0, respectively, and (M + 2H)2+ions at m/z 524.6 and 450.5, respectively (data not shown). These results are consistent with the mass spectra for Ang III and Ang (1-7) (17).
Figure 2.
LC-MS/MS chromatograms of angiotensin peptides produced by bovine adrenal cortical arteries. Isolated arteries were incubated with Ang II (100 nmol/L) and the peptides extracted and analyzed. Synthetic 13C515N1-Ang IV (top panel) was added as an internal standard (m/z 391.3→269.2, elution time = 11.92 min). Angiotensin peptides identified included Ang IV (m/z 388.8→263.4, elution time = 11.92 min), non-metabolized Ang II (m/z 349.6→255.2, elution time = 11.18 min), Ang III (m/z 311.3→228.4, elution time = 10.55) and Ang (1-7) (m/z 300.6→109.6, elution time = 4.77). The abundance of the internal standard, Ang IV, Ang III, and Ang (1-7) were amplified 10X.
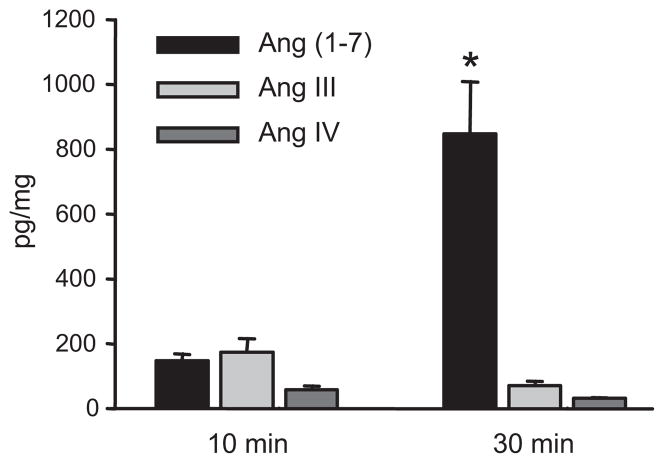
Cortical arteries were incubated with Ang II (100 nmol/L) for 10 and 30 min and angiotensin peptides were extracted and quantified by LC-MS/MS. Metabolite peptides eluted at 4.75, 10.52 and 11.95 min which comigrated with Ang (1-7), Ang III and Ang IV, respectively. The mass spectra of the 4.75 min peak had major ions of m/z 300.7 and 450.5 and the 10.52 min peak had major ions of m/z 350 and 524.6. These mass spectra are consistent with the formation of Ang (1-7) and Ang III. The signal of the 11.95 peak was too weak in the scan mode to give a clear mass spectra. Ang (1-7), Ang III and Ang IV concentrations in the buffer increased at 10 min and 30 min (Figure 3). The concentration of the Ang (1-7) significantly increased at the 30 min incubation time point as compared to the other peptides. Thus, Ang II is metabolized by adrenal cortical arteries primarily to Ang (1-7) with lesser metabolism to Ang III and Ang IV.
Figure 3.
Time course of Ang II metabolism by bovine adrenal cortical arteries. Isolated arteries were incubated with Ang II (100 nmol/L) for 10 and 30 min. The peptides were extracted and analyzed by LC-MS/MS. n=3–4. * p< 0.05 vs. other peptides.
Role of NO and AT1 and Ang (1-7) receptors in Ang II relaxations
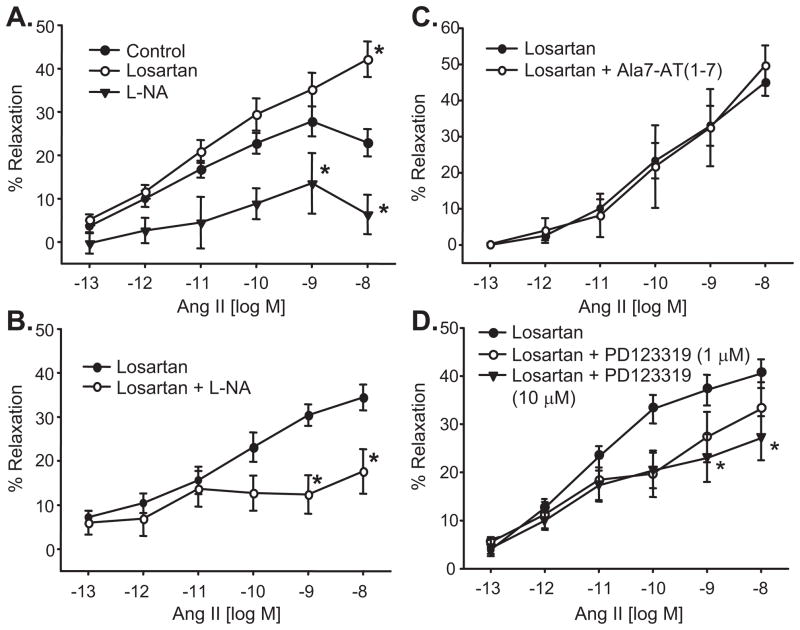
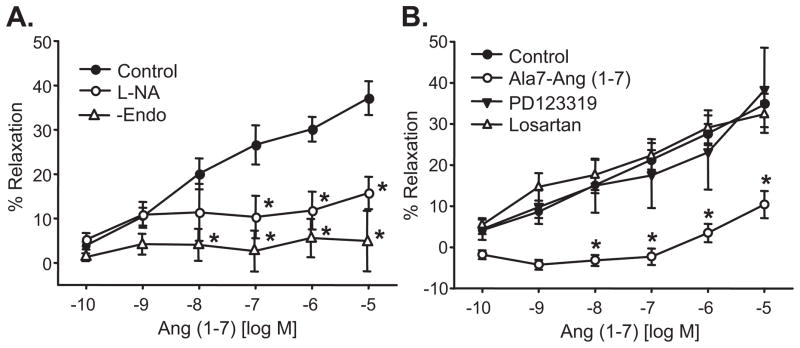
Ang II relaxations were significantly enhanced by the AT1 receptor blocker, losartan (1μmol/L) (maximum relaxation = 46±4% at 10 nmol/L) (Figure 4A). Ang II relaxations and the losartan-enhanced relaxations to Ang II were inhibited by the NO synthase inhibitor, L-NA (30μmol/L) (maximum relaxation = 15±7% and 18±5%, respectively, (Figure 4A,B). The Ang (1-7) receptor antagonist, Ala7-Ang (1-7) (1 μmol/L) was without effect (Figure 4C). The AT2 receptor antagonist, PD123319 inhibited the losartan-enhanced relaxations in a concentration-dependent manner (Figure 4D). Significant inhibition was observed at 10 μmol/L PD123319. These results suggest that Ang II relaxations are mediated in part by AT2 receptor activation and are independent of AT1 and Ang (1-7) receptors. Conversely, AT1 receptor activation causes vascular constriction. Additionally, Ang (1-7) relaxations are mediated by endothelial cell NO.
Figure 4.
Ang II-induced relaxations of isolated bovine adrenal cortical arteries. A. Effect of the NO synthase inhibitor, L-NA (30 μmol/L) and the AT1 receptor antagonist, losartan (1 μmol/L). Relaxations were performed under control conditions or after treatment with losartan or L-NA. B. Effect of L-NA on losartan-enhanced Ang II relaxations. Relaxations were performed on losartan-treated arteries before and after treatment with L-NA. C. Effect of the Ang (1-7) receptor antagonist, Ala7-Ang (1-7) (1 μmol/L) on losartan-enhanced Ang II relaxations. Relaxations were performed on losartan-treated arteries before and after treatment with Ala7-Ang (1-7). D. Effect of the AT2 receptor antagonist, PD123319 (1 and 10 μmol/L) on losartan-enhanced Ang II relaxations. Relaxations were performed on losartan-treated arteries before and after treatment with PD123319. n = 5–34, * p< 0.05 vs. control or losartan.
Ang III and Ang (1-7) relaxations
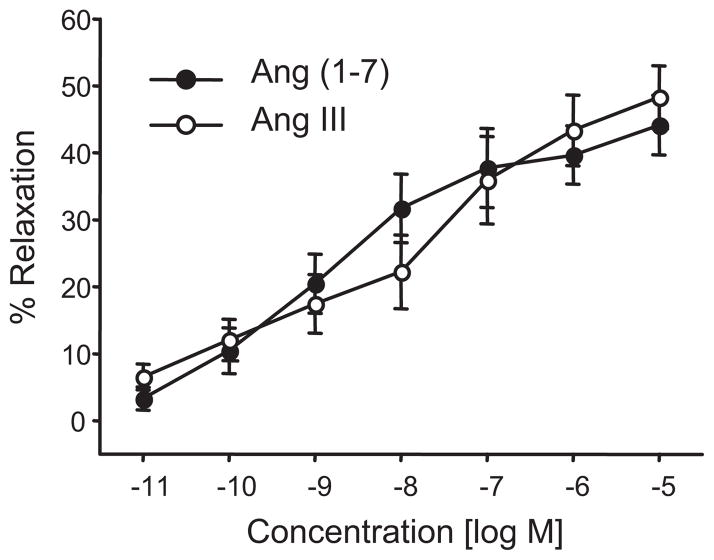
Next, we evaluated the relaxation responses of the adrenal cortical arteries to Ang III and Ang (1-7). Ang III caused concentration-related relaxations with maximal relaxations of 48±5% at 10 μmol/L (Figure 5). Similar relaxations were observed with Ang (1-7) (maximum relaxation of 44±4%). The averaged half maximal effective concentrations (EC50’s) and averaged maximal relaxations of Ang II and the angiotensin metabolites are provided in Table 1. Relaxations to Ang III and Ang (1-7) displayed similar efficacy as the losartan-enhanced Ang II relaxations but were less potent. This suggests that metabolism of Ang II to Ang III or Ang (1-7) represents an inactivation pathway to possibly limit Ang II relaxations.
Figure 5.
Concentration-dependent relaxations to Ang (1-7) and Ang III of isolated bovine adrenal cortical arteries. n = 7–10.
Table 1.
Average maximal relaxations and EC50’s of angiotensin relaxations
| Angiotensin treatment | Ang II | Ang II (Losartan-treated) | Ang III | Ang (1-7) |
|---|---|---|---|---|
| Maximum relaxation | 29±3% (10 nmol/L) | 41±3% (100 nmol/L) | 48±5% (10 μmol/L) | 39±3% (10 μmol/L) |
| EC50 | 3.4 pmol/L | 11 pmol/L | 24 nmol/L | 43 nmol/L |
Because the predominant Ang II metabolite was Ang (1-7), we further investigated the mechanisms of Ang (1-7) relaxations. Ang (1-7)-induced relaxations were eliminated by endothelial cell removal and inhibited by the NOS inhibitor, L-NA (maximum relaxation = 16±4%) (Figure 6A). Inhibition of AT1 and AT2 receptors with losartan or PD123319, respectively, did not alter the Ang (1-7) relaxations whereas the Ang (1-7) receptor antagonist, Ala7-Ang (1-7) caused significant inhibition (maximum relaxation = 10±3%) (Figure 6B). Thus, Ang (1-7) relaxations occur through the activation of Ang (1-7) receptors and similar to Ang II, the relaxations are mediated by endothelial cell NO.
Figure 6.
Ang (1-7)-induced relaxations of isolated bovine adrenal cortical arteries. A. Effect of the NO synthase inhibitor, L-NA (30 μmol/L) and endothelium removal. Responses to Ang (1-7) were performed before and after treatment with L-NA or after endothelial cell removal. B. Effect of the Ang (1-7) receptor antagonist, Ala7-Ang (1-7) (1 μmol/L), the AT1 receptor antagonist, losaratan (1 μmol/L) or the AT2 receptor antagonist, PD123319 (10 μmol/L). Responses were performed before and after receptor antagonist treatment. n = 6–21. * p< 0.05 vs. control.
Discussion
This is the first study to demonstrate Ang II metabolism in intact arteries. Importantly, these studies were performed in small resistance-sized adrenal cortical arteries which are a primary site for the regulation of adrenal cortical blood flow (22). The physiological implications for Ang II metabolism at this site are two-fold. First, because Ang II causes dynamic changes in vascular tone of these arteries (i.e. dilations at low concentrations and constrictions at high concentrations (15)), subtle changes in Ang II concentrations by vascular metabolism could alter vascular resistance and therefore adrenal cortical blood flow. Secondly, Ang II metabolism would decrease Ang II concentrations and increase metabolite concentrations presented to the steroidogenic cells. This alteration in Ang II/metabolite ratios could alter aldosterone release and secondarily, blood volume and blood pressure.
In bovine adrenal cortical arteries, relaxations to Ang II were inhibited by the removal of the endothelium. However, it was not evident if the relaxations occur through Ang II metabolism to vasoactive peptides that activate relaxing factor release or through the direct action of Ang II. Ang II metabolism to Ang III, Ang IV and Ang (1-7) was demonstrated in endothelial cells from bovine adrenal arteries and human aorta and human umbilical veins (17, 23). The contribution of the smooth muscle to the Ang II metabolism has not been clarified, although ACE2 transcriptional expression has been demonstrated in human coronary, cerebral, pulmonary, renal and mesenteric arteries (24). In the intact adrenal cortical arteries from this study, metabolism of Ang II to Ang (1-7) does not appear to contribute to the vascular relaxations to Ang II. This was evident because the Ang (1-7) receptor antagonist did not alter the relaxations to Ang II. In contrast, in rat carotid arteries, Ang II relaxations are partially dependent on Ang II metabolism to Ang (1-7) (25). Thus, it appears that the role of angiotensin metabolites in Ang II relaxation vary with the vasculature and/or species.
Ang II relaxations were mediated by endothelial cell NO. This was demonstrated in the current study and a previous study of perfused bovine adrenal cortical arteries by the inhibition of the Ang II relaxations/dilations by L-NA (15). As reviewed by Toda and colleagues (11), the role of the endothelium and NO production in Ang II and Ang (1-7) relaxations has been also demonstrated in numerous arteries from various species. Thus, our results fit the paradigm of NO as the primary effector of Ang II relaxations. Similarly, adrenal cortical arterial relaxations to Ang (1-7) were mediated by endothelial NO production. Because the relaxations induced by Ang (1-7) were blocked by the Ang (1-7) receptor antagonist, we conclude that this receptor activates endothelial cell signaling cascades that culminate with NO release.
Relaxations to Ang III and Ang (1-7) were more than 1000 fold less potent that Ang II relaxations. Therefore, Ang II metabolism to these metabolites should not significantly contribute to Ang II relaxations. However, Ang II metabolism and the impact on adrenal arterial vascular tone may be more important during times of high Ang II. Under this scenario, Ang II vascular constriction would overcome Ang II relaxation. Because Ang III and Ang (1-7) did not cause vascular constriction in the adrenal arteries, their relaxing properties could counterbalance Ang II constrictions. Alternatively, high concentrations of Ang (1-7) infusion in humans reduced forearm blood flow (26) and in rats reduced blood flow to the kidney, mesentery and skin (27).
The consequence of cortical artery Ang II metabolism on the concentrations of Ang II and Ang II metabolites that reach the adrenal steroidogenic cells depends on the steroidogenic activity of the metabolites. In this regard, Ang III was equipotent to Ang II in stimulating aldosterone and corticosterone production in conscious rats and aldosterone production from rat adrenal cortical cells (18,19). Alternatively, Ang IV and Ang (1-7) did not stimulate aldosterone production in bovine adrenal zona glomerulosa cells (16). Since the primary Ang II metabolite from the cortical arteries was Ang (1-7), we predict that vascular Ang II metabolism would reduce Ang II stimulation of steroid hormone release.
The role of Ang (1-7) as an antihypertensive peptide has not been demonstrated. In the kidney, the effects of Ang (1-7) on natriuresis and diuresis have been mixed (28). Therefore, other mechanisms must counteract the direct vasodilatory effects of Ang (1-7). Adrenal mechanisms including an increase in blood flow and the consequent increase in aldosterone release could compensate for the systemic vascular actions of Ang (1-7). Further studies will be required to address this possibility.
Perspectives.
The regulation of adrenal blood flow is multifaceted and include neural and humoral input (29). An additional level of regulation results from the interchange and cross talk between the adrenal vasculature and steroidogenic cells. Relaxing factor(s) released by the cortical steroidogenic cells influence cortical arterial tone (21). Conversely, factors originating from the adrenal vasculature influence adrenal steroidogenesis. Endothelial cell NO inhibits aldosterone secretion (30) whereas an endothelium-derived peptide stimulates aldosterone production (31). The results from the current study suggest that vascular Ang II metabolism could influence the level of Ang II-dependent regulation of vascular tone as well as modulate the concentrations of Ang II available for the stimulation of steroidogenesis.
Acknowledgments
We thank Gretchen Barg for secretarial assistance and Andrea Forgianni, Sarah Christian, Marilyn Isbell and Dr. Xiu-Yu Yi for technical assistance.
Sources of Funding
These studies were supported by a grant from the National Heart, Lung and Blood Institute (HL-83297). D.X.Z. was a postdoctoral fellow of the American Heart Association, Greater Midwest Affiliate and a recipient of the Jenkins Cardiovascular Research Fellowship. The LC-MS/MS was provided by an instrumentation grant from the National Institute of Research Resources (RR-17824).
Footnotes
Conflicts of Interest (none)
References
- 1.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 2.Keidar S, Kaplan M, Gamliel-Lazarovich A. ACE2 of the heart: From angiotensin I to angiotensin (1-7) Cardiovasc Res. 2007;73:463–469. doi: 10.1016/j.cardiores.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Krum H, Gilbert RE. Novel therapies blocking the renin-angiotensin-aldosterone system in the management of hypertension and related disorders. J Hypertens. 2007;25:25–35. doi: 10.1097/HJH.0b013e3280113950. [DOI] [PubMed] [Google Scholar]
- 5.Raizada MK, Ferreira AJ. ACE2: A new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- 6.Tipnis SR, Hooper NM, Hyde R, Karran EH, Christie G, Turner AJ. A human homolog of angiotensin converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 7.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 8.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 9.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos AS, Ferreira AJ. Angiotensin-(1-7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 11.Toda N, Ayajiki K, Okamura T. Interaction of endothelial nitric oxide and angiotensin in the circulation. Pharmacol Rev. 2007;59:54–87. doi: 10.1124/pr.59.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Ardaillou R, Chansel D. Synthesis and effects of active fragments of angiotensin II. Kidney Int. 1997;52:1458–1468. doi: 10.1038/ki.1997.476. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Ortega M, Esteban V, Egido J. The regulation of the inflammatory response through nuclear factor-κβ pathway by angiotensin IV extends the role of the renin angiotensin system in cardiovascular diseases. Trends Cardiovascul Med. 2007;17:19–25. doi: 10.1016/j.tcm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Hinson JP, Vinson GP, Whitehouse BJ. The relationship between perfusion medium flow rate and steroid secretion in the isolated perfused rat adrenal gland in situ. J Endocrinol. 1986;111:391–396. doi: 10.1677/joe.0.1110391. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier KM, Zhang DX, Edwards EM, Holmes B, Campbell WB. Angiotensin II dilates bovine adrenal cortical arterioles: role of endothelial nitric oxide. Endocrinology. 2005;146:3319–3324. doi: 10.1210/en.2005-0129. [DOI] [PubMed] [Google Scholar]
- 16.Hanke CJ, Holmes BB, Xu Y, Nithipatikom K, Campbell WB. Endothelium-derived steroidogenic factor enhances angiotensin II-stimulated aldosterone release by bovine zona glomerulosa cells. Endocrinology. 2007;148:317–323. doi: 10.1210/en.2006-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui L, Nithipatikom K, Campbell WB. Simultaneous analysis of angiotensin peptides by LC-MS and LC-MS/MS: metabolism by bovine adrenal endothelial cells. Anal Biochem. 2007;369:27–33. doi: 10.1016/j.ab.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell WB, Gomez-Sanchez CE, Adams BV, Schmitz JM, Itkovitz HD. Attenuation of angiotensin II and III-induced aldosterone release by prostaglandin synthesis inhibitors. J Clin Invest. 1979;64:1552–1557. doi: 10.1172/JCI109615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell WB, Pettinger WA. Organ specificity of angiotensin II and des-aspartyl angiotensin II in the conscious rat. J Pharmacol Exp Ther. 1976;198:450–456. [PubMed] [Google Scholar]
- 20.Zhang DX, Gauthier KM, Campbell WB. Characterization of vasoconstrictor responses in small bovine adrenal cortical arteries in vitro. Endocrinology. 2004;145:1571–1578. doi: 10.1210/en.2003-1448. [DOI] [PubMed] [Google Scholar]
- 21.Zhang DX, Gauthier KM, Falck JR, Siddam A, Campbell WB. Steroid-producing cells regulate arterial tone of adrenal cortical arteries. Endocrinology. 2007;148:3569–3576. doi: 10.1210/en.2007-0169. [DOI] [PubMed] [Google Scholar]
- 22.Vinson GP, Hinson JP, Toth IE. The neuroendocrinology of the adrenal cortex. J Neuroendocrinol. 1994;6:235–246. doi: 10.1111/j.1365-2826.1994.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 23.Santos RA, Brosnihan KB, Jacobsen DW, DiCorleto PE, Ferrario CM. Production of angiotensin-(1-7) by human vascular endothelial vascular endothelium. Hypertension. 1992;19(suppl II):II56–II61. doi: 10.1161/01.hyp.19.2_suppl.ii56. [DOI] [PubMed] [Google Scholar]
- 24.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 25.Tirapelli CR, Fukada SY, de Godoy MAF, de Oliveira AM. Analysis of the mechanisms underlying the vasorelaxant action of angiotensin II in the isolated rat carotid. Life Sci. 2006;78:2676–2682. doi: 10.1016/j.lfs.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Ueda S, Masumori-Maemoto S, Ashino K, Nagahara T, Gotoh E, Umemura S, Ishii M. Angiotensin-(1-7) attenuates vasoconstriction evoked by angiotensin II but not by noradrenaline in man. Hypertension. 2000;35:998–1001. doi: 10.1161/01.hyp.35.4.998. [DOI] [PubMed] [Google Scholar]
- 27.Sampaio WO, Nascimento AAS, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 28.Burns KD. The emerging role of angiotensin-converting enzyme-2 in the kidney. Curr Opin Nephrol Hypertens. 2007;16:116–121. doi: 10.1097/MNH.0b013e3280123c0e. [DOI] [PubMed] [Google Scholar]
- 29.Bassett JR, West SH. Vascularization of the adrenal cortex: its possible involvement in the regulation of steroid hormone release. Microsc Res Tech. 1997;36:546–557. doi: 10.1002/(SICI)1097-0029(19970315)36:6<546::AID-JEMT11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Hanke CJ, Campbell WB. Endothelial cell nitric oxide inhibits aldosterone synthesis in zona glomerulosa cells: modulation by oxygen. Am J Physiol Endocrinol Metab. 2000;279:E846–E854. doi: 10.1152/ajpendo.2000.279.4.E846. [DOI] [PubMed] [Google Scholar]
- 31.Rosolowsky LJ, Campbell WB. Endothelial cells stimulate aldosterone production from bovine adrenal zona glomerulosa cells. Am J Physiol Endocrinol Metab. 1994;266:E107–E117. doi: 10.1152/ajpendo.1994.266.1.E107. [DOI] [PubMed] [Google Scholar]