Abstract
The abuse liability of the analgesic bicifadine was investigated in animal models used to predict the abuse potential of psychostimulants in humans. Bicifadine, cocaine, d-amphetamine, bupropion, and desipramine were evaluated for the production of cocaine-like discriminative stimulus effects in rats. Cocaine, d-amphetamine, and bupropion dose-dependently and fully substituted for cocaine. Bicifadine and desipramine produced a maximum mean cocaine-lever selection of 80 and 69%, respectively, but doses yielding peak substitution strongly suppressed response rates. Microdialysis studies in normal waking rats indicated that d-amphetamine increased dopamine levels in the nucleus accumbens and striatum to a much greater degree than bicifadine, but bicifadine increased 5-hydroxytryptamine levels in the nucleus accumbens and striatum more than d-amphetamine. Bicifadine was also tested for intravenous self-administration in rhesus monkeys experienced with cocaine administration. Reinforcing effects of bicifadine were observed in only two of four subjects, whereas cocaine, d-amphetamine, and bupropion served as reinforcers in all four monkeys. When evaluated under a progressive ratio procedure, no dose of bicifadine maintained responding to the extent of cocaine, d-amphetamine, or bupropion. The discriminative stimulus effects associated with bicifadine were similar, but not identical, to those of psychostimulants. Although bicifadine maintained self-administration behavior in some subjects, its reinforcing efficacy was very low relative to cocaine, d-amphetamine, and bupropion. These results are consistent with the microdialysis findings of lower dopamine levels and higher 5-hydroxytryptamine levels after administration of bicifadine relative to d-amphetamine. Overall, the current findings support a low abuse potential of bicifadine, more resembling that of antidepressants than psychostimulants.
Abnormal levels of the monoamine neurotransmitters serotonin (5-HT), norepinephrine (NE), and dopamine (DA) have been implicated in the pathogenesis of a variety of neuropsychiatric disorders, including depression, neuropathic pain, obesity, and substance abuse. Compounds that inhibit one or more of the transporters for these monoamine neurotransmitters are used to treat many of these disorders. For example, selective inhibitors of 5-HT reuptake (e.g., fluoxetine, citalopram) are effective, albeit imperfect, therapies for major depressive disorder, generalized anxiety disorder, and obsessive-compulsive disorder (Vaswani et al., 2003). Agents that inhibit both 5-HT (SERT) and NE (NET) transporters (e.g., duloxetine and venlafaxine) not only are used to treat major depressive disorder but also are effective as analgesics, particularly in treating chronic neuropathic pain (Barkin and Barkin, 2005). Selective NET inhibitors (e.g., atomoxetine) are currently being used to treat attention deficit disorder (Kratochvil et al., 2003). Because of the involvement of DA in mesocorticolimbic pathways that subserve reward- and incentive-driven behaviors (Wise, 2002), as well as in motor pathways regulated by the caudate-putamen, compounds that inhibit dopamine transporters (DATs), alone or in combination with other monoaminergic actions, often possess a psychomotor stimulant. This profile consists of motor activation and positive motivational state, which is associated with an increased potential for abuse (Riddle et al., 2005).
Although there is evidence to indicate that blockade of dopamine uptake per se is not the sole contributory neurochemical property underlying abuse liability (Hnasko et al., 2007; Loland et al., 2008), the potential for abuse of agents that incorporate DAT inhibition as a constituent of their therapeutic mechanism of action remains. Moreover, the abuse potential associated with compounds that inhibit all three transporters (triple reuptake inhibitors) is unclear. These agents have the potential for broad therapeutic use in treating depression (Skolnick and Basile, 2007), pain (Basile et al., 2007), obesity (Tizzano et al., 2008), and substance abuse (McMillen et al., 2007).
Bicifadine (1-p-tolyl-3-azabicyclo[3.1.0]hexane) has antinociceptive properties in a wide variety of preclinical models, including both acute and persistent inflammatory pain, visceral pain, and chronic neuropathic pain (Basile et al., 2007). Clinical (phase II/III) studies have demonstrated that bicifadine is an effective analgesic in the treatment of postoperative pain (Krieter et al., 2008). Clinical efficacy in neuropathic pain has not yet been established. Bicifadine inhibits the uptake of [3H]5-HT and [3H]NE in cell lines expressing human SERT and NET with a potency ratio of approximately 2:1 (∼110 and 55 nM, respectively; Basile et al., 2007) and also inhibits [3H]DA uptake, albeit with a potency approximately 10 times lower than that for inhibiting [3H]NE up-take (∼900 nM). Nonetheless, analgesic doses of bicifadine in rodents modestly elevate striatal DA levels (Basile et al., 2007). Moreover, the involvement of DA in the analgesic actions of bicifadine is indicated by the ability of (−)-sulpiride (a D2 receptor antagonist) to reduce the antinociceptive effect of bicifadine in a rat model of neuropathic pain. Bicifadine does not cause hyperlocomotion in the open field, nor does it bind to other receptors for commonly abused substances (Basile et al., 2007). However, the results from microdialysis studies showing increased dopamine levels (Basile et al., 2007) raise the possibility that bicifadine may possess a potential for abuse. Such data will be useful in assessing the abuse-related effects of triple uptake inhibitors under development for various conditions. Therefore, we have tested the discriminative stimulus properties of bicifadine, along with antidepressants that block the NET and/or DAT (desipramine and bupropion) and commonly abused psychostimulants (cocaine, d-amphetamine) in rats trained to discriminate between cocaine and saline. In addition, we have measured bicifadine-induced changes in DA, 5-HT, and NE in the nucleus accumbens (NAcc), a brain region known to be involved in the integration of reward stimuli, after the administration of behaviorally relevant doses of bicifadine, bupropion, and d-amphetamine. For comparison, bicifadine-induced changes in DA and 5-HT were also determined in the striatum to put into context regional differences among the drugs tested that may account for differences in their pharmacological and behavioral profiles. Finally, the reinforcing properties of bicifadine relative to the abused psychomotor stimulants cocaine and d-amphetamine and the antidepressant bupropion were investigated in rhesus monkeys trained to self-administer cocaine intravenously. These studies indicate that bicifadine has an abuse liability profile more consistent with that of noradrenergic and serotonergic antidepressants than with abused psychomotor stimulants.
Materials and Methods
The behavioral studies were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). The protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and performed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The microdialysis procedures used were in accordance with the Animal Protection Act of August 21, 1997 (Poland’s Government Regulations and Laws Gazette) and the Guide for the Care and Use of Laboratory Animals.
Rat Discrimination Studies
Adult male Sprague-Dawley rats (COBS CD; Charles River Laboratories, Inc., Wilmington, MA) were trained to discriminate injections of 10 mg/kg i.p. cocaine from saline as described previously (Gold and Balster, 1996). They were individually housed with free access to water under a 12-h light/dark cycle with training and testing occurring during the light phase. Food (Rodent Diet; Harlan Teklad, Madison, WI) access was restricted and was given after session to maximize lever-pressing for food reinforcement while maintaining adult weights between 350 and 425 g. The subjects were trained daily (Monday through Friday) in 15-min sessions in standard two-lever operant conditioning chambers (MED Associates, Inc., St. Albans, VT). An injection of either 10 mg/kg cocaine (C) or saline (S) was administered before the session under a double-alternation schedule (C, C, S, S, C, C, S, S, etc.). Rats were placed in the operant conditioning chamber 10 min after injection and the session was initiated, as signaled by illumination of the chamber house light. Completion of 10 consecutive responses [FR (fixed ratio) 10] on the correct lever resulted in delivery of a 45-mg food pellet (P. J. Noyes Company, Inc., Lancaster, NH). Incorrect responding reset the FR for correct-lever responding. Initial training was continued until the subjects were responding reliably and had responded on the correct lever during a minimum of four consecutive sessions. Subsequent to acquisition of the cocaine-saline discrimination, testing with other compounds for cocaine-like discriminative stimulus effects was begun.
Test sessions were conducted on Tuesday and Friday when the subjects met the following criteria on the most recent cocaine- and saline-training sessions: 1) first FR completed on the correct lever, and 2) greater than 85% correct-lever responding over the entire session. During test sessions, completion of an FR on either lever resulted in the delivery of food reinforcement. The subjects were tested with different doses of the test drugs, in general administered in an ascending order across test days at times before the session start based on previous studies. Dose-effect curves included at least one low dose that resulted in overall lever selection and response rates similar to those for saline. The drug was then typically tested in 1/2 log or 1/4 log dose increments until a dose was reached that decreased response rates by more than 50% of saline test control rates or produced overt behavioral effects. During the course of the study, 9 or 10 rats were used to evaluate the different test drugs. Subject 4 was removed from the study before testing d-amphetamine and desipramine for reasons unrelated to the test drugs. The following dose ranges were tested: cocaine (1–17 mg/kg), bicifadine (3–10 mg/kg), bupropion (3–56 mg/kg), d-amphetamine (0.03–4.0 mg/kg), and desipramine (1–17 mg/kg). Cocaine was administered intraperitoneally 10 min before the session began. The other test compounds were administered intraperitoneally 30 min before the session began. To demonstrate retention of the cocaine-saline discrimination throughout the study, tests with 10 mg/kg cocaine and saline were done before and after each dose-response curve. Between test sessions, animals continued training with cocaine and saline injections under a double-alternation schedule. Illumination of lights, recording of responses, and pellet delivery were performed with a microcomputer programmed with MED-PC operant conditioning software (version 4.0 for Windows; MED Associates, Inc.).
Rat Discrimination Data Analysis. Test drug similarity to cocaine was determined by using the mean percentage of responses on the cocaine-associated lever during test sessions. Full substitution for cocaine was designated as greater than 80% cocaine-lever responding, partial substitution was defined as producing between 20 and 80% cocaine-lever responding, and a lack of cocaine-like discriminative stimulus effects was considered to be less than 20% cocaine-lever responding. In addition, the mean response rate for all subjects was determined for each test dose to reveal any nonspecific drug effects on behavior. Data from sessions in which responding was less than 0.05 responses per second were excluded from determination of the mean percentage cocaine-lever responding, whereas response rate averages were determined by using all test data. Selectivity for producing cocaine-like effects was assessed by determining whether doses producing substitution also produced decreases in rates of responding. The ED50 values were calculated by using linear regression analysis of the linear portion of the log10 dose-effect curves based on the means of all of the subjects. Test drugs with higher potency ratios for producing response rate versus discriminative stimulus effects were considered more selective for cocaine-like effects and thus more cocaine-like than test drugs with low potency ratios.
Microdialysis
Adult male Wistar rats (250–300 g) were housed on a 12-h light/dark cycle at a constant temperature and humidity, and they were provided with food and water ad libitum. Rats were anesthetized with ketamine (75 mg/kg i.m.) and xylazine (10 mg/kg i.m.) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). The skulls were exposed, and small holes were drilled for insertion of the microdialysis probes by using the following coordinates: 1.8 mm anterior from the bregma, 2.7 mm lateral from the sagittal suture, and −7.0 mm ventral from the dural surface (striatum); 1.7 mm anterior from the bregma, 1.5 mm lateral from the sagittal suture, and −8.0 mm ventral from the dural surface (NAcc). For striatal and accumbal recording, microdialysis probes were constructed by inserting two fused silica tubes (30 and 35 mm long, 150 μm o.d.; Polymicro Technologies Inc., Phoenix, AZ) into a microdialysis fiber (220 μm o.d.; AN69; Hospal, Bologna, Italy). The tube assembly was placed in a stainless steel cannula (22 gauge, 10 mm) forming the shaft of the probe. Portions of the inlet and outlet tubes were individually placed inside polyethylene PE-10 tubing and glued. The free end of the dialysis fiber was sealed, and 4 or 2 mm of the exposed length was used for dialysis in the striatum or NAcc, respectively. All probes were connected to a syringe pump (BAS Bioanalytical Systems, West Lafayette, IN) that delivered an artificial cerebrospinal fluid composed of 145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, and 1.2 mM CaCl2, pH 7.4, at a flow rate of 1.5 μl/min. Baseline samples were collected every 20 min after the washout period to obtain a stable extracellular neurotransmitter level. Drugs were then administered intraperitoneally, and dialysate fractions were collected for 240 min. At the end of the experiment, the rats were sacrificed and their brains were histologically examined to validate probe placement.
Neurotransmitter Analysis. DA and 5-HT were analyzed by high-performance liquid chromatography with electrochemical detection. Chromatography was performed with an LC-10 AD pump (Shimadzu Europa GmbH, Warsaw, Poland), an LC-4B amperometric detector with a cross-flow detector cell (BAS Bioanalytical Systems), and Hypersil BDS C18 analytical column (3 × 100 mm; Thermo Fisher Scientific, Waltham, MA). The mobile phase was composed of 0.1 M monochloroacetic acid adjusted to pH 3.7 with 3 M sodium hydroxide, 0.5 mM EDTA, 25 mg/liter 1-octanesulfonic acid sodium salt, 5.7% methanol, and 2.5% acetonitrile. The flow rate was 0.5 ml/min, and the applied potential of a 3-mm glassy carbon electrode was +600 mV with a sensitivity of 2 nA/V. NE was measured using an high-performance liquid chromatography system equipped with a P580 pump (Dionex, Sunnyvale, CA) connected to an injection valve with a 10-μl loop and a Hypersil BDS analytical column (2.0 × 100 mm; Thermo Fisher Scientific). The mobile phase was composed of 0.05 M KH2PO4 (adjusted to pH 3.7 with ortho-phosphoric acid), 0.5 mM EDTA, 150 mg/liter 1-octanesulfonic acid sodium salt, 10 mM NaCl, and 1.2% acetonitrile. The flow rate was 180 μl/min. NE was detected in dialysates with a radial flow detector cell coupled to a LC-4B amperometric detector (BAS Bioanalytical Systems). The applied potential of a 3-mm glassy carbon electrode was +600 mV with a sensitivity of 2 nA/V. The chromatographic data were processed by Chromax 2001 (Pol-Lab, Warsaw, Poland) software run on a personal computer. The values were not corrected for in vitro probe recovery, which was approximately 15%.
Monkey Self-Administration Studies
Four adult male rhesus monkeys were housed in fiberglass experimental chambers (1 m3; Plas Labs, Lansing, MI) with a transparent front door. The monkeys were fed high-fiber primate biscuits (Laboratory Fiber-Plus 5049 Monkey Diet; PMI Nutrition International, Richmond, IN) twice daily (morning and evening) in optimal amounts to maximize their health. The monkeys participated in a program of environmental enrichment approved by the Institutional Animal Care and Use Committee.
Silicone catheters (Cole-Parmer Instrument Co., Vernon Hills, IL) were implanted into a major vein (internal or external jugular or femoral) by using sterile technique under xylazine (1 mg/kg i.m.)/ketamine (10 mg/kg i.m.) anesthesia. The catheter was routed subcutaneously to the midscapular region and it exited through a small skin incision. After exiting the skin, the catheter was protected by a lightweight stainless-steel-lined jacket and restraining arm through which the catheter passed to the rear of the chamber and connected to a peristaltic pump capable of delivering a 1-ml, 10-s infusion. The jacket and catheter protection arm were equipped with swivel joints allowing the animals almost unlimited mobility within their cages.
Experimental Conditions. Two response levers were mounted inside the chamber door. Three stimulus lights (red, green, and white) were located approximately 10 cm above each lever. The experimental contingencies and data recording were controlled by a microcomputer located in an adjacent room using MED-PC operant conditioning software (version 4.0 for Windows; MED Associates, Inc.).
Each monkey was trained to self-administer 10 μg/kg per infusion of cocaine during daily (7 days/week) 1-h experimental sessions. Drug availability was signaled by illumination of the white stimulus light above the left lever. For baseline conditions, 10 lever presses were required to produce each 1-ml, 10-s infusion under the FR schedule. During the infusion, the white light was extinguished, the red light illuminated, and the responses recorded, but they did not count toward completion of the FR requirement. The FR 10 value was selected to maintain a clear difference between infusion rates maintained by cocaine and saline. Under these conditions, animals typically self-administered 50 to 150 injections of 10 μg/kg cocaine per session.
Testing: FR procedure. Substitution of saline or test drug solutions for cocaine occurred when rates of cocaine self-administration were stable (three consecutive baseline sessions in which the number of cocaine infusions did not vary by more than 25% from the mean for those sessions and there were no trends in infusion numbers). FR substitution tests included four consecutive sessions in which a test solution was made available for self-administration. There were no stimulus light changes or any other stimuli presented to the subjects to indicate that the solution had been changed. When the test solution failed to maintain responding above the most recent saline substitution levels, subjects were returned to cocaine self-administration for a minimum of 3 days until stable performance was again attained.
Substitution tests were conducted for the following test conditions: 1 to 56 μg/kg per infusion bicifadine; 3 to 100 μg/kg per infusion bupropion; 1 to 10 μg/kg per infusion d-amphetamine; and 0.3 to 10 μg/kg per infusion cocaine. Individual doses varied between subjects, but the subjects were tested across a range of doses sufficient to fully characterize the dose-effect curves for the test compounds. Although individual doses were presented in mixed order, the dose-effect curves were completed in the following order: cocaine, bicifadine, bupropion, and d-amphetamine. Exceptions include the 56 μg/kg per infusion dose of bicifadine that was tested after completion of the bupropion dose-effect curve and the 3 μg/kg per infusion d-amphetamine dose in three of the four subjects. Multiple tests (four or five) were conducted with saline as a negative control, one at the beginning of each dose-response curve determination and one after any new catheter implantation.
Testing: progressive ratio procedure. If the dose of test drug maintained responding at levels above those for the most recent saline control condition, the dose was provisionally considered to serve as a reinforcer, and the monkey was moved into daily testing using a progressive ratio (PR) schedule to evaluate the relative reinforcing efficacy of the test solution. On day 1 of the PR schedule testing, the ratio requirement was double the monkey’s baseline FR 10 (PR 20). Daily PR sessions progressed by an increase of 10 lever presses per session (i.e., 20, 30, 40, 50, etc.) until a break point was obtained (last ratio at which self-administration was maintained above mean saline levels). Once the break point had been reached, the subjects were returned to the cocaine baseline in the next session, and were maintained for at least three sessions until response rates were again stable. Then the monkeys returned to testing of the next test dose under a FR schedule.
If a catheter was lost during execution of this protocol, the animals were suspended from behavioral testing for a 2- to 3-week recovery period, recatheterized in another major vein, then rapidly retrained on cocaine self-administration. Once responding for cocaine was again stabilized, they resumed the testing protocol.
Monkey Self-Administration Data Analysis. FR schedule. The dependent variables were infusion numbers and pattern of response within a session. Results were analyzed separately for each subject. For cocaine baseline data, the mean (±S.E.M.) number of cocaine infusions self-administered on the last 3 days before each substitution was obtained for each test drug. For substitution tests with different doses of bicifadine, bupropion, d-amphetamine, and cocaine, data for the last 3 days of each 4-day substitution were evaluated. Data for the first substitution test day are typically excluded from analyses because responding on that day reflects the transition in behavior from the cocaine-baseline condition to the new rates of responding to the test solution. Saline data were evaluated for each individual substitution series, using data from the last three sessions and data combined from the last 3 days of all saline substitutions for each subject. The mean number of test drug infusions must have exceeded the mean of the combined saline substitution data by at least 2 S.D. for a drug to be considered a positive reinforcer. Data on the pattern of responding over the 1-h session were plotted as the percentage of the total number of injections that were self-administered in each fourth of the session. These results were based on the last 3 days of the 4-day substitution tests for saline, bicifadine, bupropion, d-amphetamine, and cocaine and the three sessions for baseline cocaine responding immediately before each test solution substitution.
PR schedule. The dependent variables were total injections per session, total responses per session, and break points. Although all data were compared between the test drugs across different reinforcing doses, the primary comparison was between break points. The break points for reinforcing doses of cocaine, bupropion, and d-amphetamine were compared for all subjects by using Kruskal-Wallis analysis. An insufficient number of bicifadine test doses in the four subjects served as positive reinforcers, preventing a statistical comparison. During determination of the dose-response curves, several doses of each test drug were provisionally designated as positive reinforcers compared with the preceding saline substitution. However, they ultimately failed to meet criteria when the analysis included all saline substitutions. These solutions were tested under a progressive ratio; however, the PR data were not included in the final analysis.
Drugs
Cocaine HCl and d-amphetamine SO4 were obtained from the National Institute on Drug Abuse (Rockville, MD), and Sigma-Aldrich (Poland), then dissolved in physiological saline (0.9% NaCl). Bupropion HCl (Toronto Research Chemicals Inc., Toronto), bicifadine HCl, and desipramine HCl (Sigma-Aldrich, St. Louis, MO) were provided by DOV Pharmaceutical, Inc. (Somerset, NJ). For rat studies, bupropion and bicifadine were dissolved in saline (0.9% NaCl). Desipramine was initially dissolved in distilled water and diluted in saline (0.9% NaCl) to obtain appropriate concentrations for the injection volume. All doses of test drug and saline control tests used an injection volume of 1 ml/kg. For the monkey self-administration studies, cocaine HCl and d-amphetamine HCl were dissolved in physiological saline (0.9%) to concentrations of 100 and 5 mg/ml, respectively. Bicifadine was reconstituted with sterile water to a concentration of 10 mg/ml. Bupropion HCl was dissolved in sterile water to a concentration of 50 mg/ml. These stock solutions were subsequently mixed with different volumes of 0.9% saline to produce the desired test concentrations. The concentrations were based on each test dose and monkey weight such that the test dose was delivered in a 1-ml infusion.
Results
Rat Discrimination. Cocaine, d-amphetamine, and bupropion dose-dependently increased cocaine-lever responding in rats, with one or more doses of each drug producing full substitution (>80% mean cocaine-associated lever responding) for the cocaine training dose (Fig. 1A). Although cocaine and bupropion produced full substitution in all subjects at one or more doses that did not suppress rates of responding (Fig. 1B), one subject failed to select the cocaine lever after dosing with d-amphetamine despite testing up to a dose that completely abolished responding (4 mg/kg; Fig. 1B). Bupropion abolished responding at the dose of 56 mg/kg (Fig. 1B).
Fig. 1.
The effects of cocaine, bicifadine, bupropion, desipramine, and d-amphetamine on cocaine-lever responding (A) and mean response rates (B) in rats trained to discriminate 10 mg/kg cocaine from saline. Values above saline (Sal) and cocaine (Coc) are the results of control tests conducted before determining each dose-response curve. One or more doses of cocaine, d-amphetamine, and bupropion fully substituted for the cocaine training dose (A). Bicifadine did substitute at the highest dose tested but with pronounced concomitant disruption of behavior (B). Each point represents the mean ± S.E.M. of the percentage cocaine-lever responding for n = 9 (A, d-amphetamine, desipramine) or 10 (B, cocaine, bupropion, bicifadine) rats except where indicated in parentheses.
The highest dose of bicifadine tested (10 mg/kg) produced a mean of 80.9% cocaine-lever responding and therefore reached criteria for full substitution. However, this dose also strongly disrupted lever-pressing behavior, as indicated by a >80% mean decrease in response rates relative to saline control rates (Fig. 1B). The mean percentage cocaine-lever responding at 10 mg/kg bicifadine was based on only 6 of 10 subjects who qualified for the inclusion of their lever selection data (response rate, >0.05 responses per second). Overall, of the 10 subjects, only 6 selected the cocaine-associated lever at one or more doses of bicifadine. Of these six subjects, only one selected the cocaine lever at doses producing minimal suppression of responding.
Administration of desipramine resulted in a maximum of 69% mean cocaine-lever responding at the highest dose tested (17 mg/kg) (Fig. 1A), but reduced the response rate by only 65% (Fig. 1B). On an individual subject basis, seven of nine subjects responded more than 80% of the time on the cocaine-associated lever at one or more doses of desipramine.
Table 1 provides a comparison of the ED50 values for the different test compounds, both for the ability to substitute for the cocaine training dose and for disruption of responding. Relative potencies for the discriminative stimulus effects (Table 1) were d-amphetamine > cocaine > bicifadine > desipramine > bupropion. In contrast, the relative potencies for response rate suppression were d-amphetamine > bicifadine > desipramine > cocaine > bupropion. Cocaine and d-amphetamine produced high ratios of selectivity for cocaine-like effects. Bicifadine had the lowest ratio of selectivity for cocaine-like effects, more similar to the results with bupropion and desipramine than with the abused stimulants.
TABLE 1.
Potency of bicifadine and reference stimulants/monoamine transport inhibitors in the rat discrimination procedure
Values represent the ED50 and 95% confidence interval of a drug in either substituting for the cocaine training dose (Stimulus Effects) or in decreasing the rates of responding relative to saline control test sessions (Response Rate Effects). Values derived from curves fitted to data points by using nonlinear regression analysis (Prism; GraphPad Software, San Diego, CA).
| Drug | Stimulus Effects | Response Rate Effects | Ratio of ED50 values |
|---|---|---|---|
| mg/kg | |||
| Cocaine | 3.6 (3.0–4.4) | >17 | >4.7 |
| Bicifadine | 5.6 (4.6–6.9) | 5.2 (4.8–6.0) | 0.9 |
| d-Amphetamine | 0.4 (0.2–0.7) | 2.9 (2.3–3.6) | 7.2 |
| Bupropion | 16.5 (14.1–19.4) | 36.8 (31.3–43.2) | 2.2 |
| Desipramine | 7.3 (3.4–15.4) | 13.8 (9.5–20.2) | 1.9 |
Rat Microdialysis. Levels of DA, 5-HT, and NE were monitored in the NAcc (Fig. 2) and striatum (Fig. 3) of rats after the administration of behaviorally active doses of bicifadine, bupropion, and d-amphetamine. For all analyses, Ftreat, Ftime, and Fint are F values for the main effect of treatment, time, and the treatment-by-time interaction, respectively. DA levels in the NAcc were significantly elevated as early as 20 to 40 min after administration of bicifadine (Ftreat5,480 = 73.9; Ftime11,480 = 22.9; Fint55,480 = 3.825, all P < 0.01), bupropion (Ftreat3,372 = 80.3; Ftime11,372 = 8.42; Fint33,372 = 3.04, all P < 0.01), or d-amphetamine (Ftreat3,264 = 252; Ftime11,264 = 26.8; Fint33,264 = 17.4, all P < 0.01), an effect that lasted between 20 and 160 min, depending on dose (Fig. 2, A–C). DA levels rose to maxima of 196 ± 25, 226 ± 20, 311 ± 31, and 540 ± 88% by 40 min after the administration of 10, 20, 40, or 60 mg/kg bicifadine, respectively. The maximum levels of DA achieved after the administration of either bupropion (369 ± 145 and 851 ± 39% at 30 and 100 mg/kg, respectively) or d-amphetamine (221 ± 43 and 1321 ± 201% at 3 and 6 mg/kg, respectively) were greater than those elicited by bicifadine. Unlike bupropion, which produced no significant increase in 5-HT levels in the NAcc (Fig. 2E, Ftreat3,372 = 4.42, P < 0.01; Ftime11,372 = 3.02, P < 0.01; Fint33,372 = 0.76, NSD), bicifadine (Fig. 2D, Ftreat5,480 = 43.7; Ftime11,480 = 27.6; Fint55,480 = 2.303, all P < 0.01) and d-amphetamine (Fig. 2F, Ftreat3, 264 = 58.7; Ftime3,264 = 17.2; Fint33,264 = 4.16, all P < 0.01) significantly increased 5-HT levels. All three agents tested increased NE levels in the NAcc. Bicifadine (Fig. 2G) produced a significant increase in NE in a dose-dependent manner (Ftreat4,384 = 19.9; Ftime11,384 = 13.2; Fint44,384 = 1.39, all P < 0.01), whereas significant increases in NE were only observed after the highest dose of bupropion (100 mg/kg) was administered (Fig. 2H, Ftreat3,372 = 79.6; Ftime11,372 = 9.27; Fint33,372 = 4.21, all P < 0.01). The effects of d-amphetamine were relatively weak and independent of dose (Ftreat3,264 = 11.35, P < 0.01; Ftime11,264 = 6.20, P < 0.01; Fint33,264 = 0.83, NSD), with a maximum increase in NE of 251 ± 21.6% above baseline attained 40 min after administration of 6 mg/kg d-amphetamine (Fig. 2I).
Fig. 2.
Relative changes in the extracellular levels of DA (A–C), 5-HT (D–F), and NE (G–I) induced by bicifadine (A, D, and G), bupropion (B, E, and H), and d-amphetamine (C, F, and I) as measured in the NAcc by using microdialysis in normal rats. Each sample of microdialysate was accumulated during a 20-min period between −40 and 180 min, with each point representing the mean percentage change (error bars omitted for clarity) from baseline levels for drug (filled symbols) and vehicle-treated (open symbols) rats. Drugs were administered at time 0 in the doses indicated (milligrams per kilogram, intraperitoneal). Bicifadine significantly elevated DA, 5-HT, and NE levels in the NAcc. Bupropion significantly elevated DA and NE levels but had variable effects on 5-HT levels. Amphetamine significantly elevated DA and 5-HT levels but had variable effects on NE levels. Absolute baseline neurotransmitter levels (in picograms per 10 μl) were 3.8 ± 0.2 (DA), 1.7 ± 0.1 (5-HT), and 1.0 ± 0.1 (NE). *, **, values significantly different from the mean baseline level, P < 0.05, 0.01, respectively, two-way ANOVA followed by Bonferroni’s post hoc comparison matrix.
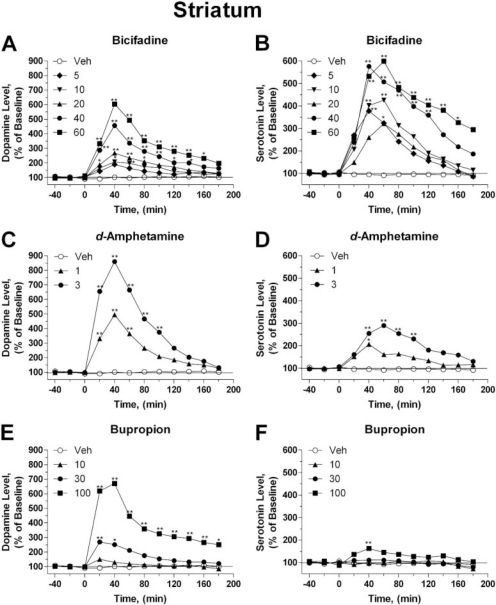
Fig. 3.
Relative changes in the extra-cellular levels of DA (A, C, and E) and 5-HT (B, D, and F) induced by bicifadine (A, B), d-amphetamine (C, D), and bupropion (E, F) as measured in the striatum by use of microdialysis in normal rats. Each sample of microdialysate was accumulated during a 20-min period between −40 and 180 min, with each point representing the mean percentage change (error bars omitted for clarity) from baseline levels for drug (filled symbols) and vehicle-treated (open symbols) rats. Drugs were administered at time 0 in the doses indicated (milligrams per kilogram, intraperitoneal). Bicifadine significantly elevated DA (A) and 5-HT (B) levels in the striatum. Amphetamine significantly elevated DA (C) and 5-HT (D) levels in the striatum. Bupropion significantly elevated DA (E) levels but had small and variable effects on 5-HT (F) levels. Absolute baseline striatal neurotransmitter levels (in picograms per 10 μl) were 10 ± 0.4 (DA) and 2.6 ± 0.1 (5-HT). *, **, values significantly different from the mean baseline level, P < 0.05, 0.01, respectively, two-way ANOVA followed by Bonferroni’s post hoc comparison matrix.
DA and 5-HT levels were significantly elevated in the striatum (Fig. 3) after administration of one or more doses of bicifadine (Fig. 3A, DA, Ftreat5,468 = 131.3; Ftime11,468 = 61.3; Fint55,468 = 8.785, all P < 0.01; and Fig. 3B, 5-HT, Ftreat5, 468 = 40.36; Ftime11,468 = 27.3; Fint55,468 = 2.305, all P < 0.01) and d-amphetamine (Fig. 3C, DA, Ftreat2,204 = 106.2; Ftime2,204 = 30.34; Fint22,204 = 10.8, all P < 0.01 and Fig. 3D, 5-HT, Ftreat2,204 = 39.4; Ftime2,204 = 5.94; Fint22,204 = 2.54, all P < 0.01). Bupropion (30, 100 mg/kg i.p.) significantly elevated DA (Fig. 3E, Ftreat3,312 = 141.6; Ftime11,312 = 18.9; Fint33,312 = 8.474, all P < 0.01) levels, but had small and variable effects on 5-HT (Fig. 3F, Ftreat3,312 = 13.78, P < 0.01; Ftime11,312 = 1.92, P < 0.05; Fint33,312 = 0.900, NSD) levels. The temporal characteristics of the DA responses to bicifadine, bupropion, and d-amphetamine were similar to those observed in the NAcc. However, although bicifadine significantly increased striatal 5-HT levels at all doses (Fig. 3B), the effects of d-amphetamine on 5-HT levels were relatively modest (Fig. 3D), whereas those of bupropion were significant only at 40 min after administration of the highest dose (Fig. 3F).
The cumulative increase in neurotransmitter released over the time course of the microdialysis study was determined by calculating the area under the curve of neurotransmitter levels (AUC). Bicifadine (40, 60 mg/kg) significantly increased the percentage elevation in DA released in the NAcc (Table 2; F5,43 = 6.334, P < 0.01). However, the relative amounts of DA released in the NAcc after administration of either bupropion (30, 100 mg/kg) or d-amphetamine (3, 6 mg/kg) were greater than observed for bicifadine (Table 2; F3,31 = 13.23, P < 0.01 and F3,21 = 18.04, P < 0.01, respectively). This was noted even at doses of bicifadine (10 mg/kg), bupropion (30 mg/kg), or d-amphetamine (3 mg/kg) that maximally substituted for cocaine (F2,23 = 16.17, P < 0.01; Fig. 1A).
TABLE 2.
AUC versus dose for DA levels in the NAcc and striatum after administration of bicifadine (5, 10, 20, 40, 60 mg/kg i.p.), bupropion (10, 30, 100 mg/kg i.p.), and d-amphetamine (1, 3, 6 mg/kg i.p. NAcc; 1, 3 mg/kg i.p. striatum)
All three compounds significantly increased the AUC for the increase in DA in the NAcc and striatum. AUC values represent the increase in neurotransmitter level versus time after each dose of drug or vehicle.
| Brain Region and Compounds | Dose | AUC ± S.E.M. |
|---|---|---|
| mg/kg | ||
| Nucleus accumbens | ||
| Bicifadine | 0 | 160 ± 86 |
| 5 | 6700 ± 1400 | |
| 10 | 6900 ± 980 | |
| 20 | 11,000 ± 1500 | |
| 40 | 19,000 ± 2400* | |
| 60 | 28,000 ± 6100** | |
| Bupropion | 0 | 150 ± 85 |
| 10 | 9500 ± 1800 | |
| 30 | 32,000 ± 5000* | |
| 100 | 84,000 ± 16,000** | |
| Amphetamine | 0 | 150 ± 85 |
| 1 | 18,000 ± 4100 | |
| 3 | 78,000 ± 15,000** | |
| 6 | 110,000 ± 9700** | |
| Striatum | ||
| Bicifadine | 0 | 1000 ± 370 |
| 5 | 7800 ± 1900 | |
| 10 | 11,000 ± 1500 | |
| 20 | 15,000 ± 2100* | |
| 40 | 28,000 ± 2100** | |
| 60 | 42,000 ± 4300** | |
| Bupropion | 0 | 1100 ± 300 |
| 10 | 3000 ± 650 | |
| 30 | 13,000 ± 1500* | |
| 100 | 51,000 ± 7200** | |
| Amphetamine | 0 | 1100 ± 300 |
| 1 | 28,000 ± 4300* | |
| 3 | 57,000 ± 8700** | |
Values significantly different from those determined in vehicle-treated animals, P < 0.05, one-way ANOVA followed by Dunnett’s post hoc test.
Values significantly different from those determined in vehicle-treated animals, P < 0.01 one-way ANOVA followed by Dunnett’s post hoc test.
Bicifadine (F5,39 = 32.13, P < 0.01), d-amphetamine (F3,22 = 14.59, P < 0.01), and bupropion (F3,31 = 28.7, P < 0.01) also significantly increased the AUC for the increase in DA levels with time in the striatum (Table 2). For the AUC comparisons, Fregion represents the F value for the main effect of region. The cumulative increase in DA levels observed in the striatum after bicifadine administration was greater than observed in the NAcc (F1,6 = 46.24, P < 0.01, slopes of the Dose × AUC lines, NAcc versus striatum, Fregion1,76 = 6.94, P = 0.01, two-way ANOVA). In contrast, the cumulative increases in striatal DA levels observed after bupropion (10, 30, and 100 mg/kg; Table 2) were lower than in the NAcc (F1,2 = 41.7, P = 0.02, slopes of the Dose × AUC lines, NAcc versus striatum after bupropion, Fregion1,51 = 7.86, P < 0.01, two-way ANOVA), whereas elevations in the NAcc induced by d-amphetamine (1, 3 mg/kg) were equivalent to those observed in the striatum.
Different profiles for the total increase in 5-HT over time were observed in the NAcc and striatum for the three test compounds (Table 3). Bicifadine-induced significant increases in 5-HT levels in the NAcc (F5,37 = 14.29, P < 0.01) tended to be greater than those observed after d-amphetamine (F3,20 = 9.2, P < 0.01), whereas bupropion had no significant effect on 5-HT levels in the NAcc (F3,30 = 1.33, NSD). The elevation in NAcc 5-HT was significantly higher (F2,24 = 9.827, P < 0.01) after a dose of bicifadine (10 mg/kg), which maximally substituted for cocaine, than after similar doses of d-amphetamine (3 mg/kg) or bupropion (30 mg/kg). The relative increase in 5-HT release in the striatum after bicifadine (F5,39 = 6.618, P < 0.01) administration was not significantly different from that observed in the NAcc. d-Amphetamine (F2,18 = 10.8, P < 0.01) and bupropion (F3,26 = 6.295, P < 0.01) significantly increased striatal 5-HT levels, but only at the highest doses tested (3 and 100 mg/kg, respectively).
TABLE 3.
AUC versus dose for 5-HT levels in the NAcc and striatum after administration of bicifadine (5, 10, 20, 40, 60 mg/kg i.p.), bupropion (10, 30, 100 mg/kg i.p.), and d-amphetamine (1, 3, 6 mg/kg i.p. NAcc; 1, 3 mg/kg i.p. striatum)
Bicifadine and d-amphetamine significantly increased the AUC for the increase in 5-HT in the NAcc and striatum. Bupropion (100 mg/kg i.p.) significantly increased the AUC for the increase in 5-HT levels in the striatum, but not the NAcc. AUC values represent the increase in neurotransmitter level versus time after each dose of drug or vehicle.
| Brain Region and Compounds | Dose | AUC ± S.E.M. |
|---|---|---|
| mg/kg | ||
| Nucleus accumbens | ||
| Bicifadine | 0 | 1500 ± 430 |
| 5 | 17,000 ± 3900 | |
| 10 | 20,000 ± 3600 | |
| 20 | 24,000 ± 4300* | |
| 40 | 25,000 ± 3100* | |
| 60 | 53,000 ± 5700** | |
| Bupropion | 0 | 1400 ± 420 |
| 10 | 1800 ± 650 | |
| 30 | 3800 ± 1100 | |
| 100 | 3500 ± 890 | |
| Amphetamine | 0 | 1400 ± 420 |
| 1 | 5200 ± 1300 | |
| 3 | 13,000 ± 2800* | |
| 6 | 24,000 ± 3600** | |
| Striatum | ||
| Bicifadine | 0 | 590 ± 480 |
| 5 | 21,000 ± 4500 | |
| 10 | 26,000 ± 4300 | |
| 20 | 18,000 ± 2500 | |
| 40 | 46,000 ± 12,000** | |
| 60 | 54,000 ± 7500** | |
| Bupropion | 0 | 1000 ± 370 |
| 10 | 3000 ± 650 | |
| 30 | 14,000 ± 1600 | |
| 100 | 51,000 ± 7300** | |
| Amphetamine | 0 | 590 ± 480 |
| 1 | 8500 ± 1400 | |
| 3 | 19,000 ± 3400** | |
Values significantly different from those determined in vehicle-treated animals, P < 0.05, one-way ANOVA followed by Dunnett’s post hoc test.
Values significantly different from those determined in vehicle-treated animals, P < 0.01 one-way ANOVA followed by Dunnett’s post hoc test.
As shown in Table 4, bicifadine (40, 60 mg/kg) and d-amphetamine administration (1, 6 mg/kg) resulted in significant, dose-dependent increases in NE levels in the NAcc (F4,39 = 2.226, P < 0.01 and F3,24 = 3.65, P < 0.05, respectively). In comparison, a significant increase in NE was recorded only at the highest dose of bupropion tested (100 mg/kg, F3,25 = 25.27, P < 0.01).
TABLE 4.
AUC versus dose for NE levels in the NAcc after administration of bicifadine (10, 20, 40, 60 mg/kg i.p.), bupropion (10, 30, 100 mg/kg i.p.), and d-amphetamine (1, 3, 6 mg/kg i.p. NAcc)
All three compounds significantly increased the AUC for the increase in NE in the NAcc and striatum at one or more doses. AUC values represent the increase in neurotransmitter level versus time after each dose of drug or vehicle.
| Brain Region and Compound | Dose | AUC ± S.E.M. |
|---|---|---|
| mg/kg | ||
| Nucleus accumbens | ||
| Bicifadine | 0 | 1200 ± 220 |
| 10 | 8600 ± 2600 | |
| 20 | 5500 ± 760 | |
| 40 | 17,000 ± 4000* | |
| 60 | 29,000 ± 7900** | |
| Bupropion | 0 | 1100 ± 200 |
| 10 | 6100 ± 1900 | |
| 30 | 13,000 ± 3300 | |
| 100 | 70,000 ± 12,000** | |
| Amphetamine | 0 | 1100 ± 200 |
| 1 | 12,000 ± 4400* | |
| 3 | 11,000 ± 2300 | |
| 6 | 14,000 ± 2300* | |
Values significantly different from those determined in vehicle-treated animals, P < 0.05, one-way ANOVA followed by Dunnett’s post hoc test.
Values significantly different from those determined in vehicle-treated animals, P < 0.01 one-way ANOVA followed by Dunnett’s post hoc test.
Monkey Self-Administration Studies. In the monkey self-administration tests, the mean number of infusions per session under cocaine baseline conditions (Coc) and during the saline substitution tests (Sal) conducted before each dose-response curve determination are shown for individual subjects on the left side of each panel in Fig. 4. The gray shading indicates the range of infusion numbers that fall within 2 S.D. of the mean number of saline infusions from the last 3 days of all of an individual subject’s saline substitution conditions. Under baseline FR 10 conditions, self-administration of cocaine (10 μg/kg per infusion) ranged from 50 to 150 infusions per session, depending on the subject. For all subjects, the mean number of cocaine infusions self-administered during three or more cocaine test doses was significantly (>2 S.D.) higher than the overall mean number of saline infusions. When saline was substituted for cocaine, infusion rates decreased substantially. For all subjects, the mean numbers of infusions for the individual saline substitution tests were within the 2 S.D. range, as illustrated by the saline data points (Fig. 4).
Fig. 4.
Results of intravenous self-administration study with cocaine, bicifadine, bupropion, and d-amphetamine in four rhesus monkeys trained to self-administer 10 μg/kg per infusion cocaine under a FR 10 schedule of reinforcement. Shown are the mean (± S.E.M) number of infusions of cocaine during baseline conditions (Coc), saline (Sal) infusions during the substitution preceding each dose-response curve determination, and during substitution tests with various doses of cocaine, bicifadine, bupropion, and d-amphetamine. The shaded area encompasses 2 S.D. of the mean number of saline infusions during the saline substitution tests determined during the study. Cocaine, d-amphetamine, and bupropion maintained self-administration significantly above saline levels at one or more doses tested in all subjects. Bicifadine only maintained responding significantly above saline in subjects M1352 and M1375.
The responses to increasing doses of bicifadine, cocaine, bupropion, and d-amphetamine by each subject are shown on the right side of each panel in Fig. 4. Two or more doses of d-amphetamine (0.3–10 μg/kg per infusion) and bupropion (3–100 μg/kg per infusion) maintained infusion levels significantly above saline levels in all subjects. For monkeys M1352, M1375, and M1382, infusion levels for these two drugs were at or above cocaine infusion levels. In contrast, evidence for reinforcing effects of bicifadine (10–56 μg/kg per infusion) was seen in only two monkeys. For these monkeys (M1352 and M1375), at least one dose of bicifadine resulted in mean infusion numbers significantly greater (>2 S.D.) than the overall mean number of saline infusions (Fig. 4). For M1352, the peak mean number of bicifadine infusions self-administrated (49.5 at 30 μg/kg per infusion) were 68.5% lower than maximal mean levels of cocaine self-administration (158.5 at 3 μg/kg per infusion). However, responding for 30 μg/kg per infusion bicifadine approximated cocaine response levels in subject M1375. Although the total number of infusions obtained varied across the daily test sessions for individual doses of the four test drugs, no consistent trends in infusion numbers were noted across days for any drug in any subject.
The percentage of the mean number of infusions received in each of four 15-min bins during the 1-h sessions were calculated for all four subjects during baseline cocaine conditions (10 μg/kg per infusion), saline substitutions, and availability of increasing doses of bicifadine (data not shown). For all subjects, differences in the distribution of infusions were found between the cocaine (positive) and saline (negative) controls. Within-session responding observed for reinforcing doses of cocaine, bupropion, and d-amphetamine maintained responding throughout the session, whereas responding for saline was primarily observed during the first 15-min bin. The patterns of responding for reinforcing doses of bicifadine in subject M1375 (30 and 56 μg/kg per infusion) were more evenly distributed throughout the session relative to saline. However, only the 30 μg/kg per infusion dose in M1375 had as consistent a distribution pattern as 10 μg/kg per infusion cocaine. For subject M1352, there was no evidence from the within-session distribution data that the 30 μg/kg per infusion dose of bicifadine maintained responding throughout the session as did cocaine, bupropion, and d-amphetamine.
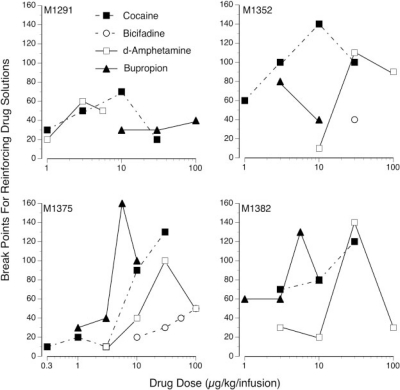
The break-point ratio values for each dose of cocaine, bicifadine, bupropion, and d-amphetamine that served as a reinforcer are shown in Fig. 5. Break points were designated as the last ratio that maintained infusion numbers above the mean number of infusions during the preceding saline substitution test. One or more doses of cocaine or d-amphetamine were most effective in maintaining self-administration behavior across sessions with escalating ratio requirements. All subjects maintained infusion levels of cocaine (3, 10, and/or 30 μg/kg per infusion) above mean saline levels for 6 to 13 days (break-point ratio values of 70 to 140). Bupropion maintained high break points in three subjects with one (M1375 and M1382) or two (M1352) doses maintaining responding across eight or more sessions. When the ratio requirement was incrementally changed for reinforcing doses of bicifadine in subjects M1352 and M1375, infusion levels fell to saline levels within 3 to 4 days (break-point ratio values of 40 or less). Comparing break points for cocaine, bupropion, and d-amphetamine in all subjects verified that break points were not significantly different among the three drugs (Kruskal-Wallis test, cocaine n1 = 15, bupropion n2 = 13; d-amphetamine n3 = 13; df = 2, H = 3.74, ). Because there were only four total substitution tests in which doses of bicifadine served as positive reinforcers, insufficient data were available to make a statistical comparison. However, visual inspection of the graphs indicates that bicifadine maintained much lower breaking points than cocaine, d-amphetamine, and bupropion.
Fig. 5.
Shown are the break-point ratio requirements reached during progressive sessions for the reinforcing doses of cocaine, bicifadine, bupropion, and d-amphetamine. There was no significant difference between break points obtained for cocaine, d-amphetamine, and bupropion (p < 0.05), whereas bicifadine sustained much lower break points.
The pattern of infusions received (Fig. 6) across progressive ratio sessions are shown for each subject. The number of infusions received during the last 3 days of each 4-day substitution test (mean ± S.E.M.) and across subsequent daily sessions as the ratio requirement was increased for each reinforcing drug and dose. For all subjects, increasing ratio requirements resulted in a decrement in infusions across sessions for all drugs. However, the rate (slope) of decrease and the number of days before reaching saline levels varied between subjects and across drug doses, consistent with the break point data (Fig. 5). For all subjects, the doses of cocaine (10 or 30 μg/kg per infusion) and d-amphetamine (3 or 5.6 μg/kg per infusion) producing the highest breaking points maintained responding above saline levels for 7 (M1291) to 16 (M1375) sessions. When reinforcing doses of bicifadine were evaluated, the infusion numbers showed a steep decline, with responding dropping from baseline FR 10 levels to saline levels within four sessions. For 10 and/or 30 μg/kg per infusion cocaine doses, total lever presses initially increased or remained constant (data not shown), reflecting the increased work required to maintain the number of infusions at or near baseline levels. Similar increases in lever pressing were noted for one or more doses of d-amphetamine and bupropion in all subjects. In the two subjects for whom bicifadine was a positive reinforcer, only M1375 emitted any appreciable increase in total lever presses with increasing ratio requirement. However, even for these doses, responding decreased after only two to three sessions at the higher workload.
Fig. 6.
Shown are the infusion numbers across increasing ratio requirements in rhesus monkeys for those doses of cocaine (Coc), bicifadine (Bic), bupropion (Bup), and d-amphetamine (Amph) serving as positive reinforcers. The FR 10 data points represent the mean values (±S.E.M) for the last 3 days of the substitution tests under baseline FR conditions.
The higher doses of cocaine (30 μg/kg per infusion) and d-amphetamine (5.6 and 10 μg/kg per infusion) caused overt psychomotor stimulation. Animals seemed normal within 2 h after cocaine exposure. However, agitation, increased loco-motor stimulation, stereotypy, and anorexia were exhibited for up to 6 h after d-amphetamine self-administration. No psychomotor stimulation was noted in any subject receiving bicifadine. Subjects M1375 and M1352 received the highest exposure levels (up to 3.6 and 3.0 mg/kg in 60 min) and were observed lying down several times during the test sessions. However, their gross behavior was normal immediately after session, and food treats were immediately consumed.
Discussion
Bicifadine is a functional triple-reuptake inhibitor with a unique analgesic profile that may be mediated, in part, by increased dopaminergic neurotransmission (Basile et al., 2007). The ability of pharmacologically relevant doses of bicifadine to raise extracellular DA levels in rat brain (Basile et al., 2007; Figs. 2 and 3) suggests that this compound may evoke psychomotor stimulant cues and present a potential abuse liability (Riddle et al., 2005). Therefore, we compared the ability of bicifadine and other uptake inhibitors, including abused psychostimulants, to substitute for cocaine in a rat discrimination study and to sustain self-administration in cocaine-experienced rhesus monkeys.
In the discrimination study, as expected, cocaine and d-amphetamine fully substituted for cocaine. Drugs that either stimulate release of DA and/or act as DAT blockers typically produce cocaine-like effects in this model (Balster et al., 1991; Kozikowski et al., 2003). Consistent with this observation, the dopaminergic actions of bupropion (Dwoskin et al., 2006) result in full generalization from cocaine in the current and previous studies (Baker et al., 1993; Katz et al., 2000). In contrast, NET-selective inhibitors such as desipramine fail to fully substitute for cocaine (Broadbent et al., 1991; Baker et al., 1993; Tella and Goldberg, 2001). For bicifadine, although it just met criteria for full substitution (>80% cocaine-lever selection) at the highest dose tested (10 mg/kg), only 6 of the 10 animals could be evaluated at this dose because of reduced lever pressing. Both cocaine and d-amphetamine produced higher maximal levels of cocaine-lever selection, and full substitution at doses that did not suppress rates. The results for bupropion were more like cocaine than the results for bicifadine, with full (100%) cocaine-associated lever selection occurring at doses that did not alter response rates. The maximum level of substitution for desipramine was only 69%. However, seven of nine rats responded on the cocaine-associated lever >80% for at least one dose. Thus, among the comparator drugs, the effects of bicifadine were more similar to desipramine than the positive controls amphetamine and cocaine.
The similarity of bicifadine to desipramine is further supported by the similar potency ratios of these two compounds in producing discriminative stimulus and response rate effects. Cocaine and d-amphetamine were 5-fold more potent in substituting for cocaine than in suppressing response rates. In contrast, desipramine and bicifadine were only 2-fold more potent or equipotent, respectively, in producing cocaine-like discriminative stimulus effects than in inhibiting responses. The lack of selectivity of a compound for cocaine-like discriminative stimulus effects may result from physical impairment or dysphoria, acting as disincentives for cocaine lever pressing or otherwise disrupting or masking the cocaine-associated discriminative stimulus. However, doses of bicifadine up to 100 mg/kg p.o. have no effect on open field activity, rotarod, or grip strength (Basile et al., 2007), evidence of a lack of effect of bicifadine on motor performance.
In the monkey self-administration study, cocaine and d-amphetamine were robust positive reinforcers, with two to four doses meeting reinforcement criteria in all four subjects together with producing the inverted U-shaped dose-effect curves typical of positive reinforcers (Young and Herling, 1986; Lile et al., 2002, 2003). Because many drugs with stimulant properties provide evidence for abuse potential in self-administration studies (Gold and Balster, 1996), the antidepressant bupropion was included as a comparator. Bupropion was a positive reinforcer in all subjects, approximating or exceeding cocaine self-administration levels at one or more doses and yielding an inverted U-shaped dose-effect curve. In general, bupropion is not abused at therapeutic doses and is not scheduled. Although the strong positive signal produced by bupropion in the current and previous studies in nonhuman primates (Bergman et al., 1989; Lamb and Griffiths, 1990) and rodents (Tella et al., 1997) may result from species differences in pharmacokinetics, it may also reflect dose ranges tested and previous drug history.
Bicifadine exhibited some evidence for reinforcement, but only in two of four monkeys. In these subjects, infusion numbers for at least one dose exceeded those of the saline control by at least 2 S.D., and the dose-effect curve was an inverted U-shape. The more stable within-session distribution of responding for bicifadine than for saline provides evidence that bicifadine was serving as a reinforcer in M1375. However, the variable results among monkeys under the FR schedule suggest that bicifadine is a less efficacious reinforcer than the comparator drugs.
Differences in efficacy among reinforcing doses of cocaine, d-amphetamine, and bupropion and those of bicifadine in the two subjects who self-administered bicifadine under the FR schedule became apparent as self-administration was evaluated over sessions with progressively increasing work requirements. PR schedules have been reliably used to evaluate the relative reinforcing efficacy of stimulant drugs (Lile et al., 2002, 2003). The most efficacious reinforcing doses of comparator drugs maintained responding across more than seven sessions. However, responding for bicifadine dropped to saline levels after only a modest increase in workload. Total lever presses increased 100 to 400% across sessions with escalating workload for the most efficacious doses of cocaine, d-amphetamine, and bupropion. In contrast, bicifadine increased lever presses by only 50 to 75% in the two subjects, and that increase was short-lived. Thus, maintenance of bicifadine responding under the FR schedule can be attributed to a weak reinforcing action, and then only in some subjects.
The rat microdialysis data may explain, in part, the weaker stimulus cues, low rates of motor activation, and low rates of bicifadine self-administration. Thus, bicifadine-induced elevations in accumbens DA levels were consistently lower than either bupropion or d-amphetamine. The substantial elevation in accumbens DA by d-amphetamine reflects not only DAT inhibition but increased DA release (Fleckenstein et al., 2007). Bupropion also increased DA levels in the NAcc to a greater extent than bicifadine. Moreover, the bupropion- and d-amphetamine-induced increases in accumbens DA are greater than in the striatum, whereas the level of DA elevations resulting from bicifadine administration were low and equivalent in both regions. This low efficacy of bicifadine in elevating accumbens DA suggests its weak reinforcing properties are due to the low potency of bicifadine as a [3H]DA uptake inhibitor relative to its ability to inhibit [3H]NE and [3H]5-HT uptake (Basile et al., 2007).
Although no neurochemical assessments were performed in monkeys, the self-administration of cocaine, d-amphetamine, and bupropion is consistent with the hypothesis that elevated DA levels are relevant to a drug’s reinforcing potential (Koob, 1992; Woolverton and Johnson, 1992). These three drugs strongly elevate DA and increase NE and 5-HT levels to a lesser extent (Reith et al., 1997). In contrast, selective NET or NET/SERT inhibitors such as desipramine and imipramine produce minimal reinforcement (Gasior et al., 2005). Moreover, coadministration of drugs that increase 5-HT levels decrease the reinforcing efficacy of cocaine and other psychomotor stimulants (Wee et al., 2005; Howell et al., 2007), possibly because of 5-HT-induced decreases in DA release (Czoty et al., 2002; Fink and Gothert, 2007). Thus, the limited reinforcing efficacy of bicifadine relative to the comparator drugs would be predicted based on its smaller elevation of NAcc DA levels, in particular, when combined with greater increases in 5-HT levels.
The lower efficacy of bicifadine than bupropion and d-amphetamine in increasing accumbens DA levels may result from a number of characteristics, including the relatively low potency of bicifadine in blocking DA uptake (IC50 ≈ 900 nM); the relatively large increases in 5-HT levels caused by bicifadine, which may serve to limit synaptic levels of DA by heterosynaptic inhibitory processes mediated via the activation of 5-HT1b, 5-HT 2C, or 5-HT3 receptors (Fink and Gothert, 2007); and its lack of releasing properties, all of which contrast with cocaine and d-amphetamine (Riddle et al., 2005; Venton et al., 2006). One may also speculate that bicifadine would have a low abuse liability because of an inability to sensitize noradrenergic and serotonergic neurons (Lanteri et al., 2008) and/or a preferential interaction with the “closed” or “inward facing” configuration of the DAT (Loland et al., 2008). The inward facing conformation differs from that bound by cocaine and does not mediate reinforcing or psychostimulant properties (Desai et al., 2005). Thus, the presence of multiple internal regulatory mechanisms attendant to the mechanism of action of triple reuptake inhibitors, as well as the intrinsic properties of bicifadine as a selective inhibitor of the “closed” conformation of the DAT, may act to limit its potential for abuse.
In summary, the behavioral profile of bicifadine in the cocaine-discrimination model resembles nonabused antidepressants more than highly abused psychostimulants. Moreover, the lower propensity for self-administration suggests that bicifadine is not an efficacious reinforcer and does not possess a high abuse potential. Whether through modulation of serotonergic pathways that may limit the amplitude of DA elevations in the NAcc and striatum, or through its interaction with the “closed” conformation of the DAT, the magnitude of the motor stimulant and reward cues associated with bicifadine are low (Carroll et al., 1990; Howell and Byrd, 1995; Czoty et al., 2002). These observations suggest that azabicyclohexanes like bicifadine would have little if any abuse potential in humans.
Acknowledgments
We thank Hua Li and Ellen Soehngen for technical assistance.
ABBREVIATIONS:
- 5-HT
serotonin
- ANOVA
analysis of variance
- AUC
area under the curve
- DA
dopamine
- DAT
dopamine transporter
- NAcc
nucleus accumbens
- NE
norepinephrine
- NET
norepinephrine transporter
- NSD
nonsignificant difference
- PR
progressive ratio
- SERT
serotonin transporter
- Veh
vehicle
Footnotes
This work was supported by DOV Pharmaceuticals, Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
References
- Baker LE, Riddle EE, Saunders RB, Appel JB. The role of monoamine uptake in the discriminative stimulus effects of cocaine and related compounds. Behav Pharmacol. 1993;4:69–79. [PubMed] [Google Scholar]
- Balster RL, Carroll FI, Graham JH, Mansbach RS, Rahman MA, Philip A, Lewin AH, Showalter VM. Potent substituted-3-beta-phenyltropane analogs of cocaine have cocaine-like discriminative stimulus effects. Drug Alcohol Depend. 1991;29:145–151. doi: 10.1016/0376-8716(91)90043-x. [DOI] [PubMed] [Google Scholar]
- Barkin RL, Barkin S. The role of venlafaxine and duloxetine in the treatment of depression with decremental changes in somatic symptoms of pain, chronic pain, and the pharmacokinetics and clinical considerations of duloxetine pharmacotherapy. Am J Ther. 2005;12:431–438. doi: 10.1097/01.mjt.0000162011.58990.94. [DOI] [PubMed] [Google Scholar]
- Basile AS, Janowsky A, Golembiowska K, Kowalska M, Tam E, Benveniste M, Popik P, Nikiforuk A, Krawczyk M, Nowak G, et al. Characterization of the antinociceptive actions of bicifadine in models of acute, persistent and chronic pain. J Pharmacol Exp Ther. 2007;321:1208–1225. doi: 10.1124/jpet.106.116483. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Broadbent J, Michael EK, Riddle EE, Apple JB. Involvement of dopamine uptake in the discriminative stimulus effects of cocaine. Behav Pharmacol. 1991;2:187–197. [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci. 2005;25:1889–1893. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Gasior M, Bergman J, Kallman MJ, Paronis CA. Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a concurrent schedule of food and i.v. drug delivery in rhesus monkeys. Neuropsychopharmacology. 2005;30:758–764. doi: 10.1038/sj.npp.1300593. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–1559. [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington DC: 1996. [Google Scholar]
- Katz JL, Izenwasser S, Terry P. Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology (Berl) 2000;148:90–98. doi: 10.1007/s002130050029. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Johnson KM, Deschaux O, Bandyopadhyay BC, Araldi GL, Carmona G, Munzar P, Smith MP, Balster RL, Beardsley PM, et al. Mixed cocaine agonist/antagonist properties of (+)-methyl 4β-(chlorophenyl)-1-methylpiperidine-3α-carboxylate, a piperidine-based analog of cocaine. J Pharmacol Exp Ther. 2003;305:143–150. doi: 10.1124/jpet.102.046318. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ, Vaughan BS, Harrington MJ, Burke WJ. Atomoxetine: a selective noradrenaline reuptake inhibitor for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Pharmacother. 2003;4:1165–1174. doi: 10.1517/14656566.4.7.1165. [DOI] [PubMed] [Google Scholar]
- Krieter PA, Gohdes M, Musick TJ, Duncanson FP, Bridson WE. Pharmacokinetics, disposition, and metabolism of bicifadine in humans. Drug Metab Dispos. 2008;36:252–259. doi: 10.1124/dmd.107.017871. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR. Self-administration in baboons and the discriminative stimulus effects in rats of bupropion, nomifensine, diclofensine and imipramine. Psychopharmacology (Berl) 1990;102:183–190. doi: 10.1007/BF02245920. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a nondopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–1734. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Birmingham AM, Wang Z, Woolverton WL, Davies HM, Nader MA. The reinforcing efficacy of the dopamine reuptake inhibitor 2beta-propanoyl-3beta-(4-tolyl)-tropane (PTT) as measured by a progressive-ratio schedule and a choice procedure in rhesus monkeys. J Pharmacol Exp Ther. 2002;303:640–648. doi: 10.1124/jpet.102.039180. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, Nader MA. The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307:356–366. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Shank JE, Jordan KB, Williams HL, Basile AS. The effect of DOV 102,677 on the volitional consumption of ethanol by the Myer’s high ethanol preferring rats. Alcohol Clin Exp Res. 2007;31:1866–1871. doi: 10.1111/j.1530-0277.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology (Berl) 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Role of monoamine transporters in mediating psychostimulant effects. AAPS J. 2005;7:E847–E851. doi: 10.1208/aapsj070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Basile AS. Triple reuptake inhibitors (“broad spectrum” anti-depressants) CNS Neurol Disord Drug Targets. 2007;6:141–149. doi: 10.2174/187152707780363285. [DOI] [PubMed] [Google Scholar]
- Tella SR, Goldberg SR. Subtle differences in the discriminative stimulus effects of cocaine and GBR-12909. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:639–656. doi: 10.1016/s0278-5846(00)00180-9. [DOI] [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Cadet JL. Differential regulation of dopamine transporter after chronic self-administration of bupropion and nomifensine. J Pharmacol Exp Ther. 1997;281:508–513. [PubMed] [Google Scholar]
- Tizzano JP, Stribling DS, Perez-Tilve D, Strack A, Frassetto A, Chen RZ, Fong TM, Shearman L, Krieter PA, Tschöp MH, et al. The triple uptake inhibitor DOV 21947 reduces body weight and plasma triglycerides in rodent models of diet-induced obesity. J Pharmacol Exp Ther. 2008;324:1111–1126. doi: 10.1124/jpet.107.133132. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unseen incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Young AM, Herling S. Drugs as reinforcers: studies in laboratory animals. In: Goldberg SR, Stolerman IP, editors. Behavioral Analysis of Drug Dependence. Academic Press; Orlando: 1986. pp. 9–67. [Google Scholar]