Abstract
Leishmania cannot synthesize purines de novo and therefore must scavenge purines from its host for survival and growth. Biochemical and genomic analyses have indicated that Leishmania species express three potential routes for the synthesis of guanylate nucleotides: 1) a two-step pathway that converts IMP to GMP; 2) a three-step pathway that starts with the deamination of guanine to xanthine, followed by phosphoribosylation to XMP and then conversion to GMP; or 3) direct guanine phosphoribosylation by HGPRT. To determine the role of the first of these pathways to guanylate nucleotide synthesis, an L. donovani line deficient in IMP dehydrogenase (IMPDH), the first step in the IMP to GMP pathway, was constructed by targeted gene replacement. The Δimpdh lesion triggered a highly restrictive growth phenotype in promastigotes in culture but did not impact parasitemias in mice. The dispensability of IMPDH in vivo is the first definitive demonstration that intracellular L. donovani amastigotes have access to a sufficient pool of guanine, xanthine, or guanylate precursors from the host.
Keywords: Leishmania donovani, gene targeting, purine salvage, inosine-5′-monophosphate dehydrogenase, guanylate nucleotides
Leishmania donovani is the etiological agent of visceral leishmaniasis, a disease that is invariably fatal if untreated. Leishmania species are digenetic, existing as the extracellular promastigote within the phlebotomine sandfly vector and as the intracellular amastigote in the phagolysosome of macrophages and other cells of the reticuloendothelial system. Drug regimens for visceral leishmaniasis are far from ideal because of toxicity, cost, intrusive routes of and prolonged administrations, and resistance. Thus, there is an acute need for new drugs and new drug targets. One pathway that has triggered considerable therapeutic interest for the treatment of parasitic diseases is that for the synthesis of purine nucleotides. While mammals synthesize purine nucleotides from amino acids and one-carbon compounds, Leishmania, as well as all protozoan parasites studied to date, are effectively auxotrophic for purines and consequently must salvage purines from their hosts [1–3]. Thus, supplementation of the growth medium with purines is an absolute nutritional requirement for Leishmania promastigotes cultured in defined medium.
The purine salvage pathway of Leishmania spp. is particularly complex, consisting of four enzymes that are capable of converting preformed host purines into the parasite nucleotide pool: 1) hypoxanthine-guanine phosphoribosyltransferase (HGPRT); 2) xanthine phosphoribosyltransferase (XPRT); 3) adenine phosphoribosyltransferase; and 4) adenosine kinase [3,4]. Genetic studies have provided powerful evidence that none of the four enzymes alone is essential and that both L. donovani promastigotes and amastigotes funnel all host purines into substrates of either HGPRT or XPRT [5–7]. Thus, HGPRT and XPRT are vital salvage enzymes, whereas adenine phosphoribosyltransferase and adenosine kinase are functionally superfluous. These same studies have implicated certain purine nucleotide interconversion enzymes as potential “Achilles heels” for purine salvage by the parasite and imply that they could be rational therapeutic targets.
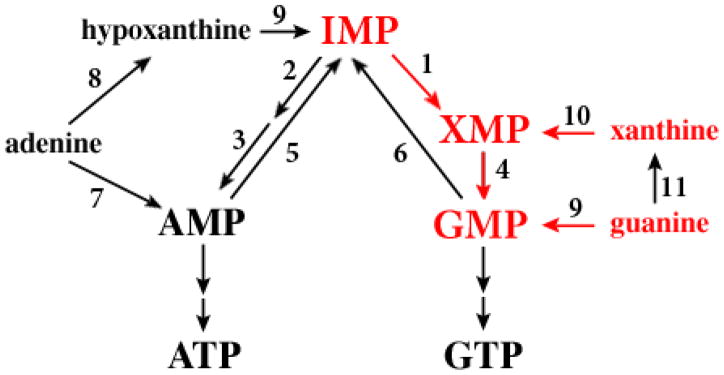
Inosine-5′-monophosphate dehydrogenase (IMPDH) (AAA29253.1) is one such nucleotide interconversion enzyme and catalyzes the irreversible conversion of IMP to XMP (Fig. 1). XMP is then converted by GMP synthetase (GMPS) to GMP, which serves as a precursor for all other guanylate nucleotides in the cell (Fig. 1). Thus, IMPDH plays a critical role in steering salvaged purines toward guanylate nucleotide synthesis and away from adenylate nucleotide synthesis, although GMP can also be reductively deaminated through GMP reductase to IMP and serve as a source of adenylate nucleotides. IMPDH is essential for mammalian cells, as well as most organisms, and has been highly touted as an antiviral, antibacterial, anticancer, and antiparasitic drug target [8]. Because L. donovani promastigotes can salvage hypoxanthine or adenine as the sole purine nutrient, a process that requires IMPDH, IMPDH is clearly operative in the insect vector stage of the parasite. Furthermore, mycophenolic acid and ribavarin, two potent and specific inhibitors of IMPDH enzymes [8,9], are also robust inhibitors of L. donovani promastigote growth when either hypoxanthine or adenine is provided as the sole purine source [10], implying that IMPDH is essential to the promastigote under these specific growth conditions. Finally, IMPDH, as well as GMP reductase, are both inhibited by metabolites of the well-characterized anti-leishmanial pyrazolopyrimidines, allopurinol, allopurinol riboside, 4-thiopurinol, and formycin B. [11,12]
Fig. 1.
Diagram of the purine salvage pathway of L. donovani. Relevant enzymes depicted include: 1, IMPDH; 2, adenylosuccinate synthetase; 3, adenylosuccinate lyase; 4, GMPS; 5, AMP deaminase; 6, GMP reductase; 7, APRT; 8, adenine deaminase; 9, HGPRT; 10, XPRT; 11, GDA. The pathways central to guanylate nucleotide synthesis are depicted in color.
The IMPDH from L. donovani has been characterized biochemically and is found in the glycosome [13], a peroxisome-like organelle unique to parasites of the Kinetoplastida order [14]. This organellar sequestration of IMPDH is mediated by a COOH-terminal tripeptide, Ala-Lys-Met (AKM) [13]. Biochemical and bioinformatic analyses of available Leishmania genomes (http://tritrypdb.org/tritrypdb), however, have revealed a second prospective pathway for guanylate nucleotide synthesis in Leishmania, one in which guanine is first converted to xanthine by guanine deaminase (GDA), xanthine is then phosphoribosylated to XMP via XPRT, and XMP is converted to GMP by GMPS [1–3] (see Fig. 1). This alternative route to guanylate nucleotide production is likely to be operational in L. donovani promastigotes since Δhgprt promastigotes can grow in exogenous guanine and guanosine [6], and XPRT, the only remaining functionally active purine salvage enzyme in Δhgprt parasites, recognizes guanine very inefficiently [13]. A third possible path for GMP synthesis in Leishmania, direct phosphoribosylation of guanine by HGPRT, does not seem robust in L. donovani since Δxprt promastigotes cannot grow on guanine as the sole source of purine in the culture medium [6]. The functionality of the two routes for GMP synthesis that have been validated in promastigotes, the IMPDH-GMPS and the GDA-XPRT-GMPS pathways, have not, however, been evaluated in amastigotes, the infectious and mammalian form of the parasite that resides within the phagolysosome of infected macrophages. Purine pool levels within the phagolysosome to which amastigotes have access are not known.
To test whether IMPDH is indispensable to Leishmania amastigotes, we generated Δimpdh null mutants in a strain of L. donovani that retains the ability to infect mice [7]. Two independent Δimpdh clones were derived from two separate IMPDH/impdh heterozygotes, IMPDH/impdh-1 and IMPDH/impdh-2, and were designated Δimpdh-1 and Δimpdh-2, respectively. The Δimpdh-1 and Δimpdh-2 clones were also complemented by integrating either a wild type IMPDH, or a mutant impdh ΔAKM copy in which the AKM glycosomal targeting signal had been removed, into the ribosomal RNA locus. These “add-back” lines were designated Δimpdh-1[pRP-IMPDH] or Δimpdh-2[pRP-IMPDH] and Δimpdh-2[pRP-impdhΔAKM] or Δimpdh-2[pRP-impdhΔAKM], respectively.
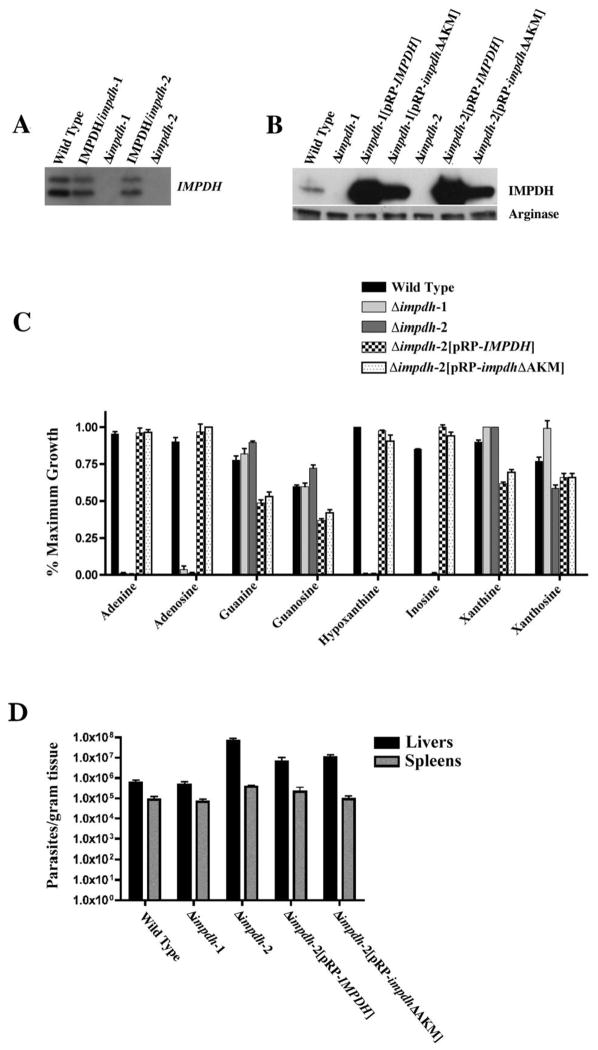
The Δimpdh lesions in the knockout lines were confirmed by Southern blot analysis of genomic DNA prepared from wild type, IMPDH/impdh-1, Δimpdh-1, IMPDH/impdh-2 and Δimpdh-2 hybridized to the IMPDH coding sequence (Fig. 2A). Equal loading of each lane in the Southern blot was ascertained by ethidium bromide staining (data not shown). The gene replacements at the IMPDH locus in the null mutants were corroborated by Western blot analysis of lysates from wild type, Δimpdh-1, Δimpdh-2 and “add-back” promastigotes (Fig. 2B) using polyclonal antisera specific for IMPDH [13]. These immunoblots confirmed the presence of IMPDH protein in wild type and “add-back” parasites and the absence of IMPDH expression in the null mutants (Fig. 2B). This IMPDH expression pattern was also observed when the promastigotes were converted to axenic amastigotes (data not shown). The glycosomal and cytosolic localizations, respectively, of IMPDH in the Δimpdh-2[pRP-IMPDH] and Δimpdh-2[pRP-impdhΔAKM] “add-backs,” were verified by indirect immunofluorescence (Fig. S1). Because IMPDH is a key branchpoint between synthesis of adenylate and guanylate nucleotides and pharmacologic ablation of IMPDH activity was lethal to L. donovani promastigotes when adenine or hypoxanthine was the sole purine source [10], the growth phenotypes conferred by the genetic lesions in the Δimpdh lines were tested under defined growth conditions. Whereas supplementation of the medium with xanthine, xanthosine, guanine, or guanosine was permissive for Δimpdh-1 and Δimpdh-2 promastigote proliferation, neither of the null mutant clones could grow in medium in which adenine, adenosine, hypoxanthine, or inosine was the singular purine source (Fig. 2C, Table S1). Conversely, wild type, Δimpdh-2[pRP-IMPDH] and Δimpdh-2[pRP-impdhΔAKM] promastigotes could replicate in all eight purines tested, although growth was generally less robust in guanine or guanosine (Fig. 2C, Table S1). Growth of Δimpdh-1[pRP-IMPDH] and Δimpdh-1[pRP-impdhΔAKM] “add-back” lines was also permissive in all purines tested (data not shown). Thus, the restrictive growth phenotype of Δimpdh promastigotes could be ascribed to the genetic lesion and not to some ancillary epigenetic change in the null parasites. Moreover, the ability of the Δimpdh-1[pRP-impdhΔAKM] and Δimpdh-2[pRP-impdhΔAKM] promastigotes to grow in adenine, adenosine, hypoxanthine, and inosine implied that glycosomal localization of IMPDH is not essential for IMPDH function in the insect vector stage of the parasite. It should be noted, however, that IMPDH expression levels are higher in the Δimpdh-1[pRP-impdhΔAKM] and Δimpdh-2[pRP-impdhΔAKM] “add-backs” than in wild type parasites (Fig. 2B), so a significant but incomplete functional deficit due to cytosolic mislocalization of the mutant impdh could have been masked by over-expression. The null genotype and restrictive growth phenotype of cultured Δimpdh parasites authenticates a single IMPDH encoding locus in L. donovani. In contrast, mammalian cells have two similar but distinct IMPDH genes [15].
Fig. 2.
Molecular characterization of Δimpdh parasites and test of IMPDH function in infectivity. Δimpdh parasites were created from previously reported IMPDH/impdh strains [18] by targeted gene replacement in the LdBob L. donovani strain that retains its ability to infect macrophages and mice after prolonged culture [7,19]. Targeting constructs were those described previously [18]. To confirm the genetic lesions, Southern and western analyses were performed. (A) Total genomic DNA (~5 μg) from wild type L. donovani (lane 1), IMPDH/impdh-1 (lane 2), Δimpdh-1 (lane 3), IMPDH/impdh-2 (lane 4) and Δimpdh-2 (lane 5) was digested with XholI, fractionated on a 1% agarose gel, and blotted onto a nylon membrane. The blot was hybridized under high stringency conditions using the IMPDH coding sequence as the probe. (B) Lysates of exponentially growing wild type, Δimpdh-1, Δimpdh-2, Δimpdh-2[pRP-IMPDH] and Δimpdh-2[pRP-impdhΔAKM] promastigotes were analyzed by immunoblotting using anti-IMPDH monospecific polyclonal antisera [13]. The amount of protein loaded onto each lane was normalized by blotting with antisera to the L. donovani arginase enzyme. (C) The ability of wild type, Δimpdh-1, Δimpdh-2, Δimpdh-2[pRP-IMPDH] and Δimpdh-2[pRP-impdhΔAKM] promastigotes to proliferate in eight different purine sources was evaluated. Exponentially growing parasites were seeded at 5 × 104 cells/mL in DME-L medium supplemented with 5% dialyzed fetal bovine serum and one of the indicated purines at a concentration of 100 μM. Cell viability was then assessed using Alamar Blue, a redox indicator that yields a colorimetric change upon its reduction triggered by metabolic activity [20]. Growth for each cell line in each purine was calculated as the mean of three replicates. Data for Δimpdh-1[pRP-IMPDH] and Δimpdh-1[pRP-impdhΔAKM] promastigotes were essentially identical to those obtained with their Δimpdh-2 counterparts and are not depicted. (D) Five groups of five Balb/c mice were infected with either 5 × 106 wild type, Δimpdh-1, Δimpdh-2, Δimpdh-2[pRP-IMPDH] or Δimpdh-2[pRP-impdhΔAKM] stationary phase promastigotes. The mice were sacrificed 4 weeks post-infection and the parasite loads in liver and spleens were quantified using limiting dilution. The limiting dilution medium for all cell lines was modified DME-L [6] containing 5% FBS and 100 μM xanthine as the purine source. Data presented are the average results and standard error from five animals.
To test whether the loss of IMPDH impacted the ability of L. donovani to establish infection in Balb/c mice, a well-established rodent model for visceral leishmaniasis in which parasite can replicate but do not cause disease [16], groups of five mice were inoculated with either wild type, Δimpdh-1, Δimpdh-2, Δimpdh-2[pRP-IMPDH], or Δimpdh-2[pRP-impdhΔAKM] stationary phase promastigotes via tail vein injection and parasite loads in livers and spleens determined after four weeks (Fig. 2D). Parasite burdens in livers and spleens of mice infected with each of the five lines were comparable indicating that IMPDH function is not essential to establish infection in mice. These data establish that Δimpdh L. donovani amastigotes can satisfy their guanylate nucleotide requirement by salvaging host purines from the phagolysosome through an IMPDH-independent route. This route presumably involves direct access to the guanylate nucleotide pool of the phagolysosome, a mechanism that would entail guanine deamination to xanthine, xanthine phosphoribosylation to XMP, and XMP conversion to GMP, or alternatively, through HGPRT-catalyzed phosphoribosylation of guanine to GMP (see Fig. 1). Whether the IMPDH-dependent route is utilized by amastigotes at all in vivo awaits additional genetic analysis. We conjecture at this point that the GDA-XPRT-GMPS pathway predominates over HGPRT as the major route of guanine salvage for at least three reasons: 1) Δxprt parasites generated in our laboratory are unable to grow in guanine, suggesting that all guanine is deaminated to xanthine, an unusable purine for a Δxprt mutant [6], since xanthine is not a substrate for HGPRT [17]; 2) we have shown by thin layer chromatography that intact L. donovani promastigotes fully convert 100% of exogenous [14C]guanosine (57 μM) to intracellular xanthine in <5 minutes; and 3) repeated attempts to generate a Δgmps cell line by targeted gene replacement have failed, even when supplied with excess amounts of guanine in the selection medium. Taken together, these investigations insinuate that GMPS, but not IMPDH, is a valid therapeutic target.
Supplementary Material
Acknowledgments
This study was supported by grant AI023682 provided by the National Institute of Allergy and Infectious Diseases. We thank Dr. Nicola S. Carter for her insightful comments on the manuscript.
Abbreviations
- GDA
guanine deaminase
- GMPS
GMP synthetase
- HGPRT
hypoxanthine-guanine phosphoribosyltransferase
- IMPDH
IMP dehydrogenase
- XPRT
xanthine phosphoribosyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berens RL, Krug, Edward C, Marr, Joseph J. Purine and Pyrimidine Metabolism. In: Marr JJ, Muller M, editors. Biochemistry and Molecular Biology of Parasites. Academic Press Ltd; London, England; San Diego, CA: 1995. pp. 89–117. [Google Scholar]
- 2.Looker DL, Berens RL, Marr JJ. Purine metabolism in Leishmania donovani amastigotes and promastigotes. Mol Biochem Parasitol. 1983;9:15–28. doi: 10.1016/0166-6851(83)90053-1. [DOI] [PubMed] [Google Scholar]
- 3.Marr JJ, Berens RL, Nelson DJ. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta. 1978;544:360–71. doi: 10.1016/0304-4165(78)90104-6. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle JV, Krenitsky TA. Purine phosphoribosyltransferases from Leishmania donovani. J Biol Chem. 1980;255:909–16. [PubMed] [Google Scholar]
- 5.Iovannisci DM, Ullman B. Characterization of a mutant Leishmania donovani deficient in adenosine kinase activity. Mol Biochem Parasitol. 1984;12:139–51. doi: 10.1016/0166-6851(84)90131-2. [DOI] [PubMed] [Google Scholar]
- 6.Boitz JM, Ullman B. Leishmania donovani singly deficient in HGPRT, APRT or XPRT are viable in vitro and within mammalian macrophages. Mol Biochem Parasitol. 2006;148:24–30. doi: 10.1016/j.molbiopara.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Boitz JM, Ullman B. A Conditional Mutant Deficient in Hypoxanthine-guanine Phosphoribosyltransferase and Xanthine Phosphoribosyltransferase Validates the Purine Salvage Pathway of Leishmania donovani. J Biol Chem. 2006;281:16084–9. doi: 10.1074/jbc.M600188200. [DOI] [PubMed] [Google Scholar]
- 8.Shu Q, Nair V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med Res Rev. 2008;28:219–32. doi: 10.1002/med.20104. [DOI] [PubMed] [Google Scholar]
- 9.Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson K, Collart FR, Huberman E, Stringer JR, Ullman B. Amplification and molecular cloning of the IMP dehydrogenase gene of Leishmania donovani. J Biol Chem. 1991;266:1665–71. [PubMed] [Google Scholar]
- 11.Marr JJ. Purine analogs as chemotherapeutic agents in leishmaniasis and American trypanosomiasis. J Lab Clin Med. 1991;118:111–9. [PubMed] [Google Scholar]
- 12.Spector T, Jones TE, LaFon SW, Nelson DJ, Berens RL, Marr JJ. Monophosphates of formycin B and allopurinol riboside. Interactions with leishmanial and mammalian succino-AMP synthetase and GMP reductase. Biochem Pharmacol. 1984;33:1611–7. doi: 10.1016/0006-2952(84)90282-x. [DOI] [PubMed] [Google Scholar]
- 13.Dobie F, Berg A, Boitz JM, Jardim A. Kinetic characterization of inosine monophosphate dehydrogenase of Leishmania donovani. Mol Biochem Parasitol. 2007;152:11–21. doi: 10.1016/j.molbiopara.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Opperdoes FR, Michels PA. The glycosomes of the Kinetoplastida. Biochimie. 1993;75:231–4. doi: 10.1016/0300-9084(93)90081-3. [DOI] [PubMed] [Google Scholar]
- 15.Garat A, Cauffiez C, Hamdan-Khalil R, Glowacki F, Devos A, Leclerc J, Lionet A, Allorge D, Lo-Guidice JM, Broly F. IMPDH2 genetic polymorphism: a promoter single-nucleotide polymorphism disrupts a cyclic adenosine monophosphate responsive element. Genet Test Mol Biomarkers. 2009;13:841–7. doi: 10.1089/gtmb.2009.0096. [DOI] [PubMed] [Google Scholar]
- 16.Wilson ME, Jeronimo SM, Pearson RD. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathog. 2005;38:147–60. doi: 10.1016/j.micpath.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Allen TE, Hwang HY, Jardim A, Olafson R, Ullman B. Cloning and expression of the hypoxanthine-guanine phosphoribosyltransferase from Leishmania donovani. Mol Biochem Parasitol. 1995;73:133–43. doi: 10.1016/0166-6851(94)00105-v. [DOI] [PubMed] [Google Scholar]
- 18.Fulwiler AL, Soysa DR, Ullman B, Yates PA. A rapid, efficient and economical method for generating leishmanial gene targeting constructs. Mol Biochem Parasitol. 175:209–12. doi: 10.1016/j.molbiopara.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, Turco SJ, Beverley SM. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997;204:205–8. doi: 10.1016/s0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.