Abstract
The hypothalamic melanocortin-4 receptor (MC4R) is a constituent of an important pathway regulating food intake and energy expenditure. We produced a monoclonal antibody (mAb) directed against the N-terminal domain of the MC4R and evaluated its potential as a possible therapeutic agent. This mAb (1E8a) showed specific binding to the MC4R in human embryonic kidney 293 cells expressing the human MC4R and blocked the activity of the MC4R under basal conditions and after stimulation with α-melanocyte-stimulating hormone (α-MSH). The inverse agonist action of Agouti-related protein was significantly enhanced in the presence of mAb 1E8a. After a single intracerebroventricular injection into the third ventricle, mAb 1E8a (1 μg) increased 24-h food intake in rats. After 7 days of continuous intracerebroventricular administration, mAb 1E8a increased food intake, body weight, and fat pad weight and induced hyperglycemia. Because the complete mAb was ineffective after intravenous injection, we produced single-chain variable fragments (scFvs) derived from mAb 1E8a. In pharmacokinetic studies it was demonstrated that these scFvs crossed the blood-brain barrier and reached the hypothalamus. Consequently, the scFv 1E8a increased significantly food intake and body weight in rats after intravenous administration (300 μg/kg). The pharmacological profile of mAb 1E8a and the fact that its scFv was active after peripheral administration suggest that derivatives of anti-MC4R mAbs may be useful in the treatment of patients with anorexia or cachexia.
Antibodies (Abs) as therapeutic agents are currently receiving renewed interest in experimental and clinical medicine. Their selectivity, potency, and efficacy at various targets have made them successful drugs in different indications, mainly cancer and autoimmune disorders (Kornbluth, 1998; Schuna and Megeff, 2000; Bohen et al., 2003). In previous studies in rats (Peter et al., 2007; Hofbauer et al., 2008) we used active and passive immunization to stimulate appetite by inhibiting the activity of the central melanocortin-4 receptor (MC4R).
The MC4R is part of an important central pathway of appetite regulation (Cowley, 2003; Ellacott and Cone, 2004; Adan et al., 2006). Its stimulation leads to an anorexigenic response, i.e., a decrease in appetite and food intake. Conversely, its blockade induces an orexigenic response, i.e., an increase in appetite and food intake. Blockade of the MC4R has been demonstrated to be effective in preventing anorexia and the concomitant loss of fat and lean body mass in rodent models of tumor-induced cachexia (Scarlett and Marks, 2005; DeBoer and Marks, 2006; Nicholson et al., 2006).
In all of our immunization experiments the N-terminal (NT) domain of the MC4R was used as an antigen because it is known to be essential for the constitutive activity of this receptor (Srinivasan et al., 2004). We demonstrated that active immunization of rats against a 16-amino acid sequence of the NT domain resulted in a mild form of obesity and insulin resistance (Peter et al., 2007). Furthermore, immunization of rats against the NT peptide prevented the decrease of body weight and the loss of appetite in an acute model of anorexia induced by the intraperitoneal application of lipopolysaccharide (Hofbauer et al., 2008). In a series of in vitro experiments, we demonstrated that anti-MC4R Abs purified from the plasma of immunized rats acted as inverse agonists in the absence of an MC4R agonist and as noncompetitive antagonists in the presence of an MC4R agonist (Peter et al., 2007). Finally, it could be shown that the passive transfer of purified polyclonal Abs from immunized rats by intracerebroventricular injection into the third ventricle of nonpretreated rats induced an increase in their food intake (Peter et al., 2007).
In the present study, we produced and characterized a monoclonal antibody (mAb) targeting the NT sequence of the MC4R and explored its therapeutic potential. This mAb (1E8a) acted in vitro as an inverse agonist and a noncompetitive antagonist. In vivo, mAb 1E8a increased food intake in rats after acute and chronic intracerebroventricular administration but was not effective after intravenous administration. To improve penetration across the blood-brain barrier, recombinant single-chain variable fragments (scFvs) were produced and evaluated in vitro and in vivo. They also acted as inverse agonists and noncompetitive antagonists and increased food intake after intravenous administration. To our knowledge, this is the first report on a pharmacologically active mAb against the MC4R and its scFv. By virtue of their pharmacological profile and pharmacokinetic properties, these agents could represent lead molecules for the development of therapies for patients with anorexia or cachexia.
Materials and Methods
Production of Monoclonal Antibodies. A peptide corresponding to the NT domain of the MC4R (KTSLHLWNRSSHGLHG, residues 11–25 of the MC4R) was used as antigen. The NT peptide shares the sequence of the rat MC4R (rMC4R) and human MC4R (hMC4R) isoform from residues 2 to 15. NT3 peptide (ASNRSGSGFCEQVFIKPEV, residues 26–44 of the MC3R) was synthesized as described previously (Peter et al., 2007).
C57BL/6 mice were immunized with 25 μg of the free NT peptide emulsified in complete Freund’s adjuvant and injected subcutaneously. Four weeks later, a booster injection of 25 μg in incomplete Freund’s adjuvant was given. Four weeks later, 10 μg of peptide dissolved in NaCl 0.9% was injected intravenously 3 days before harvesting the spleen cells for fusion. Fusion was performed with polyethylene glycol 1500 (Sigma-Aldrich, St. Louis, MO) at a ratio of two splenocytes to one SP2O myeloma cell. Hybridomas were cultivated in 96-well plates pre-coated with peritoneal macrophages of C57BL/6 mice at 1000 cells/well. A total of 5 × 105 cells was distributed per well in Isocoves modified Dulbecco’s medium supplemented with 10% heat-inactivated fetal calf serum, 200 mM glutamine, 100 mM sodium pyruvate, 1% penicillin/streptomycin (Omnilab, Mettmenstetten, Switzerland), and 3% hypoxanthine/aminopterine/thymidine (Invitrogen, Carlsbad, CA) in a humidified incubator at 37°C under an atmosphere of 5% CO2. Secreting clones were screened by enzyme immunoassay and subcloned by limiting dilution (Oi et al., 1980).
Enzyme Immunoassay. NT peptide (2 μM) was adsorbed with carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6), on 96-well maxisorp microtiter plates (NUNC A/S, Roskilde, Denmark), 50 μl/well, by incubating for 2 h at 37°C. Plates were saturated with phosphate-buffered saline (PBS) (10 mM Na2HPO4, 150 mM NaCl, 27 mM KCl, pH 7.4) supplemented with 3% dried milk (Bio-Rad Laboratories, Hercules, CA) and 0.05% Tween 20 (Fluka, Buchs, Switzerland) (PBS-T milk) for 1 h at 37°C. Immunized mice sera or 1:10 dilution of hybridoma culture supernatant were added to the plates and incubated for 1 h at room temperature (RT). Plates were then washed with PBS-T, incubated with goat anti-mouse immunoglobulin H+L horseradish peroxidase-conjugated (Bio-Rad Laboratories), and diluted 1:5000 for 1 h at 37°C. After washing the plates with PBS-T and PBS, enzymatic reactions were carried out at RT by adding 3,3′,5,5′-tetramethylbenzidine in the presence of 0.04% H2O2. Reactions were stopped after 15 min by the addition of 1 N HCl. Optical density (OD) was measured at 450 nm by using a microplate reader Multiskan RC (Thermo Fisher Scientific, Waltham, MA).
Isotyping and Purification. The isotype of the selected mAbs was determined by using a MonoAbiD kit according to the manufacturer’s instructions (Zymed Laboratories, Paris, France). The anti-NT mAbs were affinity-purified on an activated cyanogen bromide-Sepharose 4B column (GE Healthcare, Little Chalfont, Buckinghamshire, UK) with NT peptide coupled via the N terminus according to the manufacturer’s instructions. Culture supernatants were loaded on the column at 4°C. The mAbs were eluted with 0.2 M glycine (pH 2.7), collected in tubes containing 1 M Tris buffer (pH 8.0), subsequently dialyzed against PBS overnight at 4°C, and finally stored at −20°C.
Immunoprecipitation. Human embryonic kidney (HEK)-293 cells stably transfected with the hMC4R or hypothalami from male Sprague-Dawley rats were prepared as described previously (Peter et al., 2005). MC4R membrane preparations (50 μg) were incubated with purified mAb 1E8a (25 μg) or mAb 2G2 (25 μg) overnight at 4°C. The mixture was immunoprecipitated with 25 μl of protein A/G agarose. The immunoprecipitated samples were loaded on a 10% polyacrylamide gel and transferred onto nitrocellulose membranes. The presence of MC4R was detected by using polyclonal anti-MC4R Abs (Abcam plc, Cambridge, UK), and the presence of MC3R was detected by using polyclonal anti-MC3R Abs (Abcam plc) in the standard procedure described for the One-Step Complete IP-Western kit (Genscript, Piscataway, NJ).
Inhibition Experiments. Purified mAb 1E8a (25 μg) was preincubated overnight at 4°C in the presence or absence of increasing concentrations of the NT peptide (32 nM to 100 μM). MC4R membrane preparations from HEK-293 cells stably transfected with the hMC4R (50 μg) were incubated with the mAb 1E8a/NT peptide mixture overnight at 4°C. The mixtures were immunoprecipitated with 25 μl of protein A/G agarose. The immunoprecipitated samples were loaded on a 10% polyacrylamide gel and transferred onto nitrocellulose membranes. The presence of MC4R was detected by using polyclonal anti-MC4R Abs (Abcam plc) in the standard procedure described for the One-Step Complete IP-Western kit (Genscript). The Western blot films were digitalized, and resulting images were quantified by using Scion Image Beta 4.03.02 (downloaded from www.scioncorp.com). Inhibition percentage of the interaction of the mAb 1E8a with the hMC4R was calculated as follows: 1 − (OD[NT]/OD(withoutNT)) × 100, where OD[NT] is the optical density of the band corresponding to the hMC4R for a given concentration of NT peptide and OD(without NT) is the optical density of the band corresponding to the hMC4R without NT peptide.
Surface Plasmon Resonance. Assays were performed in the BIACORE 3000 system as described previously (Peter et al., 2007). Kinetic parameters of the interaction between NT peptide and anti-MC4R mAbs were measured at 25°C in two series of experiments: first, five different concentrations (0.25–4 μM) of purified mAb 1E8a were injected at a flow rate of 30 μl/min for 300 s on the immobilized NT peptide or NT3 peptide as control followed by a dissociation phase of 400 s. Subsequently, five different concentrations (0.125–1 μM) of purified mAb 2G2 were injected at a flow rate of 30 μl/min for 300 s on the immobilized NT peptides followed by a dissociation phase of 400 s. The kinetic parameters were calculated by using BIA evaluation software 4.1 (BIAcore AB, Uppsala, Sweden). All resonance unit values obtained with the control NT3 peptide were subtracted from those obtained with the MC4R NT peptide to compensate for nonspecific binding.
Cell Culture. HEK-293 cells expressing hMC4R or hMC3R were cultured in Dulbecco’s modified Eagle medium (Sigma-Aldrich) containing 10% fetal calf serum (Bioconcept, Allschwil, Switzerland), 1% penicillin/streptomycin (Invitrogen), and G418 at 600 μg/ml (Sigma-Aldrich) in a humidified atmosphere containing 5% CO2 at 37°C.
cAMP Assays. Cells were transferred to 24-well culture plates 12 h before treatment, washed for 3 h with Dulbecco’s modified Eagle medium (Sigma-Aldrich), and incubated for 30 min in PBS supplemented with 0.1% bovine serum albumin (BSA) and 3-isobutyl-1-methylxanthine (Sigma-Aldrich). Cells were treated with serial dilutions of purified mAbs or scFvs for 30 min or preincubated with fixed concentrations of mAbs or scFvs for 30 min and then treated with serial dilutions of α-melanocyte-stimulating hormone (α-MSH; 1.0 × 10−10 to 1.0 × 10−5 M) for 15 min or Agouti-related protein (AgRP; 1.0 × 10−12 to 1.0 × 10−6 M). Subsequently, cells were lysed with Biotrak cAMP lysis buffer, and cAMP was measured by using the Biotrak cAMP enzyme immunoassay system (GE Healthcare) according to the manufacturer’s instructions. Protein concentration was determined by using the BCA kit (Pierce Chemical, Rockford, IL). The concentration of cAMP was expressed in fmol cAMP/μg protein.
Immunocytofluorescence. HEK-293 cells expressing hMC4R or hMC3R were fixed for 5 min with 2% paraformaldehyde in PBS. Slides were saturated with PBS supplemented with 5% nonfat dried milk. mAbs 2G2 or 1E8a (50 μg/ml) were applied on cells for 1 h at RT. After three washes with PBS, goat anti-mouse Alexa-conjugated (1/500) (Invitrogen) was allowed to react with the fixed primary antibody for 1 h at RT. 4′,6-Diamidino-2-phenylindole, dihydrochloride (1 μg/ml; Invitrogen) was used for nuclear staining. The same magnification (40×) and exposure time (500 ms) was used for each slide.
Receptor Internalization. HEK-293 cells expressing the hMC4R were treated with 200 nM of α-MSH (Bachem, Bubendorf, Switzerland) conjugated with tretramethylrhodamine-5–6-isothiocyanate (Invitrogen) using the manufacturer’s instructions. Cells were washed with ice-cold PBS and fixed at t = 0, 10, 30, and 45 min. Concanavalin A Alexa-Fluor 488 (0.1 mg/ml; Invitrogen) and 4′,6-diamidino-2-phenylindole, dihydrochloride (1 μg/ml, Invitrogen) were applied on cells for membrane labeling and nuclear staining, respectively. The original green (concanavalin A Alexa-Fluor 488) and red fluorescence (α-MSH conjugated with tetramethylrhodamine-5-(and 6)-isothiocyanate) confocal images were converted to gray-scale. Each pixel was assigned an intensity value ranging from 0 (black) to 255 (white). The grayscale images obtained with concanavalin A fluorescence were subtracted from grayscale images obtained with α-MSH fluorescence. The resulting images were quantified by using Scion Image Beta 4.03.02 (downloaded from www.scioncorp.com). An increase of the OD signal indicated internalization of the MC4R.
Cloning of cDNA Encoding the Variable Domain of the mAbs. Total RNA was prepared from 107 freshly subcloned hybridoma cells by using the RNAnow kit (Biogentex Inc., Seabrook, TX), and first-strand cDNA was synthesized by using the iScriptcDNA Synthesis kit (Bio-Rad Laboratories). The VH and VL chain domains were amplified by polymerase chain reaction (PCR) by using the IgG primer set (Novagen, Gibbstown, NJ). The 50-μl PCR mixtures contained 50 ng of hybridoma cDNA, 20 pmol of each appropriate primer, 250 μM of each dNTP, 1× Taq buffer (Sigma-Aldrich), and 1 U Thermophilus aquaticus (Taq) polymerase. Amplification included 50 cycles of 1.5 min at 94°C, 2.5 min at 55°C, and 3 min at 72°C in a thermocycler (PTC-150; MJ Research, Watertown, MA). The amplified DNAs were ligated into the pGEMT vector (Promega, Madison, WI), and the recombinant plasmids were purified with a miniprep kit (QIAGEN, Hombrechtikon, Switzerland). The DNA sequences of the cloned VH and VL inserts were determined with the ABI PRISM Cycle Sequencing kit (Applied Biosystems, Foster City, CA) and M13 forward and reverse primers. The sequences of the V genes were determined in two independent batches of RNA preparations to ensure accuracy.
Construction of the scFv Genes. scFv proteins were created by joining their VH and VL genes together by PCR splicing with overlap extensions using oligonucleotides that encoded a 15-amino acid linker (G4-S)3 between the C terminus of the VH and the N terminus of the VL gene. The ends of the 1E8a mAb variable gene were modified by PCR using as primers, 1E8VHRev (5′-GTT CCA GCT GCA GCA GTC TGG ACC TGA GC-3′), which encodes the NT wild-type sequence of the VH containing a NcoI site and VHFor (5′-ACC ACC GGA TCC GCC TCC GCC TGA GGA GAC TGT GAG CGT-3′), which encodes the C terminus of the VH and a part of the linker. 1E8VLRev (5′-GGA GGC GGA TCC GGT GGT GGC GGA TCT GGA GGT GGC GGA AGC GAC ATT GTG ATG ACC CAG-3′) and VLFor, containing a XhoI site that encodes six His residues (5′-GCA ATT CCT CGA GTT AGT GAT GGT GAT GGT GAT GTT TTA TTT CCA GCT TGG TCC-3′) were used to amplify and modify the VL domain.
The ends of the 2G2 mAb variable gene were modified by PCR using as primers 2G2VHRev (5′-TGG CCA TGG CCG CGG GAT TGG TCC AGC TGC AGC AGT CTG GA-3′), which encodes the NT wild-type sequence of the VH containing a NcoI site, and VHFor (5′-ACC ACC GGA TCC GCC TCC GCC TGA GGA GAC TGT GAG CGT-3′), which encodes the C terminus of the VH and part of the linker. 2G2VLRev (5′-GGA GGC GGA TCC GGT GGT GGC GGA TCT GGA GGT GGC GGA AGC GAC ATT GTG ATG ACC CAG-3′) and 2G2VLFor, containing a XhoI site that encodes six His residues (5′-GCA ATT CCT CGA GTT AGT GAT GGT GAT GGT GAT GTT TTA TTT CCA GCT TGG TCC-3′), were used to amplify and modify the VL domain. The scFv gene was inserted in-frame with the PelB sequence on the expression vector pET22b (EMD Biosciences, Darmstadt, Germany) between the NcoI and XhoI sites.
Expression of scFvs. Escherichia coli Rosetta bacterias transformed with pET22b(+)-1E8a or pET22b(+)-2G2 were grown in 500 ml of medium 2×YT (1.6% bactotryptone, 1% bactoyeast extract, 0.5% NaCl, pH 7.0) containing 0.15 mM ampicillin (Applichem, Darmstadt, Germany) and 0.1 mM chloramphenicol (Gerbu Biotechnik, Gaiberg, Germany) until reaching an OD600nm of 0.6 at 37°C with agitation at 200 rpm. The expression of scFv was induced by adding 1 mM isopropyl β-d-thiogalactopyranoside (Applichem) at RT for 4 h.
Periplasmic Extraction. Five hundred milliliters of bacteria cultures was centrifuged (10 min, 10,000g, 4°C), and the pellet was resuspended in 200 ml of 30 mM Tris, 1 mM EDTA, 2% sucrose, pH 8.5). After centrifugation (10 min, 10,000g, 4°C), the pellet was resuspended in 50 ml of 5 mM MgSO4. After a last centrifugation (10 min, 10,000g, 4°C) the supernatant corresponding to the periplasmic extract (PE) was collected. This PE was dialyzed in wash buffer Ni-NTA (20 mM imidazole, 50 mM Na2HPO4, 300 mM NaCl, pH 8.0) overnight at 4°C.
Purification of scFv. The scFvs were purified from the PE on Ni-NTA columns according to the manufacturer’s instruction (QIAGEN). After elution, purified scFvs were dialyzed in PBS (10 mM Na2HPO4, 150 mM NaCl, 27 mM, KCl, pH 7.4) overnight at 4°C. The scFvs were then purified by immunoadsorption as described in Isotyping and Purification. The concentrations were determined with a Micro BCA Protein Assay kit (Pierce Chemical). The quality and purity of the purified scFv fractions were assessed by SDS-polyacrylamide gel electrophoresis analysis using 12.5% acrylamide gels followed by staining with Coomassie Brilliant Blue (Bio-Rad Laboratories) and Western blot. For Western blot analysis, the proteins were transferred from the gels onto a nitrocellulose transfer membrane using a mini-transblot system (Bio-Rad) in transfer buffer (25 mM Tris-HCl, 190 mN glycin, 20% methanol, pH 8.3). The membranes were soaked for 1 h in PBS-T supplemented with 5% nonfat milk powder and 0.1% Tween 20. This was followed by 1-h incubation with anti-His6 Ab conjugated to horseradish peroxidase 1/2000 (Sigma-Aldrich). The Ab was diluted in the blocking solution PBS-T milk. The proteins on the membranes were revealed by the classical procedure of the enhanced chemiluminescence reagents (ECL; GE Healthcare).
Radioactive Labeling and Purification. The scFv 1E8a was radioactively labeled with 131I by the iodobead method. In brief, an iodobead was incubated in 0.1 ml of phosphate buffer solution with 2 mCi of 131I for 5 min at RT. Five micrograms of scFv was then added in a volume of 2.2 μl. After 3 min of incubation at RT, the iodinated scFv (I-scFv) was separated from unincorporated 131I on a column of G-10 Sephadex. BSA was labeled by incubation with chloramine-T for 60 s and purification of the radioactively labeled albumin (I-Alb) on a column of G-10 Sephadex.
Pharmacokinetics of Brain Uptake. Male CD-1 mice were anesthetized with urethane. All animal studies were done in accordance with international standards and protocols approved by the local animal use committee. The blood-to-brain unidirectional influx rate (Ki, in units of μl/g/min) was calculated by multiple-time regression analysis (Blasberg et al., 1983; Patlak et al., 1983). In brief, the right jugular vein and left carotid artery were exposed. At t = 0, 0.2 ml of lactated Ringer’s solution containing 1% BSA (LR-BSA) and 500,000 cpm I-scFv were injected into the jugular vein. Between 2 and 180 min after the intravenous injection, blood was collected from the carotid artery, and the mouse was immediately decapitated. Two mice were studied per time point. The arterial blood was centrifuged, and serum was collected, and results expressed as the percentage of the injected dose present per ml of serum (%Inj/ml). The brain was dissected into the cortex, cerebellum, hippocampus, hypothalamus, and remainder of the brain, the regions were weighed, and the level of radioactivity was determined. Results were expressed as the brain/serum ratios (in units of μl/g) and plotted against exposure time (Expt), where
and Cp is the level of radioactivity in serum, and Cpt is the level of radioactivity in serum at time t. Expt values correct for the clearance of the test substance from the blood so that the greater the clearance from blood the greater the difference between Expt and t. Without this correction, Ki would be overestimated. Brain/serum ratios for whole brain were calculated by summing the brain region values for radioactivity and weight. The slope of the linear portion of the relation between brain/serum ratios and Expt measures Ki and the intercept of the linearity measures Vi (μl·g), the initial volume of distribution in brain.
In other mice, I-Alb was included in the intravenous injection. The percentage of the injected dose taken up per g of brain (%Inj/g) was calculated as follows:
where A is the brain/serum ratio for I-scFv, and B is the ratio for I-Alb.
Acid Precipitation. To determine whether the radioactivity in brain and serum at various times represented intact I-scFv, we performed acid precipitation on radioactivity obtained at 30 min and 4 h after intravenous injection. Whole blood was centrifuged, and 50 μl of the resulting serum was added to 100 μl of LR-BSA and then to 100 μl of 30% trichloroacetic acid. The sample was vigorously mixed and centrifuged at 5400g for 15 min at 4°C. The resultant supernatant and precipitate were separated and counted, and the results were expressed as the percentage of total counts that were precipitated. Brains were homogenized in a glass homogenizer in 3 ml of LR-BSA and then centrifuged at 5400g for 10 min at 4°C. An aliquot of 0.5 ml of the supernatant was added to 0.5 ml of 30% trichloroacetic acid, and the sample was vigorously mixed and then centrifuged at 5400g for 10 min at 4°C. The supernatant and precipitate were separated and counted, and the results were expressed as the percentage of total counts that precipitated. To correct for any degradation that might have occurred during the processing for acid precipitation, we added I-scFv to nonradioactive arterial whole blood or whole brain. These samples were then processed as above, and the percentage of total counts that were precipitated was determined. The mean of two processing controls was 96% for serum and 89% for brain. The values for the biological samples were divided by the value of the processing control and multiplied by 100 to give the reported result.
Capillary Depletion. Capillary depletion as modified for use in the mouse (Triguero et al., 1990; Gutierrez et al., 1993) was used to determine the degree to which I-scFv was sequestered by the vascular bed of the brain. Mice were anesthetized with urethane and given an injection into the jugular vein of 0.2 ml of saline containing 106 cpm I-scFv and 106 cpm I-Alb. After 2 h, arterial blood was obtained from the carotid artery. The brain was removed and emulsified in a glass homogenizer (8–10 strokes) at 4°C in a 9-fold volume of physiological buffer (10 mM HEPES, 141 mM NaCl, 4 mM KCl, 2.8 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, and 10 mM d-glucose adjusted to pH 7.4). Dextran solution was added to the homogenate to a final concentration of 26%. An aliquot was centrifuged at 5400g for 15 min at 4°C in a swinging bucket rotor. The pellet containing the brain microvessels and the supernatant containing the brain parenchyma were carefully separated. Results were expressed as capillary/serum and parenchyma/serum ratios. Values for I-Alb were subtracted from those for I-scFv to yield values corrected for residual vascular contamination and blood-brain barrier leakage.
Intracerebroventricular and Intravenous Administration. Male Sprague-Dawley rats (275–325 g) were anesthetized with isoflurane in medicinal oxygen (4% for induction and 2% for maintenance of anesthesia). A stainless-steel cannula (26 gauge, 10 mm long) was implanted into the third cerebral ventricle by using the following coordinates, relative to the Bregma: −2.3 mm anteroposterior, 0 mm lateral to the midline, and −7.5 mm ventral to the surface of the skull. The guide cannula was secured in place with three stainless-steel screws and glass-ionomer cement (3M), and a stylet was inserted to seal the cannula until use. Temgesic (Essex Chemie AG, Lucerne, Switzerland) (0.03 mg/kg) was given subcutaneously before and 2 days after surgery. Seven days after recovery from surgery, accuracy of the cannula placement in the third ventricle was tested by measuring the dipsogenic response (immediate drinking of at least 5 ml of water in 15 min) to an intracerebroventricular injection of 20 pmol of angiotensin II in 2-μl injection volume.
Purified mAbs and scFvs were slowly (1 min) injected intracerebroventricularly at 9:00 AM at a dose of 1 μg in a volume of 2 μlby using a Hamilton syringe. These doses were selected based on the results of comparative in vitro studies. After the injection of mAbs or scFvs, food intake was continuously recorded for 3 days by using an automatic food intake apparatus (TSE Systems, Bad Homburg, Germany) at 1-h intervals.
Osmotic minipumps (model 2002) (Charles River Laboratories, Les Oncins, France) were filled with mAbs 1E8a or 2G2 calculated to deliver 1 μg/day for 7 days. One week after the angiotensin II test the pumps were implanted dorsally under the skin under isoflurane anesthesia and immediately connected to the intracerebroventricular cannula via a tube prefilled with mAbs to ensure an immediate delivery. Body weight and food intake were monitored during the treatment by using an automatic food intake apparatus (TSE Systems).
In the experiments with intravenous administration, purified mAbs 1E8a or 2G2 and scFvs 1E8a and 2G2 were injected into the tail vein of rats at 9:00 AM under mild isoflurane anesthesia at a dose of 300 μg/kg. After the injection of scFv or mAb, food intake was continuously recorded with an automatic food intake apparatus (TSE Systems) at 1-h intervals for 3 days.
Data Analysis. All data are expressed as mean ± S.D. or S.E.M. as indicated. Data were analyzed by two-way analysis of variance (ANOVA) repeated measures with Bonferroni post hoc test or Student’s t test using Prism 4 software (GraphPad Software Inc., San Diego, CA). For the cAMP concentration-response experiments, the best-fitting curves were compared for their minimum, maximum, and EC50 values using the F test. Ki and EC50 were calculated by using Prism 4 software. For pharmacokinetic experiments, means are reported with standard errors. Prism 5.0 (GraphPad Software, Inc.) was used for regression analysis and comparison of slopes and regression lines. Half-time clearance from blood was calculated by multiplying by 0.301 the inverse of the slope for the relation between time and log(%Inj/ml). The Vd was computed by multiplying the antilog of the intercept by 100.
Results
Selection of mAbs and Selectivity of mAb 1E8a. The mouse serum used for hybridoma production showed a pronounced response against the immunogenic peptide derived from the N terminus of the MC4R but not against the corresponding peptide derived from the N terminus of the MC3R. Although the polyclonal response was high, only 10 clones were viable until subcloning and amplification (six IgG,κ and four IgM,κ). After purification only mAb 1E8a (IgM,κ) showed a blockade of the MC4R activity in the presence of α-MSH. mAb 2G2 (IgM,κ) was selected as an isotype-matched negative control for the characterization of mAb 1E8a, because it did not show a blockade of the MC4R activity. When the affinity of these mAbs for the NT peptide was assessed by surface plasmon resonance, a KD of 1.3 × 10−8 M for the mAb 1E8a and 3.7 × 10−8 M for the mAb 2G2 was calculated.
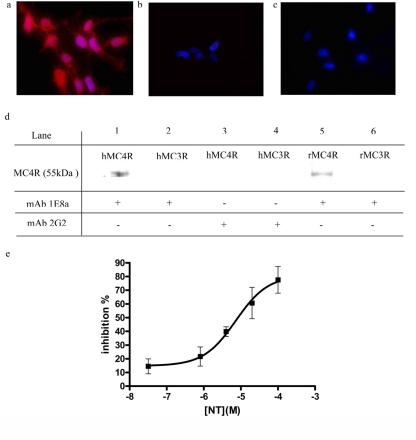
Immunocytofluorescence experiments were performed to assess the binding of the mAbs to the surface of HEK-293 cells overexpressing the hMC4R. Figure 1a shows specific membrane labeling of hMC4R expressing HEK-293 cells when using the mAb 1E8a. No labeling was observed on cells expressing the hMC3R (Fig. 1b). When HEK-293 cells expressing hMC4R were treated with mAb 2G2 no labeling could be observed (Fig. 1c). Immunoprecipitation experiments confirmed the selectivity of mAb 1E8a for the hMC4R and rMC4R. mAb 2G2 did not immunoprecipitate the hMC4R and rMC4R (Fig. 1d). The presence of NT peptide inhibited the interaction of the mAb 1E8a with the MC4R in a concentration-dependent manner (Ki of 1.3 × 10−6 M) (Fig. 1e).
Fig. 1.
Immunocytochemistry of HEK-293 cells transfected with hMC4R or hMC3R. Nuclear labeling (blue) is merged with immune labeling (red). a and b, specific membrane labeling was seen in HEK-293 cells expressing hMC4R when incubated with the mAb 1E8a (a) but not in HEK-293 cells expressing hMC3R (b). c, no labeling was observed with mAb 2G2 in hMC4R expressing cells. Magnification: 400×. d, immunoprecipitation of hMC4R and rMC4R. Lanes 1 and 2 show the results obtained with hMC4R and hMC3R, respectively, with mAb 1E8a, and lanes 5 and 6 show the corresponding results with rMC4R and rMC3R, respectively. Lanes 3 and 4 show control experiments with mAb 2G2. Purified mAb 1E8a precipitated both hMC4R (lane 1) and rMC4R (lane 5) but not hMC3R or rMC3R (lanes 2 and 6), whereas mAb 2G2 was inactive (lanes 3 and 4). e, inhibition of hMC4R immunoprecipitation by mAb 1E8a using increasing concentration of the NT peptide. Results are presented as percentage of inhibition of the signal obtained without NT peptide in function of the [NT]. Mean ± S.D. are calculated from three independent experiments. n = 5 measurements/experiment.
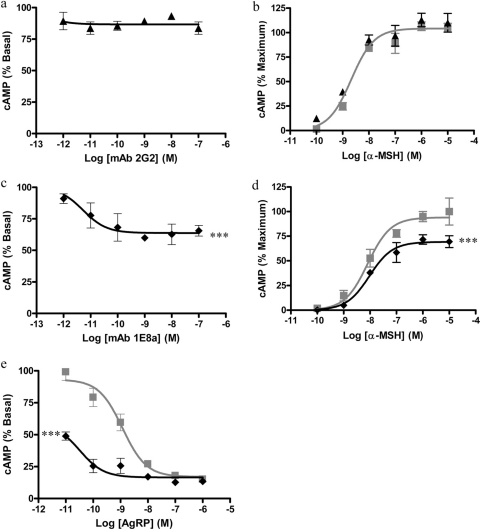
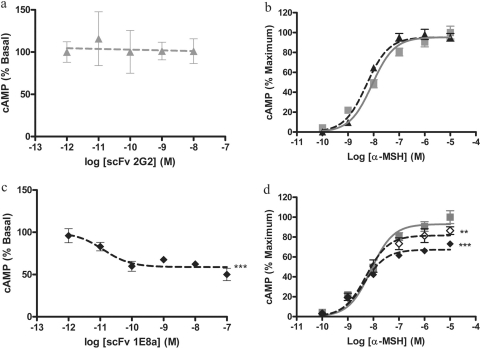
Pharmacological Activity of 1E8a and 2G2 mAbs in Vitro. When HEK-293 cells transfected with the hMC4R were exposed to increasing concentrations of mAb 2G2 (1 pM to 0.1 μM), no decrease in cAMP formation was measured (Fig. 2a). Conversely, when HEK-293 cells were exposed to increasing concentrations of mAb 1E8a (1 pM to 0.1 μM) cAMP formation was decreased in a concentration-dependent manner by up to 40% (EC50 of 2.1 × 10−12 M) (Fig. 2c).
Fig. 2.
Intracellular cAMP formation in HEK-293 cells transfected with hMC4R. a, concentration-response curves obtained with purified mAb 2G2. The mAb 2G2 had no effect on basal cAMP production. b, concentration-response curves obtained with α-MSH in the presence or absence of 100 nM of mAb 2G2 (▴) or PBS (
 ). The presence of mAb 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curves obtained with purified mAb 1E8a. The concentration-dependent decrease in the intracellular cAMP content suggests an inverse agonist effect of mAb 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 100 nM mAb 1E8a (♦) or PBS (
). The presence of mAb 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curves obtained with purified mAb 1E8a. The concentration-dependent decrease in the intracellular cAMP content suggests an inverse agonist effect of mAb 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 100 nM mAb 1E8a (♦) or PBS (
 ). The reduced maximum efficacy of α-MSH in the presence of mAb 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. e, concentration-response curves obtained with AgRP in the presence of 100 nM mAb 1E8a (♦) or PBS (
). The reduced maximum efficacy of α-MSH in the presence of mAb 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. e, concentration-response curves obtained with AgRP in the presence of 100 nM mAb 1E8a (♦) or PBS (
 ). The leftward shift of the AgRP response curve in the presence of mAb 1E8a suggests that this mAb acts in synergy with AgRP. Data are presented as means ± S.D. calculated from three independent experiments. ***, p < 0.001, F test.
). The leftward shift of the AgRP response curve in the presence of mAb 1E8a suggests that this mAb acts in synergy with AgRP. Data are presented as means ± S.D. calculated from three independent experiments. ***, p < 0.001, F test.
The presence of 100 nM mAb 2G2 did not change the concentration response curve of α-MSH (Fig. 2b), but the presence of 100 nM of mAb 1E8a significantly (p < 0.001, F test) reduced the maximum effect of α-MSH (Fig. 2d).
When HEK-293 cells transfected with the hMC4R were exposed to increasing concentrations of AgRP (1 pM to 0.1 μM) a decrease in cAMP formation was measured (Fig. 2e). In the presence of 100 nM of mAb 1E8a, a leftward shift of the AgRP concentration-response curve was observed.
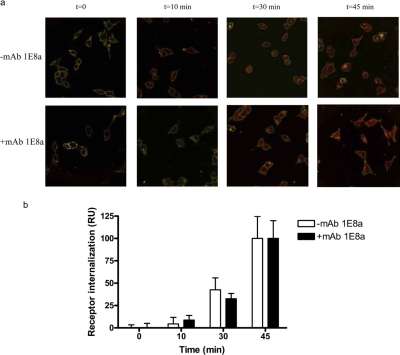
Receptor Internalization. The presence of mAb 1E8a (100 nM) did not influence the internalization of the MC4R expressed at the surface of HEK cells (Fig. 3).
Fig. 3.
Time course of agonist-induced internalization of MC4R. HEK-293 cells stably expressing hMC4R were exposed to 200 nM α-MSH coupled to rhodamine red for various lengths of time. a, confocal images show the distribution of MC4R (red) at the selected time points (t = 0, 10, 30, and 45 min) after agonist stimulation in the presence (+) or absence (−) of mAb 1E8a. b, quantitative analysis of time course of agonist-induced MC4R internalization. Digitized fluorescence intensity on the cell surface membrane and total cellular fluorescence intensity were quantified as described under Materials and Methods. The graphic represents the MC4R internalization expressed as OD as a function of time in the presence (+) or absence (−) of mAb 1E8a. The presence of mAb 1E8a did not interfere with the internalization of the hMC4R.
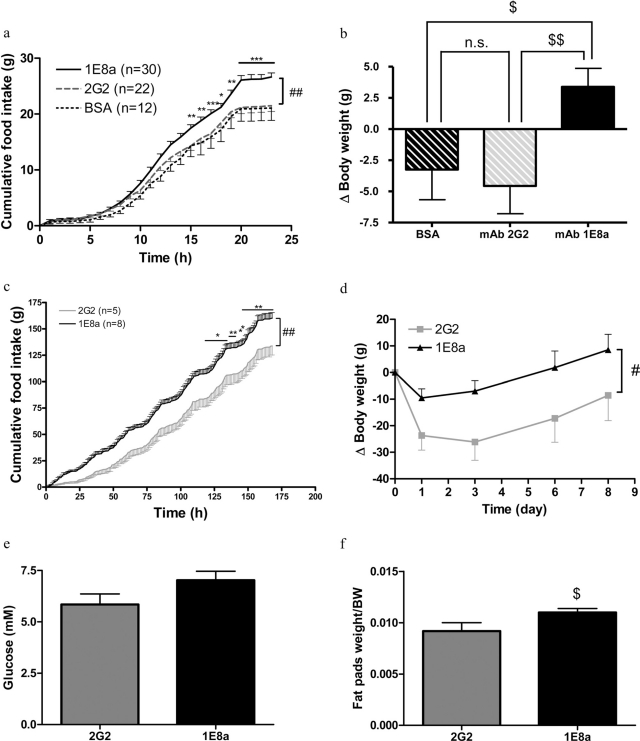
Pharmacological Activity of mAbs 1E8a and 2G2 in Vivo. Rats that received an injection of 1 μg of mAb 1E8a into the third ventricle ingested 24% more food in 24 h than rats that received mAb 2G2 or BSA as controls (Fig. 4a). The body weight of rats that received mAb 1E8a was increased, whereas body weight was reduced in rats that received either mAb 2G2 or BSA (Fig. 4b).
Fig. 4.
a, 24-h food intake in rats that received an intracerebroventricular injection of 1 μg of mAb 1E8a, BSA, or mAb 2G2. The injection of mAb 1E8a induced a significant increase in food intake. Data are presented as mean ± S.E.M. ##, p < 0.01 two-way ANOVA with repeated measures; *, p < 0.05; **, p < 0.01; ***, p < 0.001 Bonferroni post hoc test. b, body weight change of rats 24 h after intracerebroventricular injections. mAb 1E8a induced an increase in body weight compared with rats that received either mAb 2G2 or BSA. $, p < 0.05; $$, p < 0.01, Student’s t test. c, Seven-day food intake in rats, which received a continuous intracerebroventricular infusion of either 1 μg/day of mAb 1E8a or mAb 2G2. The infusion of mAb 1E8a induced a significant increase in food intake. Data are presented as mean ± S.E.M. ##, p < 0.01 two-way ANOVA with repeated measures. *, p < 0.05; **, p < 0.01, Bonferroni post hoc test. d, body weight change of rats during 7 days of continuous intracerebroventricular infusion. mAb 1E8a induced an increase in body weight compared with rats that received mAb 2G2. #, p < 0.05 two-way ANOVA with repeated measures. e and f, fasting glycemia (e) and fat pads weight (f) corrected for body weight (BW) of rats that received a continuous intracerebroventricular infusion of mAb 1E8 or mAb 2G2. $, p < 0.05, Student’s t test.
A 7-day intracerebroventricular infusion of 1E8a induced a significant increase in food intake (20%) compared with rats that received mAb 2G2 (Fig. 4c). The body weight of rats treated with mAb 1E8a was significantly less reduced than that in control rats (Fig. 4d). After 7 days of continuous intracerebroventricular infusion, a nonsignificant increase of the fasting glycemia (Fig. 4e) and a significant increase in fat pad weight were observed (Fig. 4f).
Variable Domain Cloning and scFv Expression. The scFv-encoding genes derived from the variable regions [VH and VL linked together via a short linker (G4S)3 of the mAb 1E8a and mAb 2G2, with addition of a C-terminal six-His tag encoding sequence], were inserted in-frame with the PelB sequence into the pET22b expression vector. The sequence of the single-chain construction is represented in Fig. 5. We confirmed that the cloned VL gene did not correspond to the aberrant κ transcript of the sp20 hybridomas (Carroll et al., 1988). The plasmid pET-scFv 1E8a or pET-scFv 2G2 was cloned into the Rosetta E. coli strain, and the recombinant protein was expressed and exported to the bacterial periplasm by its leader sequence PelB (Lei et al., 1987). The scFv 1E8a and scFv 2G2 were purified and concentrated by immobilized metal ion affinity chromatography and repurified by immunoadsorption to get rid of the incorrectly folded inactive recombinant proteins (Peter et al., 2003) (Fig. 6). The affinity of these scFvs for the NT peptide was assessed by surface plasmon resonance. A KD of 7.8 × 10−7 M for the scFv 1E8a and 2.4 × 10−7 M for the scFv 2G2 was calculated.
Fig. 5.
Nucleotidic and amino acid sequences of scFv 1E8a (top) and scFv 2G2 (bottom). The hypervariable loops are underlined.
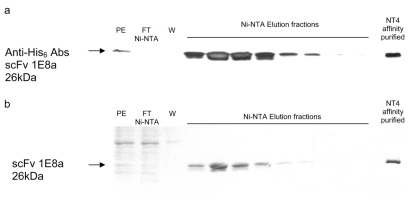
Fig. 6.
Purification of the scFv 1E8a by Ni-NTA chromatography. a, Western blot probed with an anti-His6 Ab that revealed the presence of the His6-tagged scFv 1E8a. b, SDS-polyacrylamide gel electrophoresis stained with Coomassie blue, PE, flow-through (FT), wash fraction (W), and the elution of the purified scFv protein (see Materials and Methods).
Pharmacological Effect of the scFvs 1E8a and 2G2 in Vitro. When HEK-293 cells transfected with the hMC4R were exposed to increasing concentrations of scFv 2G2 (1 pM to 0.1 μM) no decrease in cAMP formation was measured (Fig. 7a). Conversely, when HEK-293 cells were exposed to increasing concentrations of scFv 1E8a (1 pM to 0.1 μM) cAMP formation decreased in a concentration-dependent manner by up to 40% (Fig. 7c).
Fig. 7.
Intracellular cAMP production in HEK-293 cells transfected with hMC4R. a, concentration-response curve obtained with purified scFv 2G2. The scFv 2G2 had no effect on basal cAMP production. b, concentration-response curves obtained with α-MSH in the presence or absence of 100 nM scFv 2G2 (
 ) or PBS (
) or PBS (
 ). The presence of the scFv 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curve obtained with purified scFv 1E8a. The concentration-dependent decrease in cAMP formation suggests an inverse agonist effect of the scFv 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 10 nM scFv 1E8a (⋄), 100 nM scFv 1E8a (♦), or PBS (▪). The reduced maximum efficacy of α-MSH in the presence of scFv 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. **, p < 0.01; ***, p < 0.001, F test.
). The presence of the scFv 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curve obtained with purified scFv 1E8a. The concentration-dependent decrease in cAMP formation suggests an inverse agonist effect of the scFv 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 10 nM scFv 1E8a (⋄), 100 nM scFv 1E8a (♦), or PBS (▪). The reduced maximum efficacy of α-MSH in the presence of scFv 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. **, p < 0.01; ***, p < 0.001, F test.
The presence of 100 nM scFv 2G2 did not change the concentration-response curve of α-MSH (Fig. 7b), but the presence of 100 nM scFv 1E8a significantly (p < 0.001, F test) reduced the maximum effect of α-MSH (Fig. 7d).
Pharmacokinetics of scFv 1E8a. When I-scFv was injected intravenously and its clearance from blood was calculated, the relation between log(%Inj/ml) and time was highly significant (r = 0.788, n = 20, p < 0.001) with a half-life of 84.6 min and a distribution volume (Vd) of 2.15 ml. This shows that I-scFv distribution was limited to the vascular space.
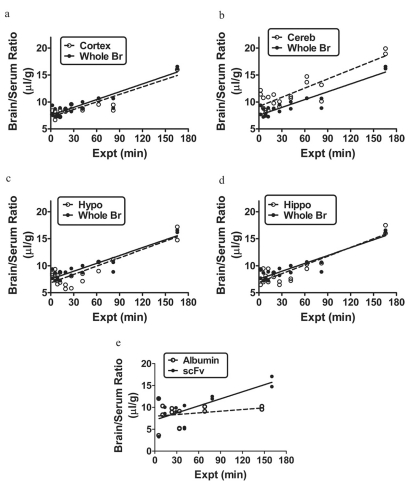
Figure 8 shows the uptake of I-scFv by cortex, cerebellum, hypothalamus, and hippocampus in comparison with whole brain. For all brain regions and whole brain, the relation between brain/serum ratios and Expt was significant (r ranging from 0.869 to 0.930, n = 20 mice/line; p < 0.001 for all curves). No statistical difference was found among the slopes, which ranged from a low of 0.046 ± 0.005 μl/g·min for cortex to a high of 0.057 ± 0.008 μl/g·min for cerebellum. The y intercept for cerebellum was greater than that for whole brain. The y intercept measures the Vi, or initial vascular space, and differences typically indicate a greater association with or binding to the luminal surface of the brain endothelial cells. Figure 8e compares I-scFv and I-Alb uptake by whole brain. For I-Alb, there was no significant correlation between brain/serum ratios and Expt, so no penetration of the blood-brain barrier was formally demonstrated. In comparison, the uptake of scFv was significant (r = 0.736, n = 13, p < 0.005) with a slope of 0.054 ± 0.015 μl/g·min. A value of approximately 0.2% Inj/g was reached at 120 min.
Fig. 8.
a–d, the uptake of I-scFv by cortex (a), cerebellum (b), hypothalamus (c), and hippocampus (d) in comparison with whole brain as a function of Expt. e, comparison of I-scFv and I-Alb uptake by whole brain.
Acid precipitation corrected for processing controls gave values for serum of 102% and 96% of radioactivity representing intact I-scFv at 30 min and 4 h, respectively. For brain, the values were 96% and 44% at 30 min and 4 h, respectively.
In capillary depletion experiments, it was found that the capillary/serum ratio was 0.57 ± 0.05 μl/g and the parenchymal/serum ratio was 1.03 ± 0.17 μl/g. This showed that the majority of I-scFv taken up by the blood-brain barrier was not sequestered by the capillary bed, but entered the brain parenchymal space.
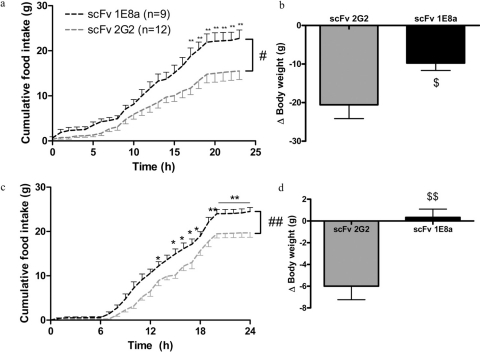
Pharmacological Activity of scFvs 1E8a and 2G2 in Vivo. One microgram of purified scFv 1E8a and scFv 2G2 was injected into the third ventricle of rats. Rats that received scFv 1E8a ingested 45% more food in 24 h than rats that received scFv 2G2 (Fig. 9a). The body weight of rats that received scFv 1E8a was significantly less decreased than that of rats that received scFv 2G2 (Fig. 9b).
Fig. 9.
a, 24-h food intake in rats that received an intracerebroventricular injection of 1 μg of scFv 1E8a or scFv 2G2. The injection of scFv 1E8a induced a significant increase in food intake. Data are presented as mean ± S.E.M. #, p < 0.05, two-way ANOVA with repeated measures and Bonferroni post hoc test; **, p < 0.01. b, body weight change in rats 24 h after injection. The injection of scFv 1E8a induced a smaller loss of body weight compared with rats that received scFv 2G2. $, p < 0.05, Student’s t test. c, 24-h food in-take in rats that received an intravenous injection of 300 μg/kg of scFv 1E8a or scFv 2G2. The injection of scFv 1E8a induced a significant increase in food intake. Data are presented as means ± S.E.M. ##, p < 0.01, repeated measures two-way ANOVA with Bonferroni post hoc test; *, p < 0.05; **, p < 0.01. d, body weight change in rats 24 h after injection. The scFv 1E8a significantly prevented the body weight loss seen in rats that received scFv 2G2. $$, p < 0.01, Student’s t test.
Purified scFv 1E8a and scFv 2G2 were injected (300 μg/kg i.v.) in rats. Rats that received scFv 1E8a ingested 33% more food in 24 h than rats that received scFv 2G2 (Fig. 9c). The body weight of rats that received scFv 1E8a was unchanged, whereas body weight was reduced in rats that received scFv 2G2 (Fig. 9d).
Discussion
The MC4R is a key receptor for the regulation of food intake and energy expenditure. Its dysfunction leads to severe obesity in rodents and humans (Ste Marie et al., 2000; Farooqi et al., 2003). Conversely, its overactivation in chronic diseases such as cancer, which is probably caused by high levels of circulating cytokines (Huang et al., 1999), leads to cachexia (Marks and Cone, 2001). Blockade of the MC4R in rodent models of cachexia showed a significant increase in lean body mass (Nicholson et al., 2006). Moreover, rats immunized against the NT domain of the MC4R were resistant to lipopolysaccharide-induced anorexia (Hofbauer et al., 2008).
In a previous study, we demonstrated that immunization against the N terminus of the MC4R in rats led to the generation of pharmacologically active Abs that acted as inverse agonists and noncompetitive antagonists (Peter et al., 2007). In the present study, we characterized the pharmacological properties of a mAb (1E8a) against this peptide sequence of the NT domain in vitro and in vivo. Another mAb (2G2) that recognized the NT peptide but not the corresponding sequence in the native receptor served as a control throughout our experiments.
The binding properties of mAbs 1E8a and 2G2 were studied in two different in vitro experiments. By using immunocytofluorescence in HEK-293 cells overexpressing the hMC3R or hMC4R, binding to the native form of the hMC4R and subtype selectivity was assessed. The mAb 1E8a bound to the hMC4R but not to the hMC3R on the cell surface, a result that was not unexpected because the N-terminal sequences of the MC3R and MC4R subtypes show a low degree of homology (Gantz et al., 1993). In general, the sequences of extracellular domains are less well conserved than those of transmembrane domains and are therefore suitable targets for a subtype-selective pharmacological approach (Mirzadegan et al., 2003).
The mAb 2G2 showed no interaction with either hMC3R or hMC4R at the cell surface. These results are consistent with our immunoprecipitation experiments that indicated that mAb 1E8a bound to hMC4R and rMC4R with high affinity, whereas the mAb 2G2 interacted only with the NT peptide but not with the corresponding sequence in the native MC4R. A specific interaction of mAb 1E8a with the MC4R was supported by the observation that increasing concentrations of the NT peptide reduced the binding of mAb 1E8a with a Ki in the micromolar range. Compared with the Kd of the mAb 1E8a/NT peptide binding, which is in the nM range, this Ki indicated as better binding of mAb 1E8a to the native conformation of the receptor than to the NT peptide.
The affinity of the mAb 1E8a and the mAb 2G2 for the NT peptide were in the same range (1.3 × 10−8 and 3.7 × 10−8 M, respectively). They are relatively high for an IgM isotype, but other high-affinity IgM mAbs have been described previously (Ballard et al., 1983; Suenaga and Abdou, 1992; Cao et al., 2004). The small difference between the affinity of mAbs 1E8a and 2G2 cannot explain why the latter did not interact with the MC4R. This might indicate that the paratope of mAb 2G2 interacted with a conformation of the NT peptide that the intact receptor is unable to adopt. In contrast, mAb 1E8a is able to recognize a common structure shared by the NT peptide and the NT domain in the native MC4R.
The pharmacological properties of mAb 1E8a were assessed by using adenylyl cyclase assays in HEK-293 cells overexpressing the hMC4R. The mAb 1E8a behaved as an inverse agonist and decreased basal activity by 40% with an EC50 in the picomolar range. It behaved as a noncompetitive antagonist in the presence of α-MSH and decreased the maximum efficacy of this agonist by 20%. A pattern of inverse agonism combined with noncompetitive antagonism has been described for other Abs at G protein-coupled receptors (Peter et al., 2003, 2004, 2007). The mAb 2G2 did not affect the constitutive activity of the MC4R or the concentration-response curve of α-MSH.
To find out whether the in vitro effects of mAb 1E8a translated into efficacy in vivo, purified mAb 1E8a was administered into the third ventricle of rats. The mAb 2G2 served as a control. When given at the beginning of the light phase mAb 1E8a increased 24-h food intake by approximately 25%. This acute effect is not too different from that obtained with SHU 9119, a peptidic MC3/MC4R antagonist (Obici et al., 2001; Peter et al., 2007). However, the main effects on food intake were seen during the dark phase. This finding is at variance with the fact that MC4R tone is highest during the light phase when satiety prevails. The fact that AgRP expression is higher during the dark phase (Lu et al., 2002), together with our observation that mAb 1E8a strongly enhanced the effects of AgRP in vitro, might offer an explanation for this result.
When mAb 1E8a was continuously infused via osmotic minipumps into the third ventricle in rats a 70% increase of food intake compared with controls was recorded during the first 24 h. This difference is much more pronounced than that seen after intracerebroventricular injection. The stronger effect of MC4R blockade in these experiments may be because the rats had not yet recovered from the minipump implantation. The effect of mAb 1E8a under these circumstances therefore may be interpreted as a treatment effect during an inflammatory response caused by a recent surgical intervention.
At the end of the 7-day treatment period with mAb 1E8a we observed a trend for an increase in glycemia and a significant increase in fat pads weight. Similar findings have been reported in MC4R knockout mice (Fan et al., 2000), rats after central administration of antisense oligonucleotides against the MC4R (Obici et al., 2001), and rats immunized with the NT peptide of the MC4R (Peter et al., 2007; Hofbauer et al., 2008).
No effects on food intake were seen after single intravenous administration of mAb 1E8a in a dose (300 μg/kg) approximately 100-fold higher than that given intracerebroventricularly (1 μg/rat) (data not shown). This excludes the contribution of peripheral MC4R but raises the question of whether intravenously administered mAbs can reach their supposed central sites of action. The absence of a central effect is consistent with the findings of Banks et al. (2002), who observed that after intravenous injection peak amounts of Abs in brain tissue are only 0.11% of the total administered dose. We therefore generated recombinant scFvs from mAb 1E8a and mAb 2G2. The scFv 1E8a acted as an inverse agonist and noncompetitive antagonist of the MC4R and showed the same efficacy as the intact mAb. The affinity of the scFv 1E8a for the NT peptide was 10 times lower than that of the intact mAb 1E8a. This difference is consistent with the higher EC50 calculated from the scFv inverse agonist activity.
The intracerebroventricular application of 1 μg of scFv 1E8a induced an increase in food intake that occurred faster and was more pronounced than that obtained with the intact mAb 1E8a. This observation could reflect a better brain penetration of scFv compared with the parent mAb. After intravenous administration in rats scFv 1E8a induced an increase of food intake, whereas the complete mAb 1E8a was ineffective. This suggests that scFv 1E8a can cross the blood-brain barrier and penetrate brain tissue to reach its central receptors. The smaller size of the scFv may provide the explanation for these observations.
Pharmacokinetic studies were performed to further address the issue of brain penetration. Uptake of I-scFv was uniform across several brain regions and exceeded that of the vascular marker albumin. This suggests a mechanism of uptake other than entry by way of extracellular pathways (Broadwell and Sofroniew, 1993), the mechanism proposed for IgG (Banks et al., 2002, 2005) and IgM molecules (Banks et al., 2007). Capillary depletion showed that approximately 1/3 of the I-scFv retained by brain was sequestered by brain endothelial cells, whereas approximately 2/3 were located in brain parenchyma. This sequestration by capillaries also suggests that a transcytotic pathway across the blood-brain barrier is more likely than leakage into brain by the extra-cellular pathways.
A long half-life from brain and enzymatic resistance contributed to brain accumulation as well. We calculated that by 2 h approximately 0.2% of the intravenously injected dose had been taken up by whole brain. However, we did not correct for degradation in brain with time, so the value may be 30 to 50% lower than this. This would still produce brain levels that are similar to or greater than those seen with other proteins that are centrally active (Banks et al., 1991, 1993, 1996) and therefore would be sufficient for a pharmacological effect. However, receptor binding studies would have been needed to clearly demonstrate that the scFv reached its presumed hypothalamic site of action at concentrations that were biologically active.
In this article we report for the first time that an anti-MC4R mAb and its scFv derivative are able to modulate the MC4R activity in vitro and in vivo. The scFv 1E8a crossed the blood-brain barrier in amounts sufficient to increase food intake after intravenous administration in rats. Such molecules therefore might represent a starting point for the development of new therapies for patients with anorexia and cachexia.
ABBREVIATIONS:
- MC4R
melanocortin-4 receptor
- rMC4R
rat MC4R
- hMC4R
human MC4R
- Ab
antibody
- mAb
monoclonal Ab
- α-MSH
α-melanocyte-stimulating hormone
- AgRP
Agouti-related protein
- scFv
single-chain variable fragment
- I-scFv
iodinated scFv
- RT
room temperature
- HEK
human embryonic kidney
- NT
N terminal
- PBS
phosphate-buffered saline
- PBS-T
PBS containing 0.05% Tween 20
- OD
optical density
- G418
(2R,3S,4R,5R,6S)-5-amino-6-[(1R,2S,3S,4R,6S)-4,6-diamino-3-[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-methylaminooxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-2-(1-hydroxyethyl)oxane-3,4-diol
- BSA
bovine serum albumin
- PCR
polymerase chain reaction
- Ni-NTA
20 mM imidazole, 50 mM Na2HPO4, 300 mM NaCl, pH 8.0
- PE
periplasmic extract
- I-Alb
radioactively labeled albumin
- LR-BSA
lactated Ringer’s solution containing 1% BSA
- %Inj/ml
percentage of the injected dose present per ml of serum
- %Inj/g
percentage of the injected dose taken up per g of brain
- Expt
exposure time
- ANOVA
analysis of variance
- SHU 9119
Asp3-Lys8-Ac-Nle-Asp-His-d-Nal(2′)-Arg-Trp-Lys-NH2
Footnotes
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
References
- Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149:815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard DW, Kranz DM, Voss EW., Jr Monoclonal IgM antibody exhibiting high-affinity binding and cryoglobulin properties. Proc Natl Acad Sci USA. 1983;80:5071–5074. doi: 10.1073/pnas.80.16.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M. Anti-amyloid β protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer’s disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol. 2007;206:248–256. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide1–27 and pituitary adenylate cyclase activating polypeptide1–38 across the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:690–696. [PubMed] [Google Scholar]
- Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1α, murine IL-1α, and murine IL-1β are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther. 1991;259:988–996. [PubMed] [Google Scholar]
- Banks WA, Pagliari P, Nakaoke R, Morley JE. Effects of a behaviorally active antibody on the brain uptake and clearance of amyloid β proteins. Peptides. 2005;26:287–294. doi: 10.1016/j.peptides.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Passage of amyloid β protein antibody across the blood-brain barrier in a mouse model of Alzheimer’s disease. Peptides. 2002;23:2223–2226. doi: 10.1016/s0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- Blasberg RG, Fenstermacher JD, Patlak CS. Transport of α-aminoisobutyric acid across brain capillary and cellular membranes. J Cereb Blood Flow Metab. 1983;3:8–32. doi: 10.1038/jcbfm.1983.2. [DOI] [PubMed] [Google Scholar]
- Bohen SP, Troyanskaya OG, Alter O, Warnke R, Botstein D, Brown PO, Levy R. Variation in gene expression patterns in follicular lymphoma and the response to rituximab. Proc Natl Acad Sci USA. 2003;100:1926–1930. doi: 10.1073/pnas.0437875100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- Cao C, Bai Y, Holloway MJ, Edgeworth RL, Jackson EA, Jr, Cotropia J, Ugen KE. Characterization of a novel human anti-HIV-1 gp41 IgM monoclonal antibody designated clone 37. DNA Cell Biol. 2004;23:836–841. doi: 10.1089/dna.2004.23.836. [DOI] [PubMed] [Google Scholar]
- Carroll WL, Mendel E, Levy S. Hybridoma fusion cell lines contain an aberrant κ transcript. Mol Immunol. 1988;25:991–995. doi: 10.1016/0161-5890(88)90005-3. [DOI] [PubMed] [Google Scholar]
- Cowley MA. Hypothalamic melanocortin neurons integrate signals of energy state. Eur J Pharmacol. 2003;480:3–11. doi: 10.1016/j.ejphar.2003.08.087. [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Marks DL. Therapy insight: use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab. 2006;2:459–466. doi: 10.1038/ncpendmet0221. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor α is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Hofbauer KG, Lecourt AC, Peter JC. Antibodies as pharmacologic tools for studies on the regulation of energy balance. Nutrition. 2008;24:791–797. doi: 10.1016/j.nut.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Huang QH, Hruby VJ, Tatro JB. Role of central melanocortins in endotoxin-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 1999;276:R864–R871. doi: 10.1152/ajpregu.1999.276.3.R864. [DOI] [PubMed] [Google Scholar]
- Kornbluth A. Infliximab approved for use in Crohn’s disease: a report on the FDA GI Advisory Committee conference. Inflamm Bowel Dis. 1998;4:328–329. doi: 10.1002/ibd.3780040415. [DOI] [PubMed] [Google Scholar]
- Lei SP, Lin HC, Wang SS, Callaway J, Wilcox G. Characterization of the Erwinia carotovora pelB gene and its product pectate lyase. J Bacteriol. 1987;169:4379–4383. doi: 10.1128/jb.169.9.4379-4383.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- Marks DL, Cone RD. Central melanocortins and the regulation of weight during acute and chronic disease. Recent Prog Horm Res. 2001;56:359–375. doi: 10.1210/rp.56.1.359. [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Benko G, Filipek S, Palczewski K. Sequence analyses of G protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42:2759–2767. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG. Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther. 2006;317:771–777. doi: 10.1124/jpet.105.097725. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi VT, Bryan VM, Herzenberg LA, Herzenberg LA. Lymphocyte membrane IgG and secreted IgG are structurally and allotypically distinct. J Exp Med. 1980;151:1260–1274. doi: 10.1084/jem.151.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Peter JC, Eftekhari P, Billiald P, Wallukat G, Hoebeke J. scFv single chain antibody variable fragment as inverse agonist of the β2-adrenergic receptor. J Biol Chem. 2003;278:36740–36747. doi: 10.1074/jbc.M306877200. [DOI] [PubMed] [Google Scholar]
- Peter JC, Nicholson JR, Heydet D, Lecourt AC, Hoebeke J, Hofbauer KG. Antibodies against the melanocortin-4 receptor act as inverse agonists in vitro and in vivo. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2151–R2158. doi: 10.1152/ajpregu.00878.2006. [DOI] [PubMed] [Google Scholar]
- Peter JC, Tugler J, Eftekhari P, Maurice D, Hoebeke J, Roegel JC. Effects on heart rate of an anti-M2 acetylcholine receptor immune response in mice. FASEB J. 2005;19:943–949. doi: 10.1096/fj.04-3042com. [DOI] [PubMed] [Google Scholar]
- Peter JC, Wallukat G, Tugler J, Maurice D, Roegel JC, Briand JP, Hoebeke J. Modulation of the M2 muscarinic acetylcholine receptor activity with monoclonal anti-M2 receptor antibody fragments. J Biol Chem. 2004;279:55697–55706. doi: 10.1074/jbc.M407213200. [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Marks DL. The use of melanocortin antagonists in cachexia of chronic disease. Exp Opin Invest Drugs. 2005;14:1233–1239. doi: 10.1517/13543784.14.10.1233. [DOI] [PubMed] [Google Scholar]
- Schuna AA, Megeff C. New drugs for the treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2000;57:225–234. doi: 10.1093/ajhp/57.3.225. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, Vaisse C. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114:1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga R, Abdou NI. Lupus-derived human monoclonal IgM anti-DNA antibody displays monospecificity, high affinity and private idiotype specificity. Lupus. 1992;1:363–368. doi: 10.1177/096120339200100605. [DOI] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]