Abstract
The benztropine analog N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane (JHW 007) displays high affinity for the dopamine transporter (DAT), but unlike typical DAT ligands, has relatively low abuse liability and blocks the effects of cocaine, including its self-administration. To determine sites responsible for the cocaine antagonist effects of JHW 007, its in vitro binding was compared with that of methyl (1R,2S,3S,5S)-3-(4-fluorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate (WIN 35428) in rats, mice, and human DAT (hDAT)-transfected cells. A one-site model, with Kd values of 4.21 (rat) and 8.99 nM (mouse) best fit the [3H]WIN 35428 data. [3H]JHW 007 binding best fit a two-site model (rat, 7.40/4400 nM; mouse, 8.18/2750 nM), although a one-site fit was observed with hDAT membranes (43.7 nM). Drugs selective for the norepinephrine and serotonin transporters had relatively low affinity in competition with [3H]JHW 007 binding, as did drugs selective for other sites identified previously as potential JHW 007 binding sites. The association of [3H]WIN 35428 best fit a one-phase model, whereas the association of [3H]JHW 007 best fit a two-phase model in all tissues. Because cocaine antagonist effects of JHW 007 have been observed previously soon after injection, its rapid association observed here may contribute to those effects. Multiple [3H]JHW 007 binding sites were obtained in tissue from mice lacking the DAT, suggesting these as yet unidentified sites as potential contributors to the cocaine antagonist effects of JHW 007. Unlike WIN 35428, the binding of JHW 007 was Na+-independent. This feature of JHW 007 has been linked to the conformational status of the DAT, which in turn may contribute to the antagonism of cocaine.
Introduction
Cocaine is a monoamine uptake inhibitor that has a high liability for abuse. Evidence that the inhibition of dopamine (DA) uptake is the mechanism mediating the abuse liability of cocaine comes from correlations of the affinity of various dopamine uptake inhibitors and several cocaine-like behavioral effects that are related to drug abuse (Ritz et al., 1987; Bergman et al., 1989). Affinities of these compounds for the dopamine transporter (DAT) are better correlated with cocaine-like effects than are affinities at the other monoamine transport sites.
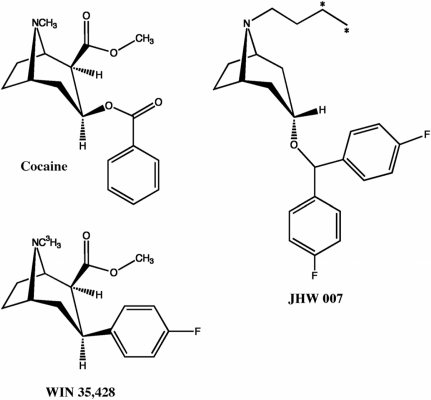
Several studies, however, have identified atypical dopamine transport inhibitors that have effects different from those of cocaine. Among the atypical dopamine transport inhibitors are benztropine (BZT) analogs that have structural similarities to cocaine (Fig. 1) and the piperazine dopamine uptake inhibitors, of which 1-[2-[bis(4-fluorophenyl)methoxy] ethyl]-4-[3-phenylpropyl]piperazine dihydrochloride (GBR 12909) is the prototype (Schmitt et al., 2008). Most BZT analogs bind to the DAT selectively among the monoamine transporters and inhibit the uptake of dopamine. However, despite their in vitro profile, many of the BZT analogs have effects that differ from those of cocaine (Tanda et al., 2009a; see reviews by Newman and Katz, 2009). For example, the increases in brain dopamine concentrations produced by BZT analogs are generally slower in onset and longer in duration and can exhibit a dose-response curve with a lower slope than that for cocaine (Tanda et al., 2005, 2009). BZT analogs are usually less effective than cocaine or other dopamine uptake inhibitors in stimulating locomotor activity in rodents (Katz et al., 1999, 2004; Ferragud et al., 2009), and although most dopamine uptake inhibitors fully substitute for cocaine in rats trained to discriminate cocaine from saline injections, many of the BZT analogs do not. BZT analogs are typically not self-administered substantially above vehicle levels in laboratory animals (Woolverton et al., 2000; Hiranita et al., 2009). Finally, certain BZT analogs block various behavioral effects of cocaine, including locomotor stimulation and self-administration (Desai et al., 2005; Ferragud et al., 2009; Hiranita et al., 2009).
Fig. 1.
Chemical structures of cocaine, WIN 35428, and the benztropine derivative, JHW 007. For WIN 35428 and JHW 007 the tritiated forms are shown.
Loland et al. (2008) have suggested that the differences in pharmacology between cocaine and several atypical dopamine uptake inhibitors are related to preferential binding of the drugs to different DAT conformations. In that study, different dopamine uptake inhibitors affected the accessibility of the sulfhydryl-reactive reagent [2-(trimethylammonium) ethyl]-methanethiosulfonate (MTSET) to a cysteine residue inserted at position 159 of the DAT (I159C). This introduced TM3 cysteine side chain has been suggested to be accessible when the transporter is in an outward-facing conformation and inaccessible when the DAT is in an inward-facing conformation (Loland et al., 2004). Cocaine and several of its analogs increased I159C reactivity to the cell-impermeant MTSET, whereas several atypical dopamine transport inhibitors, including BZT analogs, decreased MTSET reactivity. In addition, the DAT mutation Y335A, which is characterized by a change in equilibrium favoring the inward-facing conformation (Loland et al., 2002), decreased the potencies of cocaine analogs for inhibition of DA uptake much more so than analogs of BZT. These findings are consistent with an earlier study by Reith et al. (2001) that showed that cocaine and benztropine differently alter the conformation of the DAT; the study further suggested that DAT inhibitors can stabilize distinct transporter conformations, which may in turn alter the behavioral effects of the drugs. It is noteworthy that the change in potency of dopamine uptake inhibitors in cells with the Y335A mutant compared with wild-type cells was related in the Loland et al. (2008) study to the degree to which the drugs produced in vivo effects like those of cocaine.
It is currently unclear whether the binding of BZT analogs in brain differ from those of cocaine analogs, as they do in transfected cells. The present study characterized the kinetic and pharmacological characteristics of the binding sites of [3H]N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane (JHW 007) (Fig. 1), a BZT analog that has been studied in blocking the effects of cocaine. Because the effects of BZT analogs can be different from those of cocaine, the binding of [3H]JHW 007 was compared with that of [3H]WIN 35428, a ligand typically used to characterize the cocaine binding site.
Materials and Methods
Animals and Cultured Cells. Male Sprague-Dawley rats weighing 200 to 225 g and Swiss-Webster mice weighing 25 to 30 g (Taconic Farms, Germantown, NY) were used. DAT KO mice were created on a B6/Sv129 genetic background with a simian virus 40 T-antigen (TsA58) knockin at the DAT gene. Interruption of the DAT gene, absence of the DAT protein, breeding to generate homozygous DAT KO mice, and polymerase chain reaction genotyping schemes were described in a previous study (Rothman et al., 2002). Genotyping service was performed by Charles River Laboratories, Inc. (Wilmington, MA). Animals were housed in a humidity- and temperature-controlled room with a 12-h light cycle (lights on at 7:00 AM). Food and water were continuously available. N2A neuroblastoma cells stably transfected with a human DAT cDNA (gift from Dr. Margaret Gnegy, University of Michigan, Ann Arbor, MI) were cultured in Opti-MEM plus 10% fetal bovine serum, 10% penicillin/streptomycin, and 5% G418 (Geneticin) at 37°C in 5% CO2 in 750-cm2 flasks.
Materials. [3H]JHW 007 was prepared by reducing the unsaturated precursor N-(n-butenyl)-3α-[bis(4′-fluorophenyl) methoxy]-tropane (synthesized in the Medicinal Chemistry Section, National Institute on Drug Abuse Intramural Research Program), with tritium gas by American Radiolabeled Chemicals (St. Louis, MO). The label was determined by thin-layer chromatography in a chloroform/methanol/ammonium hydroxide (95:5:1) solvent system to be 99% pure and had a specific activity of 50 Ci/mmol. [3H]WIN 35428 was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA) and had a specific activity of 84 Ci/mmol. The label was determined on initial synthesis by PerkinElmer Life and Analytical Sciences to be more than 97% pure by high-pressure liquid chromatography and thin-layer chromatography, and the purity of subsequent syntheses was confirmed periodically by PerkinElmer Life and Analytical Sciences by high-performance liquid chromatography. All other drugs and reagents were obtained from Sigma-Aldrich (St. Louis, MO) or the National Institute on Drug Abuse, Drug Supply Program, or drugs were synthesized in the Medicinal Chemistry Section of the National Institute on Drug Abuse Intramural Research Program.
Membrane Preparation. Brains from rats or mice were removed, and striata were dissected and quickly frozen. hDAT/N2A neuroblastoma cells were washed with ice-cold modified sucrose phosphate buffer (0.32 M sucrose, 7.74 mM Na2HPO4, 2.26 mM NaH2PO4, pH adjusted to 7.4) and scraped into centrifuge tubes. Membranes were prepared by homogenizing tissues or cells in 20 volumes (w/v) of ice-cold modified sucrose phosphate buffer using a Brinkmann Instruments (Westbury, NY) Polytron (setting 6 for 20 s) and centrifuged at 20,000g for 10 min at 4°C. The resulting pellet was resuspended in buffer, recentrifuged, and suspended in buffer again to a concentration of 10 mg (original wet weight) per ml.
Ligand Binding Experiments. All experiments were conducted in assay tubes containing 0.5 ml of sucrose phosphate buffer, and except in kinetic studies, incubated for 120 min on ice. Each tube contained 0.5 nM [3H]JHW 007 or [3H]WIN 35428 and 1.0 mg of tissue (original wet weight). Nonspecific binding was determined by using 100 μM GBR 12909 ([3H]JHW 007 binding) or 100 μM cocaine HCl ([3H]WIN 35428 binding). For experiments with tissue from DAT KO and WT mice, JHW 007 at 100 μM was used to determine nonspecific binding. Assays were typically conducted in at least three independent experiments, each performed with triplicate observations (tubes). However, in some studies with tissue from mutant mouse lines limited tissue availability necessitated duplicate observations in each.
Kinetics of JHW 007 and WIN 35428 binding were compared in association and dissociation experiments conducted as described above, except that incubations were terminated at various times by rapid filtration. Dissociation constants were assessed by incubating tissue and ligand for 2 h, which was determined to be sufficient for either ligand to reach equilibrium. At 2 h, 100 μM cocaine ([3H]WIN 35428 assay) or GBR 12909 ([3H]JHW 007 assay) was added to the incubations, which were terminated at various times thereafter by rapid filtration with radioactivity counted as described above.
Affinities of ligands were determined as described above with homologous competitive binding of WIN 35428 and JHW 007 in striatal or cellular membranes. Homologous competitive binding experiments were conducted rather than saturation assays to use ligand most economically and minimize nonspecific binding. Membranes were incubated with a fixed concentration of radioligand and increasing concentrations of unlabeled ligand. Nonspecific binding was determined with 100 μM cocaine ([3H]WIN 35428 assay), GBR 12909 ([3H]JHW 007 assay in rodent tissue), or 10 μM JHW 007 (for JHW 007 assays in hDAT-transfected N2A neuroblastoma cell membranes).
For Na+-dependence binding assays, membranes were prepared as described above with the final suspensions in Na+-appropriate buffer. The amount of Na+ in the buffer was adjusted for final concentrations of 1, 3, 10, 30, and 100 mM sodium phosphate. Each tube contained 0.5 ml of buffer including tissue and 0.5 nM [3H]WIN 35428 or [3H]JHW 007. Incubation was terminated after 120 min by rapid filtration. Nonspecific binding was determined with 100 μM cocaine ([3H]WIN 35428 assay) or GBR 12909 ([3H]JHW 007 assay).
Competition studies were conducted by determining the inhibition of 0.5 nM [3H]WIN 35428 or [3H]JHW 007 binding to 1.0 mg of membranes by including various concentrations of competing compounds in the incubation medium. Assay tubes were incubated for 120 min on ice. Competition with the tritiated ligands by various dopamine, serotonin, or norepinephrine uptake inhibitors was determined in at least three independent experiments, each performed in triplicate.
Muscarinic M1 receptors were labeled with [3H]pirenzepine (PerkinElmer Life and Analytical Sciences). Whole brains excluding cerebellum from DAT KO and WT mice were thawed in ice-cold buffer (10 mM Tris-HCl, 320 mM sucrose, pH 7.4) and homogenized with a Brinkmann Polytron (setting 6 for 20 s) and centrifuged at 1000g for 10 min at 4°C. The resulting supernatant was then centrifuged at 10,000g for 20 min at 4°C. The resulting pellet was resuspended in a volume of 200 mg/ml in 10 mM Tris buffer, pH 7.4. Experiments were conducted in assay tubes containing 0.5 ml of buffer and incubated for 60 min at 37°C in a water bath. Each tube contained 3 nM radioligand and 20 mg of tissue (original wet weight). Nonspecific binding was determined by using 100 μM quinuclidinyl benzilate (Sigma-Aldrich). Assays were typically conducted for at least three independent experiments, each performed with triplicate or duplicate observations (tubes).
Histamine H1 receptors were labeled with [3H]mepyramine (PerkinElmer Life and Analytical Sciences). Membranes were prepared from whole brain excluding cerebellum of DAT KO and WT mice. Tissue was homogenized in 30 volumes of ice-cold 50 mM Na-K buffer (37.8 mM Na2HPO4, 12.2 mM KH2PO4, pH adjusted to 7.5 at 25°C) using a Brinkmann Polytron (setting 6 for 20 s) and centrifuged at 25,000g for 10 min at 4°C. The supernatant was discarded, and the pellet was resuspended in ice-cold Na-K buffer and centrifuged. The resulting pellet was then resuspended in Na-K buffer to give 200 mg/ml wet weight final volume. Experiments were conducted in assay tubes containing 0.5 ml of buffer and incubated for 120 min on ice. Each tube contained 2 nM radioligand and 20 mg of tissue (original wet weight). Nonspecific binding was determined by using promethazine HCl. Assays were typically conducted for at least three independent experiments, each performed with triplicate or duplicate observations (tubes).
Unless otherwise specified, all incubations were terminated by rapid filtration through Whatman (Clifton, NJ) GF/B filters, pre-soaked in 0.3% polyethyleneimine ([3H]JHW 007, [3H]pirenzepine, [3H]mepyramine) or 0.05% polyethyleneimine ([3H]WIN 35428), by using a Brandel R48 filtering manifold (Brandel Inc., Gaithersburg, MD). The filters were washed twice with 5 ml of cold buffer and transferred to scintillation vials. Beckman Ready Safe scintillation cocktail (3.0 ml) was added, and the vials were counted the next day with a Beckman 6000 liquid scintillation counter (Beckman Coulter Instruments, Fullerton, CA) at 50% efficiency.
Data Analysis. Data from association experiments were fit by nonlinear regression to a one- or two-phase exponential growth equation describing pseudo first-order association kinetics. Data from dissociation experiments were fit to a one- or two-phase exponential decay equation. In determining ligand affinities, data were fit to homologous competition curves; sigmoidal dose-effect curves were used to determine Hill slopes. Data from the replicates were globally fit by using Prism for Windows (GraphPad Software Inc., San Diego, CA).
Results
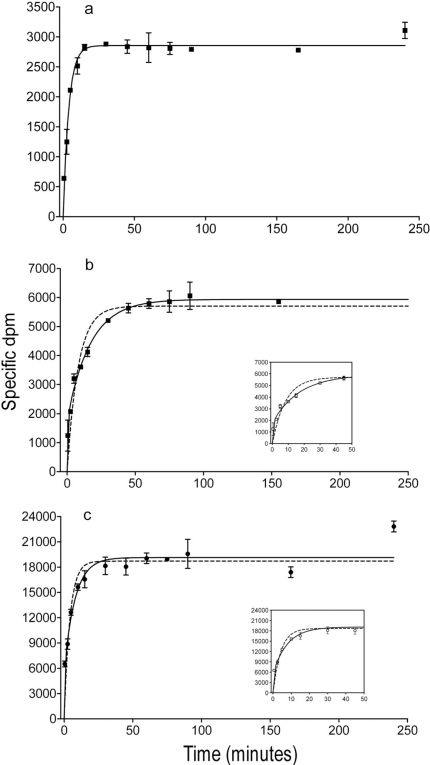
Binding association data for 0.5 nM [3H]WIN 35428 were best fit to a single-phase model with kon values reflecting half-lives of less than 3 min in rat and mouse (Fig. 2a; Table 1). Equilibrium in [3H]WIN 35428 binding was reached within a 25-min incubation period, and those conditions persisted to the last time point tested. Nonspecific binding was 5.52 and 6.95% of total binding at 240 min. The association of 0.5 nM [3H]JHW 007 was better fit to a two-phase than a single-phase model in membranes from rat (Fig. 2b; F2,68 = 43.64; p < 0.0001) and mouse (F2,65 = 42.07; p < 0.0001). Initial phases with kon values reflecting half-lives of approximately 0.1 min for both species were modeled, with second phases reflecting half-lives of 41.7 and 28.0 min in rat and mouse membranes, respectively. Equilibrium binding conditions persisted from approximately 60 min to the longest time value examined, 240 min. Nonspecific binding of [3H]JHW 007 was 10.9 and 18.1% of total binding at 240 min in rat and mouse, respectively. The association of [3H]JHW 007 to hDAT/N2A membranes (Fig. 2c) also were fit best to a two-phase model (F2,95 = 9.79; p < 0.001), with kon values reflecting half-lives of 0.326 and 12.3 min (Table 1). The difference between the one- and two-phase models was less in cells than in tissue (compare Fig. 2, b and c). Equilibrium was reached within 25 min and maintained thereafter. Nonspecific binding of [3H]JHW 007 was 8.3% of total binding at 240 min in hDAT/N2A membranes.
Fig. 2.
Association of [3H]WIN 35428 (a) or [3H]JHW 007 (b) with rat striatal membranes and [3H]JHW 007 with hDAT N2A membranes (c). The curves represent the results of a single experiment with vertical bars representing S.E.M. from three tubes. The results were selected from at least three as representative of the binding parameters resulting from a global modeling of all of the replications. Insets show the data for the first 50 min of association plotted with a changed aspect ratio to emphasize the difference between the two- and one-phase models during that time period.
TABLE 1.
Membrane binding constants for [3H]WIN35428 and [3H]JHW 007
Cells without entries indicate that single-component kinetic or single-site binding models were preferred.
| [3H]WIN 35428
|
[3H]JHW 007
|
||||||
|---|---|---|---|---|---|---|---|
| Rat | Swiss-Webster Mouse | Rat | Swiss-Webster Mouse | hDAT/N2A | DAT WT | DAT KO | |
| Association kon 1 (nM−1 · min−1) | 0.246 (±0.0220) | 0.236 (±0.0233) | 5.35 (±4.22) | 7.10 (±10.7) | 2.13 (±1.03) | N.D. | N.D. |
| Half-life (min) | 2.82 | 2.94 | 0.130 | 0.0977 | 0.326 | N.D. | N.D. |
| Y-Max (dpm) | 2560 (±42.1) | 1450 (±26.1) | 1700 (±143) | 1370 (±135) | 5180 (±888) | N.D. | N.D. |
| Association kon 2 (nM−1 · min−1) | 0.0166 (±4.49 × 10−3) | 0.0247 (±6.75 × 10−3) | 0.0563 (±0.0252) | N.D. | N.D. | ||
| Half-life (min) | 41.7 | 28.0 | 12.3 | N.D. | N.D. | ||
| Y-Max (dpm) | 2140 (±218) | 1590 (±157) | 4890 (±851) | N.D. | N.D. | ||
| Dissociation koff 1 (min−1) | 0.181 (±0.0127) | 2.51 (±1.93) | 0.0569 (±7.81 × 10−3) | 0.433 (±0.251) | 1.43 (±0.883) | N.D. | N.D. |
| Half-life (min) | 3.83 | 0.277 | 12.2 | 1.60 | 0.484 | N.D. | N.D. |
| Dissociation koff 2 (min−1) | 0.162 (±0.0185) | 0.0319 (±0.0214) | 0.0726 (±0.0135) | N.D. | N.D. | ||
| Half-life (min) | 4.28 | 21.7 | 9.54 | N.D. | N.D. | ||
| Kd 1 (nM) | 4.21 (±0.0334) | 8.99 (±0.0410) | 7.40 (±0.0533) | 8.18 (±0.057) | 43.7 (±0.0677) | 25.1 (±0.0524) | 28.4 (±0.0751) |
| Bmax 1 (fmol/mg protein) | 1860 (±27.5) | 1860 (±29.1) | 9230 (±115) | 13,200 (±241) | 52,200 (±1260) | 22,300 (±408) | 11,100 (±356) |
| Hill slope | 0.958 (±0.0268) | 0.918 (±0.0256) | 0.734 (±0.0393) | 0.550 (±0.0348) | 1.44 (±0.146) | 0.813 (±0.0482) | 0.552 (±0.0418) |
| Kd 2 (nM) | 4400 (±0.338) | 2750 (±0.195) | 2380 (±0.317) | 2000 (±0.149) | |||
| Bmax 2 (fmol/mg protein) | 787,000 (±59,200) | 1.09 × 106 (±68,900) | 459,000 (±48,500) | 493,000 (±30,600) | |||
N.D., not determined.
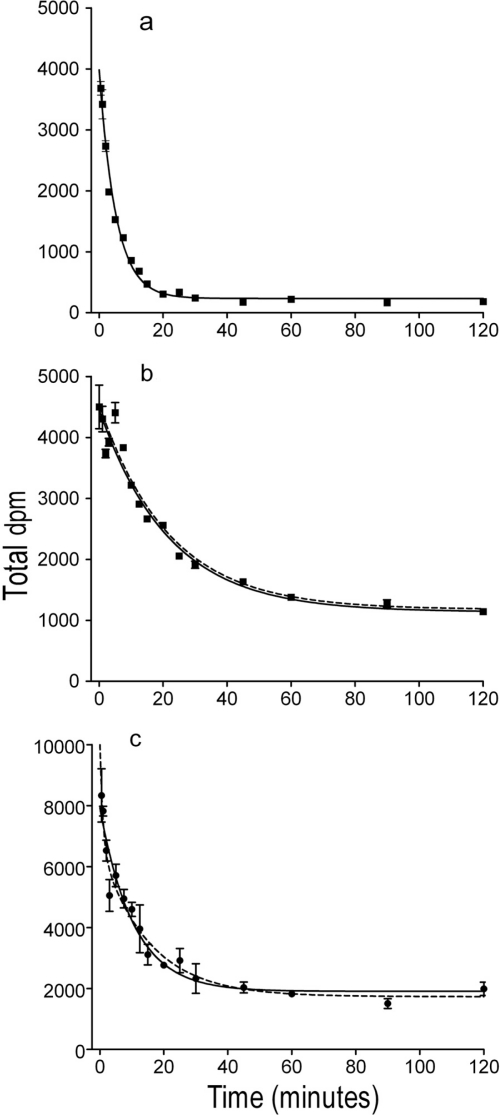
The dissociation of [3H]WIN 35428 was initiated with the addition of 100 μM cocaine and was relatively rapid (Fig. 3a). Data were best fit by a single-phase model in rat tissue (F2,136 = 2.103; p = 0.126), whereas a two-phase model (Table 1) best fit the dissociation data in mouse tissue (F2,139 = 5.163; p = 0.0069). However, the two models were virtually identical when plotted (data not shown). The koff value obtained in rat tissue reflected a half-life of 3.83 min, whereas koff values obtained in mouse tissue reflected half-lives of 0.277 and 4.28 min (Table 1), and the less preferred one-phase model had a half-life of 3.53 min. The residual binding with [3H]WIN 35428 at 120 min in tissue from either species (Fig. 3) was approximately 6 to 8% of total binding in the association experiments and was consistent with the values obtained for nonspecific binding.
Fig. 3.
Dissociation of [3H]WIN 35428 (a) or [3H]JHW 007 (b) from rat striatal membranes and [3H]JHW 007 from hDAT N2A membranes (c). The curves represent the results of a single experiment with vertical bars representing S.E.M. from three tubes. The results were selected from at least three as representative of the binding parameters resulting from a global modeling of all of the replications.
Dissociation of [3H]JHW 007 was initiated with the addition of 100 μM GBR 12909 at 240 min after the addition of radioligand, demonstrating the reversibility of the binding reaction (Fig. 3b). Data were best fit by a single-phase model in rat (F2,137 = 0.150; p = 0.861), whereas a two-phase model best fit the dissociation data in mouse (F2,136 = 4.613; p = 0.0115) and hDAT/N2A (F2,138 = 7.220; p < 0.001) membranes. The one- and two-phase dissociation models were virtually identical when plotted (Fig. 3, b and c). The koff value obtained in rat tissue reflected a half-life of 12.2 min, whereas koff values obtained reflected half-lives of 1.60 and 21.7 min in mouse tissue and 0.484 and 9.54 min in hDAT/N2A cell membranes (Table 1) or 6.22 and 6.37 min, respectively for the less preferred one-phase models. The residual binding with [3H]JHW 007 at 120 min in membranes from either species or in hDAT/N2A cells (Fig. 3) was approximately 20 to 25.0% of total binding.
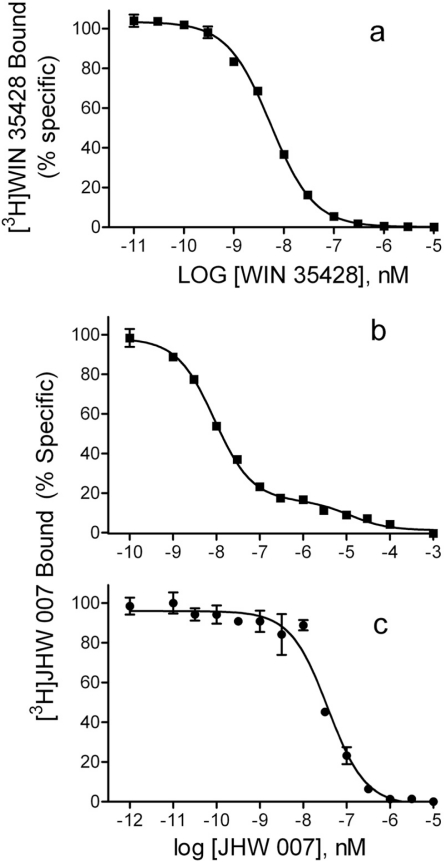
The Kd values for WIN 35428 obtained from homologous competitive binding experiments were similar in rat and mouse membranes (Table 1), and the data were better fit to a single-site model than a two-site model. Homologous competition curves (represented by Fig. 4a) indicated that the range from zero to complete displacement of [3H]WIN 35428 occurred over an approximate 100-fold domain of concentrations of unlabeled ligand. The unconstrained Hill slopes for [3H]WIN 35428 from rat tissue (F1,265 = 1.273; p = 0.2603) did not significantly differ from the value of 1.0 (Table 1). Although the value for mouse tissue did significantly differ from 1.0 (F1,112 = 73.06; p < 0.0001), the fitted value 0.918 was close to 1.0.
Fig. 4.
Homologous competition of radiotracer and nonradioactive WIN 35428 (a) or JHW 007 (b) at rat striatal membranes and JHW 007 at hDAT N2A membranes (c). The curves represent the results of a single experiment with vertical bars representing S.E.M. from three tubes. The results were selected from at least three as representative of the binding parameters resulting from a global modeling of all of the replications.
The displacement of [3H]JHW 007 from rodent membranes occurred over a larger than 10,000-fold domain of concentrations of unlabeled JHW 007, with inflections in the competition curve (represented by Fig. 4b). The modeling of the competition was better fit to a two-site than a single-site model (F2,111 = 15.71; p < 0.001), with Kd values for the two binding sites of 7.40 and 4400 nM in rat and 8.18 and 2750 nM in mouse tissue (Table 1). The lower-affinity site had a substantially greater capacity. The unconstrained Hill slopes for [3H]JHW 007 data from rat (F1,112 = 25.72; p < 0.0001) and mouse (F1,112 = 73.06; p < 0.0001) were 0.734 and 0.550, respectively, and were significantly different from the value of 1.0, further indicating multiple binding sites. Binding of [3H]JHW 007 to hDAT/N2A membranes differed from the other membranes tested in that the range from zero to complete displacement of [3H]JHW 007 occurred over a less than 100-fold domain of concentrations of unlabeled drug. The resulting competition curve modeled better for a one-site profile (Kd = 63 nM) than a two-site profile (Fig. 4c). The unconstrained Hill slope for [3H]JHW 007 binding in hDAT/N2A membranes was 1.44, which was significantly greater than the theoretical value of 1.0 (F1,168 = 12.16; p = 0.0006; Table 1).
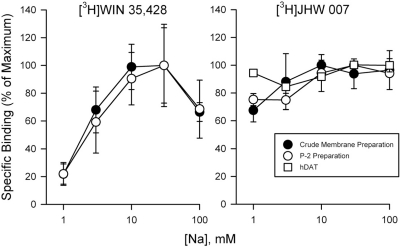
As has been shown previously, the binding of [3H]WIN 35428 in mouse and rat membranes depended on sodium concentration, with increases in binding up to concentrations of 30 mM (Fig. 5, left). This relation was obtained in both the crude membrane and P2 preparations. Linear regression of the binding of WIN 35428 up to the concentration of sodium that produced maximal binding resulted in normalized slopes of 53.6%/mM, which were significantly different from zero in both the crude membrane (F1,10 = 5.19; p = 0.0459) and the P2 preparation (F1,10 = 6.64; p = 0.0275). In contrast, the binding of [3H]JHW 007 was much less dependent on sodium concentration in either rodent tissue preparation (Fig. 5, right). In the crude membrane preparation the slope (32.3%/mM) was not significantly different from zero (F1,7 = 5.45; p = 0.0522), whereas in the P2 preparation the slope was lower still (19.0%/mM), although significantly different from zero (F1,10 = 11.5; p = 0.007). As with the rodent tissue, the slope for hDAT/N2A membranes was low (5.04%/mM) and not significantly different from zero (F1,10 = 2.086; p = 0.179).
Fig. 5.
Sodium concentration dependence of [3H]WIN 35428 (left) and [3H]JHW 007 (right) binding at crude and purified rat membranes and at membranes from cells transfected with hDAT.
Pharmacological characteristics of the specific binding of the two ligands were examined by determining the relative competition potencies of a variety of agents with affinity for monoamine transporters (Table 2). The competition by inhibitors of monoamine uptake for [3H]WIN 35428 binding uniformly fit a single-site model better than a two-site model. The Ki values for all compounds studied in competition with [3H]WIN 35428 were highly correlated in rat and mouse striatal membranes (r2 = 0.990; p < 0.001). Standard dopamine uptake inhibitors, including cocaine and its analogs, GBR 12909, mazindol, and nomifensine, all displaced [3H]WIN 35428 with relatively high affinity. The Ki values ranged from 1.12 nM for 3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1H-indole (RTI-55) to 153 nM for norcocaine in rat tissue, with similar values for mouse tissue. In contrast and as reported previously, Ki values for serotonin and norepinephrine uptake inhibitors were relatively higher than those of the known dopamine uptake inhibitors (Table 2).
TABLE 2.
Inhibition by various dopamine uptake inhibitors of specific binding of [3H]WIN35428 and [3H]JHW 007 in rodent striatal membranes
Values in parentheses are 95% confidence limits.
|
Ki Value
|
|||||
|---|---|---|---|---|---|
| [3H]WIN 35428
|
[3H]JHW 007
|
||||
| Rat | Mouse | Rat | Mouse | ||
| nM | |||||
| Dopamine uptake inhibitors | |||||
| Cocaine | Ki 1 | 87.5 (82.0–93.3) | 80.4 (69.8–92.7) | 20.2 (15.5–26.5) | 27.5 (18.7–40.6) |
| Ki 2 | 33,000 (20,100–54,000) | 19,200 (11,100–33,300) | |||
| GBR 12909 | Ki 1 | 3.24 (2.84–3.70) | 3.47 (3.03–4.04) | 11.6 (1.14–117) | 0.247 (0.0186–3.26) |
| Ki 2 | 15.5 (1.49–162) | 7.01 (5.21–9.44) | |||
| Mazindol | Ki 1 | 3.10 (2.90–3.31) | 10.4 (9.12–11.7) | 3.64 (2.48–5.33) | 19.5 (15.2–25.1) |
| Ki 2 | 557 (378–822) | 9350 (5830–15,000) | |||
| Nomifensine | Ki 1 | 30.1 (27.2–33.3) | 27.5 (25.7–29.3) | 43.5 (30.3–62.4) | 30.6 (23.9–38.9) |
| Ki 2 | 4610 (2520–8430) | 9640 (4730–19,600) | |||
| Norcocaine | Ki 1 | 153 (139–168) | 154 (144–165) | 142 (97.5–127) | 116 (93.3–144) |
| Ki 2 | 9330 (5190–16,800) | 20,900 (11,200–38,900) | |||
| WIN 35065-2 | Ki 1 | 29.4 (27.1–31.9) | 26.3 (23.4–29.6) | 26.1 (19.7–34.5) | 47.3 (28.2–79.1) |
| Ki 2 | 40,600 (17,900–92,000) | 30,000 (17,600–50,900) | |||
| WIN 35428 | Ki 1 | 11.8 (8.95–15.5) | 21.9 (12.7–37.7) | ||
| Ki 2 | 23,900 (13,100–43,600) | 20,700 (13,600–31,400) | |||
| RTI-55 | Ki 1 | 1.12 (0.993–1.26) | 0.714 (0.637–0.800) | 1.08 (0.753–1.55) | 0.831 (0.653–1.06) |
| Ki 2 | 6370 (4700–8240) | 8150 (6240–10,600) | |||
| Serotonin uptake inhibitors | |||||
| Paroxetine | Ki 1 | 560 (515–610) | 408 (374–446) | 316 (225–442) | 640 (516–793) |
| Ki 2 | 7740 (4060–14,700) | 39,100 (16,000–95,100) | |||
| Citalopram | Ki 1 | 11,400 (9820–13,200) | 8970 (7850–10,300) | 12,800a (11,100–14,900) | 4660 (851–25,500) |
| Ki 2 | 15,600 (3810–64,300) | ||||
| Fluoxetine | Ki | 2470 (2140–2860) | 1120 (979–1290) | 3480b (2990–4050) | 3600a (2420–5350) |
| Norepinephrine uptake inhibitors | |||||
| Desmethylimipramine | Ki 1 | 1960 (1670–2290) | 1310 (1120–1530) | 162 (22.8–1160) | 1010 (469–2180) |
| Ki 2 | 3860 (2470–6050) | 13,200 (4680–37,400) | |||
| Nisoxetine | Ki 1 | 163 (151–175) | 149 (135–165) | 226 (187–273) | 239 (193–297) |
| Ki 2 | 16,400 (7930–33,800) | 16,000 (10,400–25,800) | |||
| Atomoxetine | Ki 1 | 492 (456–531) | 593 (543–647) | 685 (556–845) | 573 (460–714) |
| Ki 2 | 33,800 (11,400–99,800) | 43,300 (19,100–97,900) | |||
| Benztropine analogs | |||||
| 4′-Cl-BZT | Ki 1 | 19.0 (17.5–21.6) | 17.7 (15.8–19.8) | 26.0 (23.0–29.3) | 24.6 (20.8–29.0) |
| Ki 2 | 5330 (2140–13,300) | 1190 (514–2770) | |||
| AHN 1-063 | Ki 1 | 399 (369–433) | 341 (316–368) | 270 (198–369) | 253 (206–310) |
| Ki 2 | 5060 (670–38,200) | 13,300 (2700–65,300) | |||
| JHW 007 | Ki | 12.0 (11.2–12.8) | 11.7 (10.5–13.0) | ||
These data did not converge on a two-site model. Values for a single-site model are listed.
The variance accounted for by a two-site model was greater than that accounted for by a one-site model. However, the values provided by the two-site model varied from each other by less than 5%, and the variability around the fitted constants was extremely large. Therefore, values for the constants provided by the single-site model are listed.
Analogs of benztropine, as reported previously (Newman et al., 1994; Agoston et al., 1997), displaced [3H]WIN 35428 with high affinity. JHW 007 was moderately more potent than 4′-chloro-3α-(diphenylmethoxy)tropane (4′-Cl-BZT), with 4′-chloro-3β-(diphenylmethoxy)tropane (AHN 1-063) the least potent among the drugs tested. 4′-Cl-BZT and AHN 1-063 are stereoisomers; 4′-Cl-BZT has the diphenyl-ether system attached to the tropane ring with axial (α) stereochemistry, whereas AHN 1-063 has its diphenyl-ether system in the equatorial (β) configuration. There was an approximate 20-fold stereoselectivity for 4′-Cl-BZT in rat and mouse tissue over AHN 1-063 (Table 2).
The competition of inhibitors of monoamine uptake with [3H]JHW 007 generally modeled better for two sites than for one site. The Ki values were significantly correlated in rat and mouse tissue (r2 = 0.6208; p < 0.0001), although the correlation coefficient was lower than that for [3H]WIN 35428. The cross-species comparison with [3H]JHW 007 is complicated by the preferred two-site model. When Ki values for only the high-affinity site were compared across species, the correlation among values was higher and approached that for [3H]WIN 35428 (r2 = 0.937; p < 0.0001). The correlation among low-affinity Ki values from tissue for the two species was substantially lower than that for the high-affinity site, although still significant (r2 = 0.423; p = 0.0118).
The high-affinity Ki values among known inhibitors of dopamine uptake ranged from 1.08 nM for RTI-55 to 142 nM for norcocaine in rat tissue, with similar values for mouse tissue (Table 2). In contrast high-affinity Ki values of known inhibitors of serotonin uptake were in the micromolar range in tissue from both species. The values for citalopram (in rat tissue) and fluoxetine reflected especially low affinities when the competition modeled better for one site. Competition with known inhibitors of norepinephrine uptake generally modeled better for two sites, with high-affinity values typically much lower than those for known dopamine uptake inhibitors (Table 2).
At the high-affinity [3H]JHW 007 site, 4′-Cl-BZT displaced the radiolabel with a 10-fold higher affinity than its enantiomer, AHN 1-063, in tissue from either rat or mouse (Table 2). There was an also an approximate 10-fold stereoselectivity among these drugs at the low-affinity [3H]JHW 007 site in mouse tissue, although not in tissue from rats (Table 2).
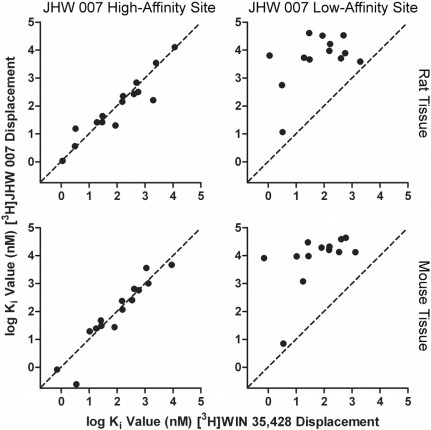
The affinities of the various monoamine uptake inhibitors for the [3H]WIN 35428 site were highly correlated with those for the high-affinity [3H]JHW 007 site in tissue from both species (Fig. 6, left). The r2 values for the correlations were 0.891 and 0.898 (both p < 0.0001), respectively, in rat and mouse tissue. Points relating the Ki values for competition against [3H]WIN 35428 to those for the [3H]JHW 007 high-affinity site fell close to the line of identity (dashed lines in Fig. 6). Points relating the Ki values for competition with [3H]WIN 35428 to those for the [3H]JHW 007 low-affinity site (Fig. 6, right) were most often far from the line of identity, and the correlations among these Ki values in rat or mouse tissue were not significant, with respective r2 values of 0.232 (p = 0.095) and 0.284 (p = 0.061).
Fig. 6.
The relationship between the affinities of various monoamine uptake inhibitors for the [3H]WIN 35428 site and the high-affinity (left) or low-affinity (right) [3H]JHW 007 sites in tissue from rat (top) or mouse (bottom) striatum. Individual values are plotted from Table 2.
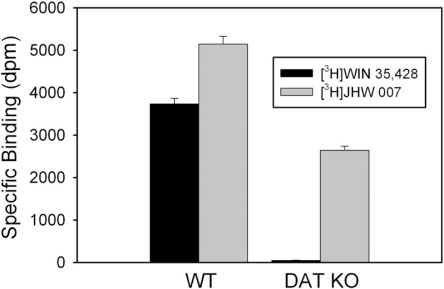
Elimination of DAT expression with the simian virus 40 T-antigen knockin at the DAT gene virtually eliminated specific binding of [3H]WIN 35428 and decreased specific binding of [3H]JHW 007 in striatal membranes (Fig. 7). Data from male and female mice were examined separately; however, there were no differences between sexes, and the results were combined for further analysis. Specific binding of [3H]WIN 35428 in striata from KO mice was 1.36% of that obtained in the same tissue from WT mice. In contrast, specific binding of [3H]JHW 007 was greater than that of [3H]WIN 35428 in tissue from WT mice and was reduced to 51.4% of controls in mice lacking DAT expression. Two-way analysis of variance indicated significance of the effects of ligand (F1,43 = 257.5; p < 0.001), genotype (F1,43 = 615.5; p < 0.001), and their interaction (F1,43 = 22.6; p < 0.001).
Fig. 7.
Specific binding of [3H]WIN 35428 or [3H]JHW 007 at WT or DAT KO striatal mouse membranes. The curves represent the results of a single experiment with vertical bars representing S.E.M. from three tubes. The results were selected from at least three as representative of the binding parameters resulting from a global modeling of all of the replications. The concentrations of radioligands for these experiments were 0.5 nM for throughout.
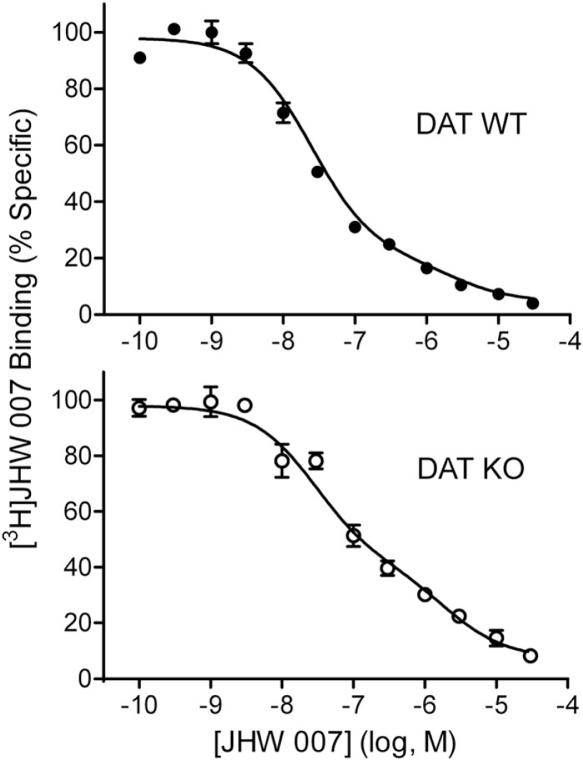
The [3H]JHW 007 binding was further characterized by comparing homologous competition in striatal membranes from DAT WT and KO mice (Fig. 8). In tissue from both lines of mice the displacement of [3H]JHW 007 occurred over a larger than 10,000-fold domain of concentrations of unlabeled ligand, with inflections in the competition curves. The unconstrained Hill slopes for [3H]JHW 007 in tissue from both lines of mice were significantly different from the value of 1.0 (WT, F1,139 = 12.7, p = 0.0005; KO, F1,138 = 76.9, p < 0.0001), further indicating multiple binding sites. The nonlinear regression of the data from both WT and KO tissue were better fit to two-site than one-site binding models (F2,138 = 16.2; p < 0.0001 and F2,137 = 54.7; p < 0.0001, respectively). The Kd values for the two binding sites were 25.1 and 2380 nM in WT and 28.4 and 2000 nM in KO mouse tissue (Table 1). The lower-affinity site had a substantially larger capacity than the high-affinity site, with considerable error associated with these values and no substantial difference among genotypes (Table 1).
Fig. 8.
Homologous competition of radiotracer and nonradioactive JHW 007 at striatal membranes from WT (top) and DAT KO (bottom) striatal mouse membranes. The curves represent the results of a single experiment with vertical bars representing S.E.M. from three tubes. The results were selected from at least three as representative of the binding parameters resulting from a global modeling of all of the replications.
Because benztropine, the parent compound of JHW 007, has affinity for muscarinic M1 and histamine H1 receptors, we examined the competition of JHW 007 with [3H]pirenzepine binding, a ligand for muscarinic M1 receptors, and [3H]mepyramine, a ligand for histamine H1 receptors (Table 3). The Ki values of JHW 007 for inhibition of the M1 ligand, [3H]pirenzepine, in whole brain excluding cerebellum from DAT WT and KO mice were 388 and 364 nM, respectively. The 95% confidence limit values did not overlap with those for either the high- or low-affinity Kd values for JHW 007 as determined from modeling the homologous competition data (Table 1). The JHW 007 Ki values for competition against the H1 ligand, [3H]mepyramine, in whole brain including cerebellum were 24.2 and 27.9 nM, respectively, in tissue from DAT WT and KO mice (Table 3). These values were similar to the high-affinity Kd for JHW 007 as determined from modeling the homologous competition data in the DAT KO mice (Table 1).
TABLE 3.
Affinities of JHW 007 in displacement of indicated radioligands for M1 and H1 receptors.
| Target | Ligand | WT Ki Value with 95% Confidence Limits | DAT KO Ki Value with 95% Confidence Limits | Ki Value at Target in Rat Brain with S.E.M. |
|---|---|---|---|---|
| nM | ||||
| Muscarinic M1 | [3H]Pirenzepine | 388 (272–554) | 364 (296 to 448) | 399 ± 28.3a |
| Histamine H1 | [3H]Mepyramine | 24.2 (19.2–30.5) | 27.9 (21.7 to 36.0) | 24.6 ± 2.43 |
Value from Katz, et al. (2004).
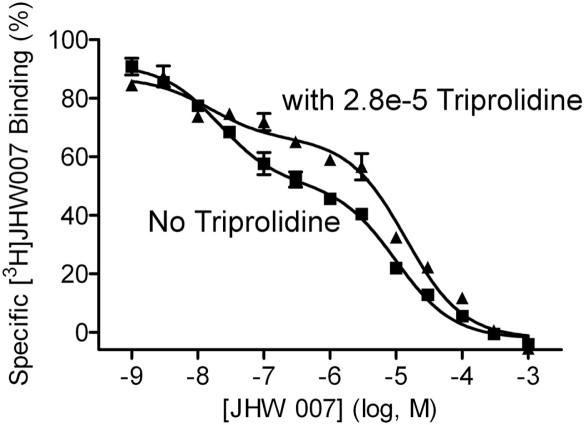
To further assess whether the [3H]JHW 007 binding was accounted for by its affinity for H1 sites, an additional set of homologous JHW 007 competition studies was conducted in striatal tissue from Sprague-Dawley rats and Swiss-Webster mice. In these studies, 5.6 μM unlabeled WIN 35428 (a concentration at more than 1000-fold the Kd of WIN 35428 at the DAT; Table 1) was added to the incubation medium to saturate the DAT cocaine binding site. Assays were conducted in parallel with and without 28 μM unlabeled triprolidine (H1 antagonist) added to saturate H1 sites. This concentration is 1000-fold greater than the Ki of triprolidine at H1 receptors (Tanda et al., 2008). In tissue from both rats and mice the homologous competition with the saturating concentration of WIN 35428 occurred over a more than 10,000-fold domain of concentrations of unlabeled ligand, with inflections in the curves (Fig. 9, squares). The nonlinear regression of the data from both rat and mouse tissues were better fit to two-site than one-site binding models (Table 4; rats: F2,226 = 93.05, p < 0.0001; mice: F2,189 = 196, p < 0.0001), with Kd values for the two binding sites of 17.8 and 12,100 nM in rat and 19.9 and 9640 nM in mouse tissue. The lower-affinity site had a substantially larger capacity than the high-affinity site, with considerable error associated with these values (Table 4).
Fig. 9.
Homologous competition of radiotracer and nonradioactive JHW 007 in the presence of a saturating concentration of WIN 35428 in the presence (▴) or absence (▪) of triprolidine. The curves represent the results of a single experiment with vertical bars representing S.E.M. from three tubes. The results were selected from at least three as representative of the binding parameters resulting from a global modeling of all of the replications. The concentrations of WIN 35428 (5.6 μM) and triprolidine (28 μM) added to the tubes were selected to saturate those sites.
TABLE 4.
Affinities of JHW 007 determined from homologous competition assays
Values are in nM with 95% confidence limits.
| Rat
|
Mouse
|
|||
|---|---|---|---|---|
| WIN 35428 Onlya | WIN 35428 and Triprolidineb | WIN 35428 Onlya | WIN 35428 and Triprolidineb | |
| Kd 1 | 17.8 (10.0–31.6) | 28.3 (6.97–115) | 19.9 (14.7–27.0) | 13.2 (5.48–32.0) |
| Kd 2 | 12,100 (7480–19,600) | 11,600 (7110–19,000) | 9640 (6370–14,600) | 8750 (6410–11,900) |
5.6 μM unlabeled WIN 35428 (final concentration) in all tubes.
5.6 μM unlabeled WIN 35428 and 28 μM unlabeled triprolidine (final concentrations) in all tubes.
The homologous competition of JHW 007 in the presence of the same saturating concentration of WIN 35428 and 28 μM triprolidine also occurred over a larger than 10,000-fold domain of concentrations of unlabeled JHW 007 (Fig. 9, triangles). There was evidence of a diminished capacity of the high-affinity site (Fig. 9, compare squares and triangles), although a high-affinity site remained evident in the curve. The nonlinear regression of the data from both rat and mouse tissues was better fit to two-site than one-site binding models, with Kd values for the two binding sites of 28.3 and 11,600 nM in rat and 13.2 and 8750 nM in mouse tissue (Table 4). These fits were preferred over a single-site model (rat: F2,113 = 19.1, p < 0.0001; mouse: F2,113 = 51.7, p < 0.0001).
A previous study (Katz et al., 2004) suggested sites in addition to the DAT at which JHW 007 might be binding. Those sites are shown in Table 5, along with ligands used to target those sites selected for their known affinity. The competition against [3H]JHW 007 binding in striatum from DAT WT and KO mice was typically obtained at concentrations higher than 10 μM, the exceptions being that with 3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1H-indole (L-741626) and rimcazole. IC50 values for displacement of [3H]JHW 007 were substantially higher than the Ki values for these compounds at the target sites as reported in the literature. The decrease in potency of rimcazole in tissue from DAT KO compared with WT mice probably is related to its affinity for the DAT (e.g., Ukairo et al., 2005).
TABLE 5.
Potencies of various ligands in displacement of [3H]JHW 007 from striatal tissue of WT and DAT KO mice
All values are expressed as nM concentrations. The listed Ki values are those obtained for these compounds as reported in the literature.
| Target | Displacer | IC50 Value (95% confidence interval)
|
Literature Ki Values at Target (± S.E.M.) | |
|---|---|---|---|---|
| DAT KO | WT | |||
| Dopamine D3 | PD 128907 | >10,000 | >10,000 | 8.48 ± 0.11 (Cussac, 2000) |
| Dopamine D2 | L-741626 | 8240 (6200–11,000) | 5130 (3780–6980) | 7.52 ± 0.01 (Cussac, 2000) |
| Histamine H1 | (+)-Chlorpheniramine | >10,000 | >10,000 | 10 (Hill and Young, 1980) |
| Muscarinic M2 | AQ-RA 741 | >10,000 | >10,000 | 8.37 ± 0.04 (Dorje, et al., 1990) |
| 5-HT2A | Ketanserin | >10,000 | >10,000 | 8.86 ± 0.08 (Hoyer, et al., 1985) |
| Sigma | Rimcazole | 4790 (3490–6570) | 1770 (1160–2710) | 836 ± 44 (DeHaven-Hudkins, et al., 1992) |
| Na channel | Tetracaine | >10,000 | >10,000 | 1700 ± 0.6 (Postma and Catterall, 1984) |
Discussion
Binding to the DAT is considered the initial action by which cocaine produces the effects that confer its abuse liability (Carroll et al., 1999). However, we previously reported BZT analogs that have high DAT affinity and selectivity, without preclinical predictors of abuse liability (Newman and Katz, 2009; Tanda et al., 2009a). JHW 007 is one such BZT analog that also antagonizes cocaine-induced increases in extracellular dopamine (Tanda et al., 2009b) and locomotion (Desai et al., 2005), as well as cocaine self-administration (Hiranita et al., 2009). To investigate features of the pharmacology of JHW 007 that may contribute to its cocaine antagonist effects, the present study examined the in vitro binding of [3H]JHW 007 and compared it with that of [3H]WIN 35428, a ligand used to label the cocaine binding site (Carroll et al., 1999). The binding of JHW 007 in native tissue differed from that of WIN 35428 in modeling better for more than one binding site, whereas models of WIN 35428 binding, consistent with previous results (e.g., Reith and Selmeci, 1992), preferred a single site.
JHW 007 exhibited a two-phase association with rodent striatal and hDAT-transfected N2A neuroblastoma cell membranes. A rapid phase with a half-life of seconds was followed by a slower component with a half-life of tens of minutes. In a previous study, in vivo displacement of the DAT ligand 3β-(4-[125I]iodophenyl) tropane-2β-carboxylic acid isopropyl ester ([125I]RTI-121) (a cocaine analog) by JHW 007 had a slow time course, with maximal displacement not evident after 4 h. Nonetheless, at the earliest times there was as much as 20% displacement of specific [125I]RTI-121 binding (Desai et al., 2005). Furthermore, evidence suggests that the fast association component of JHW 007 binding is responsible for its cocaine antagonist effects. A recent study examined cocaine–JHW 007 combinations on extracellular dopamine concentrations in the nucleus accumbens shell in mice (Tanda et al., 2009b), an area thought to be intimately involved in the abuse liability of cocaine (Pontieri et al., 1995). Those combinations were generally infra-additive, whereas combinations of cocaine and WIN 35428 were supra-additive. Furthermore, infra-additive effects of JHW 007 and cocaine were more prominent early compared with later after JHW 007 injection. Finally, antagonism of the locomotor-stimulant effects of cocaine was obtained as rapidly as 10 min after injection (R. I. Desai et al., submitted manuscript). These antagonist effects with the infra-additive effects on nucleus accumbens dopamine suggest a relation between its atypical effects and the faster association rate of JHW 007.
DAT translocation of neurotransmitter depends on sodium ions, and the binding of dopamine uptake inhibitors to the DAT, including [3H]WIN 35428 in the present study, is regulated by the concentration of sodium in the buffer (Chen et al., 2002). In contrast, the binding of [3H]JHW 007 was largely independent of sodium concentration. Previous studies showed “atypical” inhibitors such as benztropine, GBR 12909, and buproprion, to be less sensitive to sodium in intact cells (Schmitt et al., 2008). Because results from native tissue indicated multiple-site [3H]JHW 007 binding, hDAT/N2A membranes that showed single-site binding were studied. The largely sodium-independent nature of JHW 007 binding in hDAT/N2A membranes indicates that the JHW 007 interaction with the DAT differs from that of WIN 35428. In addition, JHW 007 binding was sodium insensitive in both membranes and synaptosomes and therefore does not depend on a membrane potential or sodium gradient (Chen and Reith, 2003).
A DAT model incorporating JHW 007 suggests a binding site in the DAT central cavity, preserving an D79-Y156 hydrogen bond that contributes to occluding this binding pocket (Beuming et al., 2008), leaving the DAT in a closed (inward facing) conformation. Reith et al. (2006) suggest that the DAT in an outward facing conformational state is poised for binding Na+, which in turn facilitates binding of cocaine analogs. Accordingly, the potential of JHW 007 to shift the DAT to an inward facing conformational equilibrium (Loland et al., 2008) would hinder that sodium-induced facilitation of ligand binding.
The high-affinity JHW 007 binding site in tissue from rodents seems similar to the cocaine binding site labeled with [3H]WIN 35428. The two ligands are mutually displaceable, and binding of cocaine analogs and other recognized DAT ligands had a higher affinity in displacing [3H]JHW 007 than did recognized serotonin and norepinephrine transport inhibitors, as has been reported previously for [3H]WIN 35428 binding (e.g., Madras et al., 1989). Furthermore, there was a high correlation between binding affinities of displacers at the JHW 007 high-affinity site and the site labeled by [3H]WIN 35428. The difference in dependence on sodium between [3H]WIN 35428 and [3H]JHW 007 binding may reflect different points of attachment to the DAT (binding domains) as suggested in the modeling studies by Beuming et al. (2008).
Some studies have found multiple binding sites using [3H]WIN 35428 (e.g., Madras et al., 1989; but see Reith and Selmeci, 1992). However, the multiple-site binding of [3H]JHW 007 differs from that occasionally identified for WIN 35428. The binding of WIN 35428 was virtually eliminated, whereas substantial binding of JHW 007 remained in tissue from DAT KO mice. Because the elimination of DAT protein was previously confirmed (Rothman et al., 2002) in these KO mice, sites other than those associated with the DAT contribute to the multiple-site binding in DAT KO tissue. Furthermore, the binding of JHW 007 to membranes from cells transfected with hDAT shows no evidence for multiple-site binding. Thus, JHW 007 binds with high affinity to a single DAT site, as do cocaine analogs, although with differing binding domains, and to multiple sites that are not located on the DAT protein.
The identities of the non-DAT JHW 007 high- and low-affinity binding sites were addressed in several experiments. Displacement of the M1 ligand, [3H]pirenzepine, and the H1 ligand, [3H]mepyramine, in DAT KO tissue gave Ki values that were similar to those previously obtained at these targets. The values for muscarinic M1 receptors did not match the values for either the JHW 007 high- or low-affinity sites; however, the Ki value for displacement of [3H]mepyramine approximated the value for the non-DAT high-affinity JHW 007 site. The multiple-site binding of [3H]JHW 007 in striatum was not caused entirely by H1-receptor binding, because H1 binding capacity in rat striatum seems to be much lower than that for JHW 007 (Chang et al., 1978; Palacios et al., 1978; Bouthenet et al., 1988), and homologous JHW 007 competition studies in the presence of saturating concentrations of unlabeled triprolidine remained biphasic. Furthermore, other potential displacers representing various binding sites at which JHW 007 has some identified activity (Katz et al., 2004) largely failed to displace [3H]JHW 007 binding with any substantial potency. Thus there is a previously unidentified high-affinity striatal JHW 007 binding site that does not correspond to known candidate sites for the compound.
The present results also suggest a low-affinity non-DAT JHW 007 binding site in striatum. The moderate stereoselectivity of 4′-Cl-BZT and AHN 1063 in striatal tissue from mice, but not rats, suggests that the low-affinity site may be different in the two species. Rothman et al. (2002) previously identified a high-affinity binding site for the cocaine analog [125I]RTI-55 that was distinct from known monoamine transporter sites, but that binds many monoamine uptake inhibitors. That site seems to be pharmacologically distinct from the present low-affinity JHW 007 site; among compounds that were examined in both studies there is no significant correlation of Ki values. In addition, RTI-55 had micromolar affinity for the low-affinity JHW 007 site, but was in the 10-nM range for the site examined by Rothman et al. (2002).
Another potential low-affinity site for JHW 007 binding is the “piperazine-acceptor” site. In characterizing the binding of the DAT ligand [3H]GBR 12935, a second site was characterized that was not a neurotransmitter receptor, was 6-OH-DA insensitive, and was distributed uniformly throughout brain tissue (Andersen et al., 1987). In addition, BZT, the parent compound of JHW 007, has been reported to label the piperazine-acceptor site (Hirai et al., 1988). Because GBR 12909 was used to define nonspecific binding, and the piperazine-acceptor site is sensitive to GBR 12909 (Andersen et al., 1987), further studies will address whether [3H]JHW 007 is labeling that site.
In summary, JHW 007 has a complex binding profile that includes the DAT and high- and low-affinity non-DAT sites that may contribute to its cocaine antagonist effects. The high-affinity JHW 007 DAT site seems to closely related to the cocaine recognition site. However, the binding of JHW 007 differs from WIN 35428 binding. First, the association of JHW 007 occurs in two phases, with the fast association rate possibly contributing to its cocaine antagonist effects based on previous functional studies. In addition, JHW 007 binding contrasts with WIN 35428 binding to the DAT because the former is relatively Na+-independent. That independence may reflect a shift of the DAT to an inward-(cytoplasmic-) facing conformation that in turn decreases the affinity of the DAT for cocaine and may thereby contribute to the cocaine antagonist effects of JHW 007.
Acknowledgments
We thank Drs. Su-Min Li for helping with some of the binding experiments; Greg Agoston for synthesizing the precursor to [3H]JHW 007; Margaret Gnegy for providing hDAT N2A neuroblastoma cells; Linda Werling for providing advice and guidance throughout the studies; and the two reviewers for providing instructive comments.
ABBREVIATIONS:
- DA
dopamine
- DAT
DA transporter
- hDAT
human DAT
- 4′-Cl-BZT
4′-chloro-3α-(diphenylmethoxy)tropane
- AHN 1-063
4′-chloro-3β-(diphenylmethoxy)tropane
- AQ-RA 741
11-[[4-[4-(diethylamino)butyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
- BZT
benztropine
- GBR 12909
1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-[3-phenylpropyl]piperazine dihydrochloride
- JHW 007
N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane
- KO
knockout
- L-741626
3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1H-indole
- MTSET
[2-(trimethylammonium) ethyl]-methanethiosulfonate
- PD 128907
(S)-(+)-(4aR,10bR)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydro-chloride
- RTI-55
3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1H-indole
- WIN 35428
methyl (1R,2S,3S,5S)-3-(4-fluorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- WIN 35065-2
(-)-2β-carbomethoxy-3β-phenyltropane tartrate
- WT
wild type
- [125I]RTI-121
3β-(4-[125I]iodophenyl) tropane-2β-carboxylic acid isopropyl ester
Footnotes
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. Novel N-substituted 3α-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem. 1997;40:4329–4339. doi: 10.1021/jm970525a. [DOI] [PubMed] [Google Scholar]
- Andersen PH, Jansen JA, Nielsen EB. [3H]GBR 12935 binding in vivo in mouse brain: labelling of a piperazine acceptor site. Eur J Pharmacol. 1987;144:1–6. doi: 10.1016/0014-2999(87)90002-1. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet ML, Ruat M, Sales N, Garbarg M, Schwartz JC. A detailed mapping of histamine H1-receptors in guinea-pig central nervous system established by autoradiography with [125I]iodobolpuramine. Neuroscience. 1988;26:553–600. doi: 10.1016/0306-4522(88)90167-4. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- Chang RS, Tran VT, Snyder SH. Histamine HI-receptors in brain labeled with 3H-mepyramine. Eur J Pharmacol. 1978;48:463–464. doi: 10.1016/0014-2999(78)90177-2. [DOI] [PubMed] [Google Scholar]
- Chen N, Reith ME. Na+ and the substrate permeation pathway in dopamine transporters. Eur J Pharmacol. 2003;479:213–221. doi: 10.1016/j.ejphar.2003.08.070. [DOI] [PubMed] [Google Scholar]
- Chen N, Sun L, Reith ME. Cationic interactions at the human dopamine transporter reveal binding conformations for dopamine distinguishable from those for the cocaine analog 2α-carbomethoxy-3α-(4-fluorophenyl)tropane. J Neurochem. 2002;81:1383–1393. doi: 10.1046/j.1471-4159.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Sezgin L, Millan MJ. The novel antagonist, S33084, and GR218,231 interact selectively with cloned and native, rat dopamine D(3) receptors as compared with native, rat dopamine D(2) receptors. Eur J Pharmacol. 2000;394:47–50. doi: 10.1016/s0014-2999(00)00149-7. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Fleissner LC, Ford-Rice FY. Characterization of the binding of [3H](+)-pentazocine to sigma recognition sites in guinea pig brain. Eur J Pharmacol. 1992;227:371–378. doi: 10.1016/0922-4106(92)90153-m. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci. 2005;25:1889–1893. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörje F, Friebe T, Tacke R, Mutschler E, Lambrecht G. Novel pharmacological profile of muscarinic receptors mediating contraction of the guinea-pig uterus. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:284–289. doi: 10.1007/BF00169439. [DOI] [PubMed] [Google Scholar]
- Ferragud A, Velázquez-Sánchez C, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, Canales JJ. A dopamine transport inhibitor with markedly low abuse liability suppresses cocaine self-administration in the rat. Psychopharmacology. 2009;207:281–289. doi: 10.1007/s00213-009-1653-x. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Young JM. Histamine H1-receptors in the brain of the guinea-pig and the rat: differences in ligand binding properties and regional distribution. Br J Pharmacol. 1980;68:687–696. doi: 10.1111/j.1476-5381.1980.tb10861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Kitamura N, Hashimoto T, Nakai T, Mita T, Shirakawa O, Yamadori T, Amano T, Noguchi-Kuno SA, Tanaka C. [3H]GBR-12935 binding sites in human striatal membranes: binding characteristics and changes in parkinsonians and schizophrenics. Jpn J Pharmacol. 1988;47:237–243. doi: 10.1254/jjp.47.237. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther. 2009;329:677–686. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Engel G, Kalkman HO. Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (−)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur J Pharmacol. 1985;118:13–23. doi: 10.1016/0014-2999(85)90658-2. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. Novel 3α-diphenyl-methoxy-tropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther. 1999;288:302–315. [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. Effects of N-substituted analogues of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther. 2004;309:650–660. doi: 10.1124/jpet.103.060525. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Grånäs C, Javitch JA, Gether U. Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. J Biol Chem. 2004;279:3228–3238. doi: 10.1074/jbc.M304755200. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci USA. 2002;99:1683–1688. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Spealman RD, Fahey MA, Neumeyer JL, Saha JK, Milius RA. Cocaine receptors labeled by [3H]2β-carbomethoxy-3β-(4-fluorophenyl)tropane. Mol Pharmacol. 1989;36:518–524. [PubMed] [Google Scholar]
- Newman AH, Katz JL. The benztropines: atypical dopamine uptake inhibitors that provide clues about cocaine’s mechanism at the dopamine transporter. Topics Med Chem. 2009;4:95–129. [Google Scholar]
- Newman AH, Allen AC, Izenwasser S, Katz JL. Novel 3α-(diphenylmethoxy)tropane analogs: potent dopamine uptake inhibitors without cocaine-like behavioral profiles. J Med Chem. 1994;37:2258–2261. doi: 10.1021/jm00041a002. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Schwartz JC, Garbarg M. High affinity binding of 3H-histamine in rat brain. Eur J Pharmacol. 1978;50:443–444. doi: 10.1016/0014-2999(78)90151-6. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma SW, Catterall WA. Inhibition of binding of [3H]batrachotoxinin A 20-α-benzoate to sodium channels by local anesthetics. Mol Pharmacol. 1984;25:219–227. [PubMed] [Google Scholar]
- Reith ME, Selmeci G. Radiolabeling of dopamine uptake sites in mouse striatum: comparison of binding sites for cocaine, mazindol, and GBR 12935. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:309–318. doi: 10.1007/BF00168692. [DOI] [PubMed] [Google Scholar]
- Reith ME, Berfield JL, Wang LC, Ferrer JV, Javitch JA. The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem. 2001;276:29012–29018. doi: 10.1074/jbc.M011785200. [DOI] [PubMed] [Google Scholar]
- Reith ME, Zhen J, Chen N. The importance of company: Na+ and Cl− influence substrate interaction with SLC6 transporters and other proteins. Handb Exp Pharmacol. 2006;175:75–93. doi: 10.1007/3-540-29784-7_4. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Carroll FI, Morales M, Rowley DL, Rice KC, Dersch CM, Donovan DM. Studies of the biogenic amine transporters. 10. Characterization of a novel cocaine binding site in brain membranes prepared from dopamine transporter knockout mice. Synapse. 2002;44:94–105. doi: 10.1002/syn.10060. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith ME. Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties. J Neurochem. 2008;107:928–940. doi: 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH, Katz JL. Effects of 4′-chloro-3α-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther. 2005;313:613–620. doi: 10.1124/jpet.104.080465. [DOI] [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA, Katz JL. Cocaine-like neurochemical effects of antihistaminic medications. J Neurochem. 2008;106:147–157. doi: 10.1111/j.1471-4159.2008.05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Katz JL. Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol. 2009a;57:253–289. doi: 10.1016/S1054-3589(08)57007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL. Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharmacol Exp Ther. 2009b;330:802–809. doi: 10.1124/jpet.109.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukairo OT, Bondi CD, Newman AH, Kulkarni SS, Kozikowski AP, Pan S, Surratt CK. Recognition of benztropine by the dopamine transporter (DAT) differs from that of the classical dopamine uptake inhibitors cocaine, methylphenidate, and mazindol as a function of a DAT transmembrane 1 aspartic acid residue. J Pharmacol Exp Ther. 2005;314:575–583. doi: 10.1124/jpet.105.085829. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Wilcox KM, Paul IA, Kline RH, Newman AH, Katz JL. 3′ and 4′-Chloro-substituted analogs of benztropine: intravenous self-administration and in vitro radioligand binding studies in rhesus monkeys. Psychopharmacology. 2000;147:426–435. doi: 10.1007/s002130050012. [DOI] [PubMed] [Google Scholar]