Abstract
Multiple lines of evidence indicate that the platinum-containing cancer drugs enter cells, are distributed to various subcellular compartments, and are exported from cells via transporters that evolved to manage copper homeostasis. The cytotoxicity of the platinum drugs is directly related to how much drug enters the cell, and almost all cells that have acquired resistance to the platinum drugs exhibit reduced drug accumulation. The major copper influx transporter, copper transporter 1 (CTR1), has now been shown to control the tumor cell accumulation and cytotoxic effect of cisplatin, carboplatin, and oxaliplatin. There is a good correlation between change in CTR1 expression and acquired cisplatin resistance among ovarian cancer cell lines, and genetic knockout of CTR1 renders cells resistant to cisplatin in vivo. The expression of CTR1 is regulated at the transcriptional level by copper via Sp1 and at the post-translational level by the proteosome. Copper and cisplatin both trigger the down-regulation of CTR1 via a process that involves ubiquitination and proteosomal degradation and requires the copper chaperone antioxidant protein 1 (ATOX1). The cisplatin-induced degradation of CTR1 can be blocked with the proteosome inhibitor bortezomib, and this increases the cellular uptake and the cytotoxicity of cisplatin in a synergistic manner. Copper and platinum(II) have similar sulfur binding characteristics, and the presence of stacked rings of methionines and cysteines in the CTR1 trimer suggest a mechanism by which CTR1 selectively transports copper and the platinum-containing drugs via sequential transchelation reactions similar to the manner in which copper is passed from ATOX1 to the copper efflux transporters.
Cisplatin (DDP), carboplatin, and oxaliplatin are widely used chemotherapeutic agents. All three are highly polar molecules that do not readily diffuse across lipid membranes (Hall et al., 2008). In the high chloride environment of plasma, they remain largely in their native form, but once inside the cell, in which the chloride concentration is much lower, they become aquated and form adducts with DNA and react with nucleophilic sites in a variety of targets (Kelland, 2007). Although a variety of ion pumps and transporters can influence the uptake of the platinum-containing drugs (Hall et al., 2008), the mechanism by which any of these actually move drug across the plasma membrane has remained largely undefined until recently. Ishida et al. (2002) showed that yeast lacking copper transporter 1 (yCTR1) had reduced accumulation of DDP; this was confirmed by this laboratory in yeast (Lin et al., 2002) and mammalian cells (Holzer and Howell, 2006). Independent laboratories have now documented that CTR1 is a major influx transporter for the platinum drugs in multiple cell systems and that knockout of CTR1 renders cells resistant to all of the platinum drugs in vitro and to DDP in vivo. Like copper (Dancis et al., 1994; Ooi et al., 1996; Petris et al., 2003), in many types of cells, DDP triggers the rapid degradation of CTR1 and does so at much lower concentrations and more rapidly than copper (Holzer et al., 2006b). This can be blocked by inhibiting the proteosome, resulting in increased uptake and cytotoxicity of DDP both in vitro and in vivo (Jandial et al., 2009). This brief review focuses on recent evidence relating to the mechanism by which CTR1 transports DDP.
Copper Homeostasis
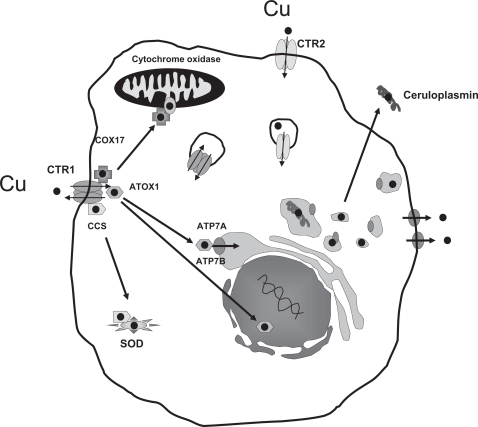
Figure 1 presents a schematic diagram of the copper homeostasis system in mammals. Copper is an essential micronutrient; it is a cofactor of many enzymes involved in processes such as biosynthesis of neuroendocrine peptides and neurotransmitters, detoxification of free radicals, and formation of connective tissues and blood vessels. However, the intracellular form of copper, Cu+, is highly toxic because it reacts with molecular oxygen or hydrogen peroxide to produce free radicals. The copper homeostasis system is a complex network of proteins that bind and deliver Cu+ to the copper-dependent proteins and protect cells from the harmful effects of excess copper. Cu+ enters cells via the copper transporter 1 (CTR1) and is handed to pathway-specific chaperones such as antioxidant protein 1 (ATOX1), copper chaperone for superoxide dismutase, and cytochrome c oxidase assembly homolog that deliver it to various organelles for transfer to copper-requiring enzymes. The central feature of the copper transporters and chaperones is the presence of unique cysteine-, methionine-, or histidine-rich domains called metal binding sequences. These metal binding sequences bind Cu+ via metal-sulfur or metal-nitrogen bonds in a protective pocket and hand it to the next protein through an intimate protein-protein transchelation reaction such that copper is virtually never free in the cell. Thus, the concentration of free copper in the cell is maintained at <10−18 M (Rae et al., 1999). The copper homeostasis system is highly conserved, and orthologs from mammalian cells can complement copper transport deficits in yeast. In addition to CTR1, other copper transporters, including CTR2 and the divalent metal transporters divalent metal transporter 1, account for some component of copper uptake in mammalian and other eukaryotic cells (Arredondo et al., 2003).
Fig. 1.
Schematic diagram of the major mammalian copper homeostasis pathways.
Structure and Copper Transport Function of CTR1
The main copper uptake transporter in human cells is CTR1, a 190-amino acid protein of 28 kDa with three transmembrane domains that forms a stable homotrimer in membranes. Transport of copper by CTR1 is energy-independent (Lee et al., 2002a) but is influenced by temperature, pH, and K+ (Lee et al., 2002b). Although the exact mode of Cu+ internalization by CTR1 is not clear, the trimer seems to form a central pore that functions as a channel (Aller et al., 2004; Eisses et al., 2005; De Feo et al., 2007,2009). Crystallographic analysis of human CTR1 showed that the permeation pathway formed by the association of three CTR1 molecules involves a series of rings of methionines potentially capable of chelating copper in a trimeric configuration (De Feo et al., 2007, 2009) There are two rings each containing three methionines stacked on top of each other in the narrowest part of the pore, and a ring of three cysteines at the bottom of the pore. The first ring is formed by the Met154 contributed by the three monomers and the second is formed by the three Met150 and the ring of cysteines by Cys189. As shown schematically in Fig. 2, the pore seems to have a truncated cone shape being ≈8 Å at the extracellular entrance and ≈22 Å at the intracellular end. Transmembrane domain (TM) 2 lines the pore and TM1 is pinched between TM2 and TM3 from the same monomer. Toward the intracellular side, contacts between subunits are mediated by interactions between TM2 and TM3 from adjacent monomers, whereas close to the extracellular side of the membrane all interactions between monomers are mediated by TM2. Thus, TM2 is the single most important contributor to the pore itself, as suggested by mutagenesis data (Puig et al., 2002; Guo et al., 2004; Eisses et al., 2005). TM3 is involved in tight helical packing; the GXXXG motif allows TM3 to approach TM1 of the same monomer.
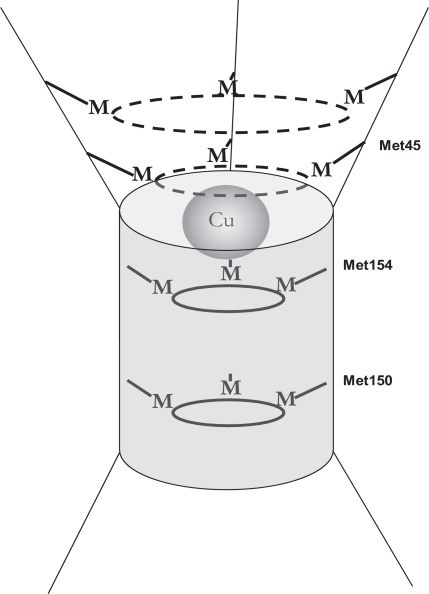
Fig. 2.
Schematic illustration of the trimeric pore of CTR1. The diagram illustrates the concept that, when assembled as a trimer, CTR1 provides a series of stacked rings of methionines, histidines, and cysteines that participate in transchelation reactions to ensure the regulated movement of copper through CTR1.
The passage of copper seems to be associated with conformational changes in CTR1 (Fig. 3). First, fluorescence resonance transfer studies showed that the C termini move during copper transport in yeast CTR1 (Sinani et al., 2007), although whether this occurs with hCTR1, which has a very much shorter cytoplasmic tail, is unclear. Second, high-extracellular copper causes clearance of CTR1 from the cell surface possibly because the occupancy of extracellular Cu+-binding sites is communicated to the intracellular side of the membrane (Guo et al., 2004; Molloy and Kaplan, 2009). Finally, tryptic digestion of the human CTR1 (hCTR1) in the absence or presence of copper results in different proteolytic cleavage patterns (Eisses and Kaplan, 2002). However, the methionine residues that form the ring around the pore entrance are not static. Rather, they form a flexible structure as shown by mutational studies (Puig et al., 2002; Eisses et al., 2005), and the short loop connecting this region to TM3 allows the status of the methionine rings to be communicated very efficiently to other parts of the monomer and trimer. Therefore, this region seems to be a good candidate to serve as a conformational switch (De Feo et al., 2009). The N-terminal domain may act as a “gate” or “concentrator” for copper ions, whereas Cys189 at the C-terminal end functions as a switch to open and close the pore (Wu et al., 2009a) and His139 and possibly other charged amino acids in the transmembrane domain serve to delay movement through the pore (Eisses and Kaplan, 2005). Cys189 also controls the multimerization of CTR1 (Eisses and Kaplan, 2002; Lee et al., 2007; Sinani et al., 2007). The relative binding affinities are such that the C-terminal end of CTR1 can transfer copper to ATOX1 and presumably also to copper chaperone for superoxide dismutase and cytochrome c oxidase assembly homolog (Xiao et al., 2001), although binding of ATOX1 to CTR1 has not actually been demonstrated.
Fig. 3.
Schematic diagram of the movement of copper through the CTR1 pore via transchelation involving Met40, Met45, Met150, and Met154. [Redrawn from De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, and Unger VM (2009) Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA 106:4237–4242. Copyright © 2009 National Academy of Sciences of the United States of America. Used with permission.]
Knockout of both alleles of CTR1 produces an embryonic lethal phenotype (Kuo et al., 2001; Lee et al., 2001). Although this may be due to the deficiency of copper, recent evidence indicates that CTR1 plays a structural role in fibroblast growth factor signal transduction that controls the induction of neurectoderm (Haremaki et al., 2007). Although CTR1s from various species have different lengths and share various degrees of homology, they contain some highly conserved sequences and have been shown to complement each other’s function (Puig et al., 2002; Petris, 2004). In fact the human variant of CTR1 was discovered by virtue of its ability to complement copper transport defects in Saccharomyces cerevisiae yCtr1 mutants (Zhou and Gitschier, 1997; Lee et al., 2000). In vertebrates, CTR1 is expressed ubiquitously in all tissues (Zhou and Gitschier, 1997). Its subcellular localization varies among different cell types. In some cell lines, hCTR1 is found predominantly at the plasma membrane, whereas in most others, it is found in perinuclear intracellular vesicles (Klomp et al., 2002). hCTR1 becomes N- and O-linked glycosylated to produce a mature protein of 35 kDa (Maryon et al., 2009). Mutational studies have shown that the O-linked glycosylation protects CTR1 against proteolysis and loss of the majority of its function (Maryon et al., 2009).
The details of how copper is transported across the membrane remain unknown. Studies in which different domains of CTR1 have been mutated have identified some parts of the molecule that are essential, but the data from various laboratories are not completely consistent (Puig et al., 2002; Guo et al., 2004; Eisses et al., 2005; Sinani et al., 2007; Liang et al., 2009). Nevertheless, they are generally in agreement with the model developed from electron crystallography and suggest that the methionine-containing motifs at the N-terminal end serve as binding sites for copper, whereas those in the transmembrane segments serve to conduct the copper inward. In addition, a number of charged residues in transmembrane segments, such as Glu68, Glu84, His139, Gly171, and Gly187, regulate copper uptake (Liang et al., 2009), possibly by contributing to helix packing interactions and oligomerization of CTR1 trimers (Aller et al., 2004). Tryptophan scanning of the third transmembrane segment of yCtr3 showed that other conserved residues, Ile196, Ser198, Cys199, and Arg207, may have a role in protein folding and structural integrity (Aller et al., 2004). Finally, sequences in the C-terminal domain, particularly 178KK179 and Cys189 are also known to regulate copper uptake to varying degrees (Eisses et al., 2005). The role of the cytosolic and extracellular loops of CTR1 in the transport of copper is largely unknown, but the conformation of the entire molecule is likely to change when these are altered; for example, a reduced uptake of copper was observed when Tyr156 in the short extracellular loop was converted to alanine (Eisses et al., 2005). Conversion of Met69 and Met81 in the first transmembrane segment to isoleucine increased copper uptake by 150% (Eisses et al., 2005), indicating that these methionine residues are somehow involved in copper coordination. The 178KK179 residues at the C-terminal end influence copper uptake (Eisses et al., 2005), and our work and that of others (Liu et al., 2007) indicate that these residues are involved in the ubiquitination of CTR1. It is most interesting that whereas N-terminal deletions decrease the value of Km for copper, C-terminal deletions increase the Km (Wu et al., 2009b).
Regulation of CTR1 Expression by Copper
The expression of CTR1 is regulated at both transcriptional and translational levels. CTR1 is down-regulated by intracellular copper; analysis of the hCTR1 promoter demonstrated that the Sp1 transcription factor is involved in the basal and copper-dependent regulation of CTR1 expression. The zinc finger domain of Sp1 was shown to function as a sensor of copper in the regulation of CTR1 (Song et al., 2008). CTR1 expression is also regulated at the post-translational level. Elevated concentrations of copper are known to trigger endocytosis of CTR1, and in some types of cells this is accompanied by degradation (Petris et al., 2003), whereas in others, it is not (Liang et al., 2009; Molloy and Kaplan, 2009). It has been hypothesized that degradation may serve to limit the accumulation of toxic levels of the metal (Guo et al., 2004; Holzer et al., 2004a; Liu et al., 2007; Molloy and Kaplan, 2009). In yeast, the degradation of CTR1 requires the E3 ubiquitin ligase Rsp5 (Liu et al., 2007), of which neural precursor cell-expressed developmentally down-regulated 4 (NEDD4-1) and neural precursor cell-expressed developmentally down-regulated 4-like (NEDD4-2) are the human homologs. It is now apparent that, in addition to cytosolic proteins, numerous transmembrane proteins are also ubiquitinated and that this plays an essential role in their endocytosis and subsequent degradation (Rotin et al., 2000). A major function of the NEDD4/Rsp5 family of ubiquitin ligases is to regulate the stability of yeast and mammalian transmembrane proteins by ubiquitination. Rsp5 is involved in endocytosis and sorting of numerous transmembrane proteins in yeast, and its candidate human homologs NEDD4-1 and NEDD4-2 seem to function similarly in mammalian cells. For example, NEDD4-2 binds the epithelial Na+ channel, ubiquitinates it, and promotes its endocytosis from the plasma membrane and subsequent degradation (Staub et al., 1996). In the case of yeast, most if not all transporters become phosphorylated upon ligand binding, and the phosphorylation then discloses sites, typically PY motifs, that then bind the WW motifs in the NEDD4 family members. NEDD4-2 binds to a PY motif located in its own HECT domain and thus protects itself from autoubiquitination until a target is found. NEDD4 family-interacting protein 1 and NEDD4 family-interacting protein 2 are NEDD4 family member adaptor proteins. These proteins have three transmembrane domains and localize to the Golgi, Golgi-derived transport vesicles, endosomes, and microvesicular bodies, the subcellular sites of biosynthetic, secretory, and endocytic trafficking. Both bind to NEDD4-1 and NEDD4-2 and are predicted to regulate their activity by directing them to specific targets. NEDD4-1 and NEDD4-2 are strong candidates for the ubiquitination of hCTR1 that occurs in response to DDP exposure.
CTR1 Controls Uptake of the Platinum-Containing Drugs
There is now quite a large body of evidence, derived from multiple cell models, that CTR1 regulates the cytotoxicity of DDP by affecting drug uptake (Lin et al., 2002; Holzer et al., 2004a,b, 2006a,b; Holzer and Howell, 2006; Larson et al., 2009; Jandial et al., 2009; Safaei et al., 2009) and that the copper exporters ATP7A (Samimi et al., 2003, 2004a,b; Samimi and Howell, 2006) and ATP7B (Katano et al., 2002, 2003, 2004; Safaei et al., 2004, 2008; Safaei, 2006) contribute to DDP resistance by enhancing efflux. The absence of CTR1 expression renders yeast and mammalian cells resistant to copper, DDP, carboplatin, and oxaliplatin (Lin et al., 2002; Holzer et al., 2004b, 2006a; Larson et al., 2009), whereas overexpression of CTR1 was found to sensitize cells to the toxic effects of these agents (Holzer et al., 2004b; Noordhuis et al., 2008). These changes in sensitivity are accompanied by proportionate changes in the uptake of all four agents; time course studies showed that CTR1 mainly regulates the initial phase of DDP uptake (Larson et al., 2009). That CTR1 regulates the cellular accumulation of DDP has now been confirmed in several other laboratories (Song et al., 2004, 2008; Pabla et al., 2009; Liang et al., 2009). In vivo studies in rats showed that DDP, carboplatin, and oxaliplatin cause atrophy in those cells in dorsal root ganglia that expressed high levels of CTR1 but not those with low-level expression (Liu et al., 2009). Finally, it was recently demonstrated that knockout of CTR1 completely eliminates responsiveness of tumors to DDP in vivo in a murine model (Larson et al., 2009).
CTR1 and Acquired Resistance to the Platinum-Containing Drugs
Because CTR1 functions as a major platinum drug transporter whose knockout produces resistance to the platinum drugs in vitro and in vivo in model systems, and because acquired DDP resistance is commonly accompanied by defects in DDP uptake, one might reasonably expect the expression of CTR1 to decrease during the acquisition of DDP resistance. However, a survey of tumors and cell lines with acquired DDP resistance, using immunohistochemical and quantitative reverse transcriptase-polymerase chain reaction, has only identified a few cases in which this occurs (Matsumoto et al., 2007; Zisowsky et al., 2007; Noordhuis, 2008). New insight regarding the role of trafficking factors and glycosylation of CTR1 provides a possible explanation for this discrepancy. It turns out that failure to glycosylate CTR1 at Thr27 renders the protein susceptible to proteases that excise the N-terminal domain and inactivate CTR1’s transport function but leave the rest of the protein in the membrane (Maryon et al., 2009). The finding that platinum drug-resistant cells are defective in glycosylation (Liang et al., 2003; Nakagawa et al., 2008; Zhang et al., 2009) and the observation that treatment of tumor-bearing animals with DDP causes a reduction in sialic acid content of tumors (Nicol and Prasad, 2002) raises the intriguing possibility that the CTR1 in DDP-resistant cells, although expressed at a normal level, is not functional with respect to DDP transport. Further evidence is provided by the observation that exposure to DDP increases sialidase activity (Sodhi and Prasad, 1985) and releases sialic acid-containing proteins from cells (Sarna et al., 1988). It is interesting that when DDP-resistant cells were transfected with a wild-type CTR1, the molecular weight of the exogenous protein was smaller than projected (Song et al., 2004), and DDP resistance was not reversed, consistent with failure of full glycosylation of CTR1 (Beretta et al., 2004). In addition, resistant cells are known to have altered trafficking patterns and mislocalize many important membrane proteins (Liang et al., 2003); the mechanism that localizes CTR1 to the cell surface is likely to have been altered in DDP-resistant cells. Thus, a re-examination of the function of CTR1 in DDP-sensitive and -resistant cells lines, rather than just its expression, is now required.
Modulation of CTR1 by Cisplatin and Proteosome Inhibitors
Exposure of human ovarian cancer cells to DDP causes rapid degradation of CTR1, resulting in the paradox that DDP down-regulates its own influx transporter. This effect has been observed at even low concentrations of DDP (2 μM) and documented by Western blotting, confocal microscopy, and fluorescence-activated cell sorting analysis. DDP-induced degradation of CTR1 has now been documented in most but not all types of cells (Holzer et al., 2004a; Holzer and Howell, 2006; Larson et al., 2009; Safaei et al., 2009). That the down-regulation of CTR1 by DDP is functionally significant is shown by the fact that it reduces the subsequent uptake of 64Cu in the absence of DDP (Larson et al., 2009). The rapid degradation produced by DDP provides an explanation for why the greatest effect of CTR1 on DDP transport is observed during the early phase of DDP uptake (Holzer et al., 2004b; Liu et al., 2007). DDP-induced down-regulation of CTR1 involves endocytosis, and a careful analysis of various endocytotic pathways suggested that macropinocytosis is the dominant route by which CTR1 is removed from the plasma membrane (Holzer et al., 2004a). Immunoprecipitation and confocal microscopy using mouse cells that have been treated with bortezomib and DDP showed that exposure to DDP caused polyubiquitination of CTR1. Western blots identified several CTR1 bands with molecular masses as large as 130 kDa in lysates of DDP-treated cells (Safaei et al., 2009). In the case of yCTR1, there is evidence that copper is transferred directly from its C-terminal domain to the CXXC motif in ATOX1 for subsequent delivery to copper-transporting ATPase α (ATP7A) and copper-transporting ATPase β (ATP7B) in the secretory compartment (Xiao et al., 2004). However, there are substantial differences in the structure of the C-terminal domain between yeast and mammalian CTR1, and exactly how this occurs is not well defined for either protein. In the case of mammalian CTR1, the interaction is likely to occur at Cys189, which has been reported to be involved in the stabilization of the CTR1 multimer (Eisses and Kaplan, 2002; Lee et al., 2007). The DDP-induced degradation of CTR1 requires ATOX1 because CTR1 fails to undergo either down-regulation or ubiquitination in ATOX1−/− cells (Safaei et al., 2009). ATOX1 is thus important both for the trafficking of CTR1 to the cell surface and its endocytosis and degradation upon exposure to DDP. It is noteworthy that ATOX1 has been shown recently to bind DDP at Cys12 and Cys15, the same sites that chelate copper (Boal and Rosenzweig, 2009).
In human ovarian carcinomas and mouse fibroblasts, the proteasome inhibitors bortezomib and lactacystin were found to prevent the DDP-induced down-regulation of CTR1 (Holzer et al., 2004a; Holzer and Howell, 2006; Jandial et al., 2009; Safaei et al., 2009) independent of transcriptional and translational events (Holzer and Howell, 2006). Bortezomib reduced degradation and increased DDP uptake by 1.6- to 2.4-fold in a concentration-dependent manner. Median effect analysis demonstrated a combination index of 0.37 at 50% cell kill, indicating a high level of synergy. The effect of bortezomib was muted in cells lacking both alleles of CTR1, demonstrating that bortezomib was working primarily through its effect on blocking CTR1 degradation. Intraperitoneal administration of bortezomib produced a peritoneal/plasma area under the curve ratio of 252 in a murine model. Intraperitoneal administration of bortezomib before intraperitoneal DDP increases platinum accumulation in peritoneal tumors by 33% (p = 0.006). These data have provided the basis for the development of a phase I trial of the combination of intraperitoneal bortezomib and carboplatin in patients with recurrent ovarian cancer.
Mechanism of Copper and DDP Transport by CTR1
The molecular details of how CTR1 transports copper and DDP remain to be defined, although the importance of CTR1 in DDP transport is well documented (Kelland, 2007). Electron microscopic analysis of CTR1 crystals suggests that the pore formed by the CTR1 trimer is only 8 Å in diameter (De Feo et al., 2009). This is large enough to accommodate Cu+ but initially seems too small to allow passage of the platinum-containing drugs. However, transmembrane proteins of this type can be quite flexible, and the wall of the pore is potentially quite expandable. The observation that changing His139 to arginine, a change that preserved the charge but altered the size, markedly increased copper transport supports this contention (Eisses and Kaplan, 2005). The electronic microscopic analysis supports the concept that the CTR1 trimer folds in such a way as to create a series of stacked rings, each containing three methionines or cysteines, that can chelate Cu+. Whether the methionine motifs in the extracellular N-terminal domain form additional stacked rings is unknown, but it has been reported that the N-terminal domains bind to each other and that the interaction requires both of the methionine rich regions (Met7–Met12 and Met40–Met45) (Klomp et al., 2003). Both Cu+ and the platinum-containing drugs are soft Lewis acids that readily form weak bonds with methionine. One potential mechanism by which CTR1 transports Cu+ is through a series of transchelation reactions that pass the Cu+ from one ring of methionines to the next and eventually to the ring of cysteines at the bottom of the pore. Indeed, it is through such a translation reaction that Cu+ is believed to be transferred from the chaperone ATOX1 to the copper efflux transporters ATP7A and ATP7B (Petris, 2004). Cu+ has a tetrahedral coordination configuration, whereas the platinum drugs have a square planar structure. However, rather than their chelation configuration, it may be the ability of each to bind to one or more sulfur atoms of the methionine rings in CTR1 and to undergo transchelation reactions, which makes them transportable by CTR1 (Fig. 3). In the case of tetrahedral Cu+, the transchelation reaction may involve binding to all three methionines in a given ring plus one methionine of the next ring. In the case of DDP, presumably transport would involve initial chelation to two of the methionines in a given ring followed by release of one platinum-sulfur bond and pivoting of the molecule so that it can bind to a methionine sulfur in the next lower ring. The molecular distances between the rings formed by the Met154 and Met150 seem to be short enough to permit this type of movement. One of the features of CTR1 is that it transports Cu+ and the platinum drugs but not Cu2+. Although Cu+ and Pt2+ are both considered soft acids in the Hard-Soft Acid-Base formalism of Pearson (Martell and Hancock, 1996) and should favor bonding with methionine, Cu2+ is expected to form a weaker bond because of its harder acidic character. We speculate that it is the unique chemistry of the metal-sulfur bond that determines the selectivity of CTR1 for Cu+ and the platinum drugs rather than the specific chelation configuration. This model would predict that Cu2+ and other harder acids that form weaker bonds with methionine would fail to be transported because they would not be able to effectively enter the chelation environment. On the other hand, softer acids that form stronger methionine-sulfur bonds might effectively plug the CTR1 pore. Indeed, Ag(I) forms a stronger bond with sulfur than Cu+ and has been reported to be an inhibitor of CTR1-mediated copper transport (Lee et al., 2002a). Possessing a reasonably labile bonding interaction with sulfur atoms on methionine and cysteine, Pt2+, like Cu+, may bind strongly enough to enter the pore and bind with the first ring (Met154) but not so strongly that it cannot be effectively passed along to the second ring (Met150) and subsequently across the membrane. The higher affinity of the metal ions for cysteine versus methionine (Zimmermann et al., 2005) may control the rates of transport, and the greater affinity of Pt2+ for thiols (Reedijk, 1999) may be responsible for its slower transport rate relative to Cu+ (Lin et al., 2002).
How the C-terminal Cys189 might be involved in a series of sequential transchelation reactions that move copper and DDP through the pore is a matter of speculation. In the model proposed by De Feo et al. (2009), these cysteines are quite far away from the methionine rings above them. Nevertheless, there is biochemical evidence that the cysteines bind Cu+ and are important for copper transport, making it likely that the cysteines can accept Cu+ from the ring of methionines located in the top part of the pore. In addition, it has been proposed that the cysteines function as a gate that controls movement through the other parts of the pore (Xiao and Wedd, 2002; Xiao et al., 2004; De Feo et al., 2009).
Despite the fact that Cu+ and DDP seem to be substrates for the CTR1 transporter, there is strong evidence that the molecular details of the mechanism by which CTR1 imports DDP and copper are somewhat different. For example, fluorescence resonance energy transfer analysis of the interaction between yCTR1 monomers showed that although copper brought them closer together in a manner that correlated with cellular copper transport, DDP did not. This may be a reflection of the ability of Cu+ to bind three or four sulfur atoms, whereas DDP and its variants are expected to only form bis-adducts (two sulfur atoms). Chelation to three or four sulfurs would be expected to produce a tighter, more compact complex. A variant of yCTR1 defective in copper transport nevertheless still enhances cellular DDP accumulation. Some N-terminal methionine-rich motifs that are dispensable for copper transport are required for DDP uptake (Sinani et al., 2007). Very few data are available from mutational studies that address potential differences between DDP and copper transport. A recent study by Liang et al. (2009) showed that the MXXXM motifs in the N-terminal domain are important for both copper and DDP uptake in small-cell lung cancer cells, but the kinetics of transport are different for copper and DDP. Deletion of the Met43 group results in a decrease in Vmax for transport of both Cu+ and DDP, but the value of Km is only reduced for copper and not for DDP. These studies also demonstrated that the Gly167 and Gly179 are important to both the transport of copper and DDP (Liang et al., 2009). Electrospray ionization mass spectrometry has demonstrated that DDP (Sze et al., 2009; Wu et al., 2009b), carboplatin, and oxaliplatin (Wu et al., 2009b) can all bind with a stoichiometry of 1:1 and 2:1 to the methionines and histidine-rich motif in the N-terminal domain of CTR1 in a process that involves the removal of all of the ligands of DDP, although like the multimerization of CTR1, this occurs very slowly relative to the transport of DDP and requires high concentrations of the drugs. In addition, DDP but not copper increases the fraction of CTR1 that is found in the trimeric rather than the monomeric state (Guo et al., 2004; Liang et al., 2009). However, both the binding of DDP to the N-terminal domain and the multimerization of CTR1 occur only after prolonged periods of incubation at concentrations of DDP that are far higher than those found in patient plasma, and the relevance of these reactions to the regulation of DDP transport is not apparent. Although these studies clearly show that CTR1, and particularly the methionines in the transmembrane domains, are important to DDP transport, they do not explain the difference in the mode of DDP and copper transport by CTR1.
Concepts and Implications
One of the remarkable features of the copper homeostasis system is that copper ions are essentially never free in the cell. They are always bound to a transporter, chaperone, or enzyme. The observation that both copper and DDP are substrates for CTR1 and the speculation that CTR1 transports both via a series of transchelation reactions suggest that perhaps the platinum drugs are distributed to various cellular compartments in part via chaperones and transchelation reactions in a manner similar to copper. It has already been established that complexes of DDP with methionine can donate the DDP to the Asn7 position of guanine in DNA (van Boom et al., 1999). This concept presupposes that, like Cu+, the platinum-containing drugs cannot diffuse across cellular membranes very well because of their polarity. Knockout of CTR1 markedly reduces DDP uptake in both yeast and mammalian cells, and cisplatin and oxaliplatin can be encapsulated in liposomes from which they do not leak. These facts further support this idea (Kelland, 2007). However, although they do not diffuse readily across artificial membranes, the ability of these drugs to diffuse across actual cellular membranes with their much greater complexity remains unknown.
If the transport and trafficking of the platinum-containing drugs is handled by the cellular highways that evolved to manage copper homeostasis, one would predict that the proteins that deliver these drugs to the nucleus, mitochondria, and microsomal compartments, all of which are known to accumulate platinum after drug exposure. ATOX1 translocates to the nucleus in response to copper exposure (Itoh et al., 2008) and has also recently been reported to bind DDP (Boal and Rosenzweig, 2009), raising the question of whether it could be involved in the delivery of DDP to DNA and/or to the copper efflux transporters ATP7A and ATP7B, both of which mediate DDP resistance when overexpressed. Although the level of expression of CTR1 itself has been established as a determinant of the cytotoxicity of the platinum drugs, the concept that intracellular chaperones are responsible for the trafficking of these drugs identifies altered expression of these proteins or defective drug binding or movement as potential determinants of sensitivity to these important chemotherapeutic agents.
ABBREVIATIONS:
- DDP
cisplatin
- CTR1
copper transporter 1
- ATOX1
antioxidant protein 1
- TM
transmembrane domain
- NEDD4-1
neural precursor cell-expressed developmentally down-regulated 4
- NEDD4-2
neural precursor cell-expressed developmentally down-regulated 4-like
Footnotes
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
References
- Aller SG, Eng ET, De Feo CJ, Unger VM. Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem. 2004;279:53435–53441. doi: 10.1074/jbc.M409421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo M, Muñoz P, Mura CV, Nùñez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol. 2003;284:C1525–C1530. doi: 10.1152/ajpcell.00480.2002. [DOI] [PubMed] [Google Scholar]
- Beretta GL, Gatti L, Tinelli S, Corna E, Colangelo D, Zunino F, Perego P. Cellular pharmacology of cisplatin in relation to the expression of human copper transporter CTR1 in different pairs of cisplatin-sensitive and -resistant cells. Biochem Pharmacol. 2004;68:283–291. doi: 10.1016/j.bcp.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Boal AK, Rosenzweig AC. Crystal structures of cisplatin bound to a human copper chaperone. J Am Chem Soc. 2009;131:14196–14197. doi: 10.1021/ja906363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A, Haile D, Yuan DS, Klausner RD. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem. 1994;269:25660–25667. [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, Unger VM. A structural perspective on copper uptake in eukaryotes. Biometals. 2007;20:705–716. doi: 10.1007/s10534-006-9054-7. [DOI] [PubMed] [Google Scholar]
- Eisses JF, Chi Y, Kaplan JH. Stable plasma membrane levels of hCTR1 mediate cellular copper uptake. J Biol Chem. 2005;280:9635–9639. doi: 10.1074/jbc.M500116200. [DOI] [PubMed] [Google Scholar]
- Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem. 2002;277:29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- Eisses JF, Kaplan JH. The mechanism of copper uptake mediated by human CTR1: a mutational analysis. J Biol Chem. 2005;280:37159–37168. doi: 10.1074/jbc.M508822200. [DOI] [PubMed] [Google Scholar]
- Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem. 2004;279:17428–17433. doi: 10.1074/jbc.M401493200. [DOI] [PubMed] [Google Scholar]
- Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- Haremaki T, Fraser ST, Kuo YM, Baron MH, Weinstein DC. Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2007;104:12029–12034. doi: 10.1073/pnas.0701413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 2006;66:10944–10952. doi: 10.1158/0008-5472.CAN-06-1710. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Katano K, Klomp LW, Howell SB. Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res. 2004a;10:6744–6749. doi: 10.1158/1078-0432.CCR-04-0748. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006a;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Samimi G, Katano K, Naerdemann W, Lin X, Safaei R, Howell SB. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol. 2004b;66:817–823. doi: 10.1124/mol.104.001198. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Varki NM, Le QT, Gibson MA, Naredi P, Howell SB. Expression of the human copper influx transporter 1 in normal and malignant human tissues. J Histochem Cytochem. 2006b;54:1041–1049. doi: 10.1369/jhc.6A6970.2006. [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem. 2008;283:9157–9167. doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res. 2009;15:553–560. doi: 10.1158/1078-0432.CCR-08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, Kuo YM, Rochdi M, Howell SB. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- Katano K, Safaei R, Samimi G, Holzer A, Rochdi M, Howell SB. The copper export pump ATP7B modulates the cellular pharmacology of carboplatin in ovarian carcinoma cells. Mol Pharmacol. 2003;64:466–473. doi: 10.1124/mol.64.2.466. [DOI] [PubMed] [Google Scholar]
- Katano K, Safaei R, Samimi G, Holzer A, Tomioka M, Goodman M, Howell SB. Confocal microscopic analysis of the interaction between cisplatin and the copper transporter ATP7B in human ovarian carcinoma cells. Clin Cancer Res. 2004;10:4578–4588. doi: 10.1158/1078-0432.CCR-03-0689. [DOI] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Klomp AE, Juijn JA, van der Gun LT, van den Berg IE, Berger R, Klomp LW. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem J. 2003;370:881–889. doi: 10.1042/BJ20021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1) Biochem J. 2002;364:497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA. 2001;98:6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CA, Blair BG, Safaei R, Howell SB. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol. 2009;75:324–330. doi: 10.1124/mol.108.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Peña MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002a;277:4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem. 2002b;277:40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254:87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Howell SB, Opella SJ. NMR and mutagenesis of human copper transporter 1 (hCtr1) show that Cys-189 is required for correct folding and dimerization. Biochim Biophys Acta. 2007;1768:3127–3134. doi: 10.1016/j.bbamem.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XJ, Shen DW, Garfield S, Gottesman MM. Mislocalization of membrane proteins associated with multidrug resistance in cisplatin-resistant cancer cell lines. Cancer Res. 2003;63:5909–5916. [PubMed] [Google Scholar]
- Liang ZD, Stockton D, Savaraj N, Tien Kuo M. Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol. 2009;76:843–853. doi: 10.1124/mol.109.056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Okuda T, Holzer A, Howell SB. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol. 2002;62:1154–1159. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- Liu J, Sitaram A, Burd CG. Regulation of copper-dependent endocytosis and vacuolar degradation of the yeast copper transporter, Ctr1p, by the Rsp5 ubiquitin ligase. Traffic. 2007;8:1375–1384. doi: 10.1111/j.1600-0854.2007.00616.x. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Jamieson SM, Subramaniam J, Ip V, Jong NN, Mercer JF, McKeage MJ. Neuronal expression of copper transporter 1 in rat dorsal root ganglia: association with platinum neurotoxicity. Cancer Chemother Pharmacol. 2009;64:847–856. doi: 10.1007/s00280-009-1017-6. [DOI] [PubMed] [Google Scholar]
- Martell A, Hancock R. Metal Complexes in Aqueous Solutions. Plenum Press; New York: 1996. [Google Scholar]
- Maryon EB, Zhang J, Jellison JW, Kaplan JH. Human copper transporter 1 lacking O-linked glycosylation is proteolytically cleaved in a Rab9-positive endosomal compartment. J Biol Chem. 2009;284:28104–28114. doi: 10.1074/jbc.M109.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Tanaka T, Kurokawa H, Matsuno K, Hayashida Y, Takahashi T. Effect of copper and role of the copper transporters ATP7A and CTR1 in intracellular accumulation of cisplatin. Anticancer Res. 2007;27:2209–2216. [PubMed] [Google Scholar]
- Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem. 2009;284:29704–29713. doi: 10.1074/jbc.M109.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Ohira M, Hayashi S, Abe S, Saito S, Nagahori N, Monde K, Shinohara Y, Fujitani N, Kondo H, et al. Alterations in the glycoform of cisplatin-resistant human carcinoma cells are caused by defects in the endoplasmic reticulum-associated degradation system. Cancer Lett. 2008;270:295–301. doi: 10.1016/j.canlet.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Nicol BM, Prasad SB. Sialic acid changes in Dalton’s lymphoma-bearing mice after cyclophosphamide and cisplatin treatment. Braz J Med Biol Res. 2002;35:549–553. doi: 10.1590/s0100-879x2002000500006. [DOI] [PubMed] [Google Scholar]
- Noordhuis P, Laan AC, van de Born K, Losekoot N, Kathmann I, Peters GJ. Oxaliplatin activity in selected and unselected human ovarian and colorectal cancer cell lines. Biochem Pharmacol. 2008;76:53–61. doi: 10.1016/j.bcp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15:3515–3523. [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petris MJ. The SLC31 (Ctr) copper transporter family. Pflugers Arch. 2004;447:752–755. doi: 10.1007/s00424-003-1092-1. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277:26021–26030. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- Reedijk J. Why does cisplatin reach guanine-n7 with competing s-donor ligands available in the cell? Chem Rev. 1999;99:2499–2510. doi: 10.1021/cr980422f. [DOI] [PubMed] [Google Scholar]
- Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J Membr Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- Safaei R. Role of copper transporters in the uptake and efflux of platinum containing drugs. Cancer Lett. 2006;234:34–39. doi: 10.1016/j.canlet.2005.07.046. [DOI] [PubMed] [Google Scholar]
- Safaei R, Katano K, Samimi G, Naerdemann W, Stevenson JL, Rochdi M, Howell SB. Cross-resistance to cisplatin in cells with acquired resistance to copper. Cancer Chemother Pharmacol. 2004;53:239–246. doi: 10.1007/s00280-003-0736-3. [DOI] [PubMed] [Google Scholar]
- Safaei R, Maktabi MH, Blair BG, Larson CA, Howell SB. Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J Inorg Biochem. 2009;103:333–341. doi: 10.1016/j.jinorgbio.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei R, Otani S, Larson BJ, Rasmussen ML, Howell SB. Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol. 2008;73:461–468. doi: 10.1124/mol.107.040980. [DOI] [PubMed] [Google Scholar]
- Samimi G, Howell SB. Modulation of the cellular pharmacology of JM118, the major metabolite of satraplatin, by copper influx and efflux transporters. Cancer Chemother Pharmacol. 2006;57:781–788. doi: 10.1007/s00280-005-0121-5. [DOI] [PubMed] [Google Scholar]
- Samimi G, Katano K, Holzer AK, Safaei R, Howell SB. Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol. 2004a;66:25–32. doi: 10.1124/mol.66.1.25. [DOI] [PubMed] [Google Scholar]
- Samimi G, Safaei R, Katano K, Holzer AK, Rochdi M, Tomioka M, Goodman M, Howell SB. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res. 2004b;10:4661–4669. doi: 10.1158/1078-0432.CCR-04-0137. [DOI] [PubMed] [Google Scholar]
- Samimi G, Varki NM, Wilczynski S, Safaei R, Alberts DS, Howell SB. Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin Cancer Res. 2003;9:5853–5859. [PubMed] [Google Scholar]
- Sarna S, Bhola RK, Sodhi A. Release of protein bound sialic acid from fibrosarcoma cells after cis-dichlorodiammine platinum (II) treatment: the possible role in tumor regression. Pol J Pharmacol Pharm. 1988;40:295–302. [PubMed] [Google Scholar]
- Sinani D, Adle DJ, Kim H, Lee J. Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem. 2007;282:26775–26785. doi: 10.1074/jbc.M703973200. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Prasad SB. Differential binding of conA and WGA on the cell surface, the role of sialic acid in their expression and the increased activity of sialidase after cis-platin treatment. Experientia. 1985;41:93–95. doi: 10.1007/BF02005893. [DOI] [PubMed] [Google Scholar]
- Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, Kuo MT. Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol. 2008;74:705–713. doi: 10.1124/mol.108.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3:1543–1549. [PubMed] [Google Scholar]
- Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Sze CM, Khairallah GN, Xiao Z, Donnelly PS, O’Hair RA, Wedd AG. Interaction of cisplatin and analogues with a Met-rich protein site. J Biol Inorg Chem. 2009;14:163–165. doi: 10.1007/s00775-008-0452-x. [DOI] [PubMed] [Google Scholar]
- van Boom S, Chen BW, Teuben JM, Reedijk J. Platinum-thioether bonds can be reverted by guanine-N7 bonds in Pt(dien)2+ model adducts. Am Chem Soc. 1999;38:1450–1455. [Google Scholar]
- Wu X, Sinani D, Kim H, Lee J. Copper transport activity of yeast Ctr1 is down-regulated via its C terminus in response to excess copper. J Biol Chem. 2009a;284:4112–4122. doi: 10.1074/jbc.M807909200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liu Q, Liang X, Yang X, Wang N, Wang X, Sun H, Lu Y, Guo Z. Reactivity of platinum-based antitumor drugs towards a Met- and His-rich 20mer peptide corresponding to the N-terminal domain of human copper transporter 1. J Biol Inorg Chem. 2009b;14:1313–1323. doi: 10.1007/s00775-009-0576-7. [DOI] [PubMed] [Google Scholar]
- Xiao D, Vera MD, Liang B, Joullié MM. Total synthesis of a conformationally constrained didemnin B analog. J Org Chem. 2001;66:2734–2742. doi: 10.1021/jo001640n. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Loughlin F, George GN, Howlett GJ, Wedd AG. C-terminal domain of the membrane copper transporter Ctr1 from Saccharomyces cerevisiae binds four Cu(I) ions as a cuprous-thiolate polynuclear cluster: sub-femtomolar Cu(I) affinity of three proteins involved in copper trafficking. J Am Chem Soc. 2004;126:3081–3090. doi: 10.1021/ja0390350. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wedd AG. A C-terminal domain of the membrane copper pump Ctr1 exchanges copper(I) with the copper chaperone Atx1. Chem Commun Camb. 2002;6:588–589. doi: 10.1039/b111180a. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Li ZQ, Yang YP, Li XW, Ji JF. Tunicamycin suppresses cisplatin-induced HepG2 cell apoptosis via enhancing p53 protein nuclear export. Mol Cell Biochem. 2009;327:171–182. doi: 10.1007/s11010-009-0055-z. [DOI] [PubMed] [Google Scholar]
- Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann T, Zeizinger M, Burda JV. Cisplatin interaction with cysteine and methionine, a theoretical DFT study. J Inorg Biochem. 2005;99:2184–2196. doi: 10.1016/j.jinorgbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Zisowsky J, Koegel S, Leyers S, Devarakonda K, Kassack MU, Osmak M, Jaehde U. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem Pharmacol. 2007;73:298–307. doi: 10.1016/j.bcp.2006.10.003. [DOI] [PubMed] [Google Scholar]