Abstract
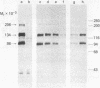
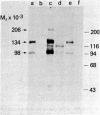
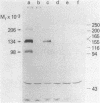
Insulin receptors of human lymphocytes (IM-9 line) were biosynthetically labeled with [3H]glucosamine, [3H]galactose, [3H]fucose, or [3H]mannose. After solubilization in Triton X-100, cell extracts were immunoprecipitated with serum from a patient containing autoantibodies to the insulin receptor. Na-DodSO4/polyacrylamide gel electrophoresis of the immunoprecipitates under reducing conditions showed the presence of major labeled subunits of apparent Mr 134,000 and 98,000 and a minor component of Mr 206,000. The ratio of activity in the 134,000 versus 98,000 Mr bands varied from 2:1 for mannose to 1.2:1 for galactose. In addition, the receptor subunits could be demonstrated when the cell surface of intact lymphocytes was labeled with NaB3H4 by using either the galactose oxidase (acts on nonreducing terminal galactose and N-acetylgalactosamine) technique or the periodate (oxidizes sialic acid) technique. With the periodate treatment, NaB3H4 labeled preferentially the Mr 98,000 band. With the galactose oxidase procedure, on the other hand, NaB3H4 labeled only the Mr 134,000 band; prior treatment with neuraminidase increased the labeling of this band and also revealed the Mr 98,000 subunit. These data demonstrate that the major subunits of the insulin receptor are complex glycoproteins that have differences in the nonreducing ends of the carbohydrate chains. In the Mr 134,000 subunit, there appear to be more exposed galactosyl or N-acetylgalactosaminyl (or both) residues, whereas the Mr 98,000 subunit appears to have a higher degree of sialylation. These labeling techniques provide new tools to examine the role of the carbohydrate moiety in insulin receptor function and turnover.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P., Tell G. P. Insulin-like activity of concanavalin A and wheat germ agglutinin--direct interactions with insulin receptors. Proc Natl Acad Sci U S A. 1973 Feb;70(2):485–489. doi: 10.1073/pnas.70.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. L., Buell D. N., Sox H. C. Proliferation and differentiation of lymphoid cells: studies with human lymphoid cell lines and immunoglobulin synthesis. Ann N Y Acad Sci. 1971 Dec 31;190:221–234. doi: 10.1111/j.1749-6632.1971.tb13537.x. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Allison J. P., Hixson D. C., Walborg E. F., Jr Resolution and partial characterization of the major plasma membrane sialoglycoproteins of Novikoff tumor cells. J Biol Chem. 1979 Sep 25;254(18):9247–9253. [PubMed] [Google Scholar]
- Harrison L. C., Flier J. S., Roth J., Karlsson F. A., Kahn C. R. Immunoprecipitation of the insulin receptor: a sensitive assay for receptor antibodies and a specific technique for receptor purification. J Clin Endocrinol Metab. 1979 Jan;48(1):59–65. doi: 10.1210/jcem-48-1-59. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Hedo J. A., Harrison L. C., Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981 Jun 9;20(12):3385–3393. doi: 10.1021/bi00515a013. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kasuga M., van Obberghen E., Yamada K. M., Harrison L. C. Autoantibodies against the insulin receptor recognize the insulin binding subunits of an oligomeric receptor. Diabetes. 1981 Apr;30(4):354–357. doi: 10.2337/diab.30.4.354. [DOI] [PubMed] [Google Scholar]
- Kosmakos F. C., Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980 Oct 25;255(20):9860–9869. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massaqué J., Czech M. P. Multiple redox forms of the insulin receptor in native liver membranes. Diabetes. 1980 Nov;29(11):945–947. doi: 10.2337/diab.29.11.945. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Rosen O. M., Chia G. H., Fung C., Rubin C. S. Tunicamycin-mediated depletion of insulin receptors in 3T3-L1 adipocytes. J Cell Physiol. 1979 Apr;99(1):37–42. doi: 10.1002/jcp.1040990106. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Van Eijk R. V., Mühlradt P. F. Carbohydrate incorporation in plasma membranes of mouse thymocytes stimulated by concanavalin A. Eur J Biochem. 1977 Aug 15;78(1):41–54. doi: 10.1111/j.1432-1033.1977.tb11712.x. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Ksauga M., Le Cam A., Hedo J. A., Itin A., Harrison L. C. Biosynthetic labeling of insulin receptor: studies of subunits in cultured human IM-9 lymphocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1052–1056. doi: 10.1073/pnas.78.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor of rat adiopocyte plasma membrane. J Biol Chem. 1978 Mar 25;253(6):1743–1745. [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry. 1980 Jan 8;19(1):70–76. doi: 10.1021/bi00542a011. [DOI] [PubMed] [Google Scholar]