Abstract
This review summarizes research on the chronobiology and neurobiology of winter seasonal affective disorder (SAD), a recurrent subtype of depression characterized by a predictable onset in the fall/winter months and spontaneous remission in the spring/summer period. Chronobiological mechanisms related to circadian rhythms, melatonin, and photoperiodism play a significant role in many cases of SAD, and treatment of SAD can be optimized by considering individual differences in key chronobiological markers. Converging evidence also points to a role for the major monoamine neurotransmitters serotonin, norepinephrine, and dopamine in one or more aspects of SAD. Ultimately, as with other psychiatric illnesses, SAD is best considered as a complex disorder resulting from the interaction of several vulnerability factors acting at different levels, the various genetic mechanisms that underlie them, and the physical environment. Models of SAD that emphasize its potential role in human evolution will also be discussed.
Keywords: seasonal affective disorder, pathophysiology, circadian rhythm, melatonin, photoperiodism, monoamine neurotransmitter, genetics
Abstract
Esta revisión resume la cronobiología y la neurobiología del trastorno afectivo estacional invernal (TAE), un subtipo de depresión recurrente caracterizado por una aparición predecible en los meses de otoño e invierno y una remisión espontánea en el período de primavera y verano. Los mecanismos cronobiológicos relacionados con los ritmos circadianos, la melatonina y el fotoperíodo juegan un papel significativo en muchos casos de TAE, y el tratamiento de este cuadro se puede optimizar al tener en consideración las diferencias individuales en los marcadores cronobiológicos clave. También existen evidencias que apuntan al papel de los principales neurotransmisores monoaminérgicos serotonina, noradrenalina y dopamina en uno o más aspectos del TAE. Últimamente el TAE, como otras patologías psiquiátricas, se considera más bien un trastorno complejo que se debe a la interacción de diversos factores de vulnerabilidad (distintos mecanismos genéticos y el ambiente físico) que actúan a diferentes niveles. También se discuten modelos de TAE que enfatizan su potencial papel en la evolución humana.
Abstract
Cet article résume les travaux sur la chronobiologie et la neurobiologie des troubles affectifs saisonniers (TAS) de l'hiver, un sous-type de dépression récurrente caractérisé par un début prévisible en automne/hiver et une rémission spontanée au printemps/été. Des mécanismes chronobiologiques liés aux rythmes circadiens, la mélatonine et le photo-périodisme jouent un rôle significatif dans de nombreux cas de TAS, le traitement du TAS pouvant être optimisé en tenant compte des différences individuelles au niveau des marqueurs chronobiologiques. Des données convergentes soulignent le rôle des principaux neurotransmetteurs monoaminergiques comme la sérotonine, la noradrénaline et la dopamine au niveau d'un ou de plusieurs aspects du TAS. Comme pour les autres maladies psychiatriques, le TAS est à envisager comme un trouble complexe issu de l'interaction de plusieurs facteurs de vulnérabilité agissant à différents niveaux, de mécanismes génétiques variés qui les sous-tendent, et de l'environnement physique. Nous examinerons également des modèles du TAS qui soulignent le rôle potentiel de ce trouble dans l'évolution de l'espèce humaine.
Winter seasonal affective disorder (SAD) Is a mood disorder characterized by the predictable onset of depression in the fall/winter months, with spontaneous remissions in the spring/summer period.1 The typical patient with SAD is a premenopausal woman who experiences carbohydrate craving, hypersomnia, and prominent fatigue during winter depressive episodes.1 Many adults experience similar but milder vegetative symptoms in the fall/winter months,2,3 often referred to as “subsyndromal SAD.” This suggests that seasonality may be a dimensional process rather than a discrete syndrome. Based on the energy-conserving nature of the core symptoms of SAD, various evolutionary theories of SAD and seasonality have been proposed.4-8 The possibility that obesity in the context of SAD might reflect a ”seasonal thrifty phenotype“ has also been suggested.9
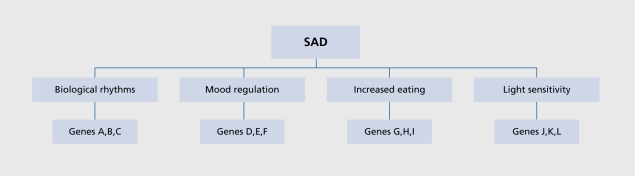
To date, research on the biology of SAD has had two major foci. One major body of work has attempted to delineate one or more chronobiological factors contributing to SAD and seasonality, with an emphasis on circadian rhythms, melatonin, and photoperiodic mechanisms. The second major body of work has used a variety of approaches to examine other brain processes that might play a role in SAD, with a primary focus on major brain neurotransmitters such as serotonin, norepinephrine, and dopamine. Ultimately, as with other psychiatric illnesses, it might be best to consider SAD as a complex disorder that results from the interaction of several vulnerability factors acting at different levels, and the various genetic mechanisms that underlie them (Figure 1). The following paragraphs will summarize work to date on the chronobiology /neurobiology of SAD, and are followed by a general discussion and directions for future work.
Figure 1. Schematic model of the biology of seasonal affective disorder (SAD). SAD is depicted as a complex phenotype shaped by multiple vulnerability factors acting at the level of biological rhythms, mood and appetite regulation, light sensitivity etc. Each of these intermediate phenotypes is in turn shaped by multiple genes. As with other complex phenotypes, it is unlikely that a single vulnerability factor or gene can account for SAD - rather it is the interaction of multiple factors interacting with the environment that shapes the ultimate phenotype.
Chronobiological mechanisms
Photoperiodism and day length
As the most distinguishing feature of SAD is its inherent rhythmicity and sensitivity to environmental light conditions, chronobiological mechanisms have been a major focus for research in this area. Based on the marked similarity between the core symptoms of SAD and energyconserving strategies implemented by various species at northern latitudes, the “latitude” or “photoperiodic” hypothesis of SAD was one of the first to be examined. According to this hypothesis, if one could demonstrate a clear association between the prevalence of SAD and increasing latitude, this would strongly support the notion that biological adaptations tied to the short days of winter are the primary factor that distinguishes SAD from other mood disorders. In one of the first large studies to test this hypothesis, the Seasonal Pattern Assessment Questionnaire (SPAQ),10 a screening tool which assesses the seasonality of six core symptoms of SAD and the degree to which seasonality is problematic, was mailed to randomly selected individuals in four areas of the United States differing in latitude.11 It was found that rates of winter SAD and subsyndromal SAD were significantly higher at higher latitudes, while no correlation was found between latitude and summer SAD, a rarer form of seasonal depression thought to be mediated by heat and humidity. The authors concluded that winter SAD was likely triggered by light deprivation during the short days of fall and winter associated with more northern latitudes.11
Michalak and Lam reviewed 22 studies performed in either the general population or in specific subpopulations to look at the relationship of latitude to SAD.12 In the general population in particular, there was a correlation of 0.66 between latitude and rates of SAD, which would support the latitude hypothesis overall However, one of the paradoxes in studying a possible relationship between SAD and latitude is that over the course of time, populations that are less impacted by the short days of winter may choose to remain at a northern latitude, while more sensitive populations might be expected to migrate South. If so, this would likely weaken the correlation between latitude and rates of SAD in large epidemiological studies. One example of this potential confounding factor is demonstrated in a study of SAD and seasonality in Icelanders. Magnusson and Axelsson examined the prevalence of SAD in Icelanders who had migrated to Manitoba, Canada, and found their rates of SAD to be much lower than in other ethnic populations living at a similar latitude.13 Based on this finding, the authors concluded that Icelanders might be genetically protected from SAD. This demonstrates the importance of considering gene-environment interactions when analyzing prevalence rates for SAD at a given location. A similar finding was reported by Levitt and Boyle14 in a Canadian population. In this study, eight different strata were identified in the Province of Ontario, based on latitude. A negative correlation between latitude and rates of SAD was found, contradictory to the prediction of the latitude hypothesis. The authors themselves noted the possibility that this could be explained by a tendency of genetically protected individuals to remain at more northern latitudes, whereas others would tend to migrate southward. Another factor to consider in studies of this type is that relative to urban dwellers, rural communities may have significantly more exposure to natural light in the wintertime, based on their daily routines. Yet another complicating factor is local weather conditions, which might greatly affect light availability independent of latitude.
Taking these factors into consideration, a robust test of the latitude hypothesis might require large-scale studies using within-subject designs to look at seasonal mood changes in genetically homogenous groups who migrate North or South. It would be important to study populations travelling in both directions, as immigration is itself associated with the risk for depression. A consistent pattern of within-subject increases in seasonality with northern migration above the equator, and decreased seasonality with the opposite direction of migration, would lend further support to the latitude hypothesis.
Melatonin
Another strategy to test the photoperiodic hypothesis of SAD is to study the hormone melatonin, which is secreted by the pineal gland in response to ambient darkness. In animals, the circadian pacemaker in the suprachiasmatic nucleus (SCN) regulates seasonal changes in various aspects of behavior, including food intake and reproduction, by transmitting a melatonin signal of day length. This signal is expressed through the duration of melatonin secretion at night, which is longer in winter than in summer. Over the course of the year, the SCN is able to track changing times of dawn and dusk. Various central and peripheral sites can respond to the melatonin signal produced in this way to help an organism adapt to seasonal environmental conditions.
While the anatomical circuitry that mediates this photoperiodic mechanism is present in humans,15 its functional significance in our species remains controversial With respect to SAD, demonstrating differences in this system between SAD patients and matched controls would lend support to the hypothesis that SAD is a chronobiological disorder tied to changes in the photoperiod across seasons. One approach to examining a possible photoperiodic model of SAD has been to compare melatonin rhythms in SAD patients and normal controls across the winter and summer seasons. Two studies failed to show differences in the melatonin rhythm between SAD patients and controls across seasons,16,17 although another did report high daytime melatonin levels during winter depressive episodes.18 In the latter study, the difference between patients and controls in the winter disappeared both after light treatment and in the subsequent summer. Koorengevel et al19 used a forced desynchrony protocol to examine unmasked circadian pacemaker characteristics in SAD. No significant differences were observed between SAD patients and controls in the melatonin-derived period across seasons. In a large sample of patients with SAD and matched healthy volunteers, Wehr et al found that patients with SAD had a longer duration of nocturnal melatonin secretion in winter than in the summer, while healthy controls did not,20 which might reflect a vestigial photoperiodic mechanism present in SAD patients but not controls. The authors concluded that SAD patients might be able to generate a biological signal of change of season, similar to that which is seen in other mammals to regulate seasonal changes in behavior and reproduction.
Other studies have looked at responsiveness to different light therapy protocols as a way of testing the photoperiodic hypothesis. The very first studies of light therapy in SAD were specifically designed to extend the photoperiod in winter by giving light exposure both early in the morning and later in the evening. While this artificial method of extending the photoperiod did lead to significant improvement, subsequent studies showed that such extension of the photoperiod was not sufficient to treat SAD, and that early-morning light on its own was effective in many cases. The latter does not necessarily refute the photoperiod extension hypothesis, however, in that early-morning light might still extend the photoperiod by decreasing the duration of night.
In summary, for the reasons outlined above, testing the photoperiod or latitude hypothesis of SAD has proven to be quite challenging methodologically Taken as a whole, the data does suggest that over large changes in latitude there is a positive correlation between higher latitude and rates of seasonal mood change in the population in general. Furthermore, Wehr et al's study20 suggests that SAD patients have a greater seasonal fluctuation in their melatonin rhythm than do normal controls, similar to what is seen in animals who rely on photoperiodic signals. Light therapy studies have produced mixed results, although these could in theory be explained by an ability of morning light to extend the photoperiod early in the day.
Circadian rhythms and the phase shift hypothesis
In mammals, internal circadian rhythms are generated by the SCN of the hypothalamus. The periodicity of the SCN is controlled by a number of cellular proteins which are coded for by PERIOD (per) genes. Entrainment of internally generated circadian rhythms to the light-dark cycle requires one or more time cues, or zeitgebers, such as light. Light signals are transmitted through the eyes to the SCN via the retinohypothalamic tract. While it was previously thought that circadian rhythms were entrained through light signals coming from the visual system per se, recent studies have identified a novel class of intrinsically light sensitive retinal ganglion cells that send axonal projections to the SCN independently of rod and cone photoreceptors.21 These SCN-projecting cells express newly discovered photopigments such as melanopsin22 and cryptochromes.23 Circadian rhythms have been an important focus for SAD research. Lewy et al proposed a phase shift hypothesis of SAD, suggesting that seasonal depression occurs when intrinsic circadian rhythms, such as the melatonin and temperature rhythms, are phase delayed relative to the external clock and/or sleep/wake cycle.24 According to this model, light therapy should exert its therapeutic effect by correcting these phase abnormalities. A further prediction of this hypothesis is that morning light therapy should be superior to evening light therapy for the larger group of SAD patients who start with a phase delay, based on the ability of morning light to cause a corrective phase advance. This contrasts the effect of evening light, which would further delay circadian phase.
Regarding the first component of the phase shift hypothesis, several studies have looked for a phase delay in circadian rhythms in SAD patients relative to controls. While both positive and negative studies have been reported,16,25-32 it could be argued that one or more masking effects may have limited some of these results. One study that used a stringent constant routine protocol to minimize the problem of masking effects did find circadian phase delays in a small number of hypersomnic patients.33 However, given the intensive nature of the constant routine protocol, dim light melatonin onset (DLMO), which can be measured using salivary samples, is a more practical means of assessing circadian phase in larger samples. Using this methodology, a recent study in 68 SAD patients found that 71% were in fact phase delayed, while 29% were phase advanced, suggesting that the phase advanced subtype might be more common than previously thought.34
Regarding the question of whether light therapy works by correcting a phase delay in circadian rhythms, two meta-analyses35,36 do support the prediction that morning light, which phase advances circadian rhythms, is more effective than light administered at other times of day. Melatonin given in the evening, a different means of producing a phase advance, has also been shown to have therapeutic effects in SAD patients, particularly for subjects having a phase delay at baseline.34
Other work has shown that while the ability of light to produce a phase advance contributes to its clinical efficacy, this is not limited to subjects who are phase delayed to start with. Rather, it is the size of the phase advance relative to one's sleep rhythm that is most important, with the optimal response achieved with light given 8.5 hours after melatonin onset or 2.5 hours after the sleep midpoint, defined as the midpoint between sleep onset and time of awakening.37 Yet another study which used rectal core body temperature as the key dependent measure found a weak correlation between phase advance with light and therapeutic response in SAD patients.38
Taking these various findings into consideration, and giving additional weight to the more recent studies with large sample sizes and rigorous methodologies, it would appear that circadian phase abnormalities do play a role in many cases of SAD, and that the ability of morning light to produce a phase advance is an important component of its therapeutic effect. While it was initially thought that only phase-delayed SAD patients would benefit from this effect, it would now appear that optimizing treatment based on circadian time can benefit a broader range of patients.37 Use of the DLMO as a marker of circadian phase has great potential benefit in terms of optimizing treatment schedules.
Clock genes, circadian rhythms, and SAD
Another potential focus for future research may be to identify clock genes which contribute to SAD via altered circadian rhythms. Preliminary studies of clock gene variants related to SAD and seasonality have begun to emerge.39 However, as is the case with all genetic association studies, replication and clearer delineation of the relevant phenotypes are needed before firm conclusions can be drawn. Optimizing light therapy treatment based on particular clock gene variants is another important goal for SAD genetics work.
Brain neurotransmitter studies
In parallel with work in nonseasonal depression, a number of approaches have been implemented to study the role of brain neurotransmitters, particularly the monoamines serotonin, norepinephrine, and dopamine, in the etiology and pathophysiology of SAD. One challenge in work of this type is to look for changes that distinguish SAD from other types of depression.
Serotonin
The largest body of work on brain neurotransmitter function in SAD has focused on the serotonin system. Of the monoamine neurotransmitters, serotonin has the clearest seasonal rhythm in its metabolism and availability,40-42 with most such measures pointing to decreased levels/activity in the winter months. To more directly assess serotonergic function in SAD, various probes of the serotonin system have been used. Earlier studies used hormonal responses to challenges with serotonergic agonists to assess the status of serotonin receptors, with mixed results overall43-47 Subjective responses to the drug may be a better indicator of actual brain receptor functioning in that hormonal responses are mediated at the level of the pituitary gland. Studies with the nonspecific serotonin agonist meta-chlorophenyl-piperazine (mCPP) have been relatively consistent in this regard, demonstrating that during depressive episodes, SAD patients tend to report increased activation or euphoria relative to controls following administration of this drug.43,46,47 These altered subjective responses, which appear to resolve after light therapy, are likely to be a state marker of winter depression. Interestingly, a study of m-CPP in nonseasonal depression demonstrated no differences in subjective responses in patients compared with controls, and only minor changes in neuroendrocine responses,48 suggesting that altered serotonin receptor function in SAD may be relatively specific. On the other hand, the eating disorder bulimia nervosa has also been associated with altered responses to serotonergic agonists such as m-CPP,49-51 suggesting that some serotonin receptor changes may be associated with increased appetitive behavior, independently of depression, across psychiatric disorders. It is well established that serotonin has a major role in suppressing various aspects of feeding behavior.52 Depletion of tryptophan, the amino-acid precursor of serotonin, has also been used to assess brain serotonergic functioning in various psychiatric populations. This uses a specialized diet which includes various amino acids other than tryptophan. Imaging studies suggest that this procedure is capable of rapidly lowering brain tryptophan levels by over 80% within just a few hours.53 Tryptophan depletion does not worsen depressive symptoms in untreated SAD patients during the fall/winter period, suggesting a possible floor effect in terms of decreased serotonergic functioning and lowered mood.54 However, similar to patients with nonseasonal depression,55,56 SAD patients who are in short-term clinical remission do show a brief relapse of depressive symptoms in response to tryptophan depletion.57,58 This procedure may also produce a brief relapse of symptoms when patients are in their summer remitted state,59 although negative findings have also been reported.60
Tryptophan depletion may have particularly strong effects in triggering the appetitive symptoms of SAD.57 Subjective loss of control of eating following tryptophan depletion has also been demonstrated in recovered patients with bulimia nervosa,61 adding further evidence for serotonergic involvement in the increased eating behavior manifest in these disorders. The fact that SAD patients report distinct subjective responses to high-carbohydrate meals,62 which can enhance serotonin turnover via increased tryptophan uptake into the brain,63 adds further support to this hypothesis.
There have been relatively few brain imaging studies looking at serotonin function in SAD; however, one study using single photon emission computed tomography (SPECT) showed reduced availability of brain serotonin transporters, the proteins responsible for reuptake of serotonin into presynaptic neurons, in drug-free patients with SAD during a winter depressive episode.64 These findings were clearest in the thalamus and hypothalamus, a finding that has also been reported in nonseasonal atypical depression.65
Given the large body of evidence linking serotonin dysfunction to SAD, several genetic association studies have looked at whether variation in serotonin genes contributes to one or more aspects of SAD (reviewed by Sohn and Lam66). The largest body of work has been done on the serotonin transporter gene repeat length polymorphism (5-HTTLPR) which is known to be functional in humans.67 Initial positive associations between this gene and SAD and/or seasonality were found68,69; however, the largest study to date, using pooled data from three separate samples, did not find a positive association between 5-HTTLPR and the diagnosis of SAD.70 One study did find an association between the hypofunctional s-allele of HTTLPR and the atypical symptoms of SAD,71 providing more evidence that in some cases, low serotonin activity may contribute to appetitive symptoms independently of the overall diagnosis of SAD. A possible link between altered tryptophan responses and the hypofunctional “s” allele of HTTLPR has been suggested in nonseasonal depression.72 However, a similar study in SAD patients was negative.73 Examining effects of tryptophan depletion in individuals with differing variants of the tryptophan hydroxylase-2 gene, which is expressed in brain, would also be of great interest, although no studies of this type have been reported to date.
Catecholamines
To determine whether catecholamine dysfunction can also explain the clinical manifestation of SAD, Neumeister et al administered both tryptophan depletion and catecholamine depletion protocols, in random order, to patients with SAD in remission after light therapy.74 Sham depletions were also included in this protocol. Both active depletions caused a temporary relapse of depressive symptoms, demonstrating that catecholamines, in addition to serotonin, are likely to play a role in SAD. In further support of this hypothesis, catecholamine depletion can also cause a temporary relapse in depressive symptoms in SAD patients during their summer remission.75
Dopamine has unique characteristics that might play a role in particular aspects of the SAD syndrome. For example, dopamine is known to play a critical role in light/dark adaptation at the level of the retina, where it has a mutually inhibitory relationship with melatonin. Among the various components of the dopamine system, the D4 receptor may be of particular interest in this regard. Electroretinography (ERG) studies suggest that SAD patients have a reduced B-wave amplitude76 which might reflect low retinal dopaminergic activity; strikingly, virtually the same attenuation of the B-wave response has been found in D4 knockout mice, who do not express D4 receptors.77 As a hypofunctional 7-repeat variant of the D4 gene exon 3 VNTR polymorphism has been well characterized in humans,78 a reasonable hypothesis for future work is that blunted ERGs in patients with SAD are mediated by this hypofunctional dopamine receptor gene (DRD4) variant.
While no studies to date have looked at the relationship between D4 receptors and ocular mechanisms in SAD, the hypofunctional 7-repeat allele of DRD4 has been associated with childhood dysphoria and inattention, and various manifestations of overeating and obesity, in female SAD probands.7,9,79 Neuroimaging further points to decreased availability of striatal dopamine transporter binding sites in symptomatic patients with SAD.80 As both the D4 receptor and dopamine transporter are expressed in brain areas that comprise the natural reward pathway,81 and given the fundamental role of dopamine in brain reward processes, it is reasonable to hypothesize that dopamine plays a unique role in the appetitive symptoms of SAD, distinct from those of serotonin. It is highly plausible that altered dopamine activity contributes to the rewarding aspects of highly palatable foods in SAD, while low serotonin activity contributes to overeating via effects on satiety mechanisms.
Fatigue and low levels of subjective arousal are also highly characteristic of SAD patients, which could reflect hypoactivity of both dopamine and norepinephrine in the brain. One study has shown blunted norepinephrine responses to a pharmacological challenge in untreated SAD patients compared with normal controls,82 while another has found an increase in plasma levels of norepinephrine following light treatment for SAD.83 A negative correlation between resting cerebrospinal fluid levels of norepinephrine metabolites, and depression ratings in SAD patients has also been described.84
Conclusion and future directions
Significant progress has been made on delineating the chronobiology and neurobiology of SAD and seasonality, though much more work is needed to refine our understanding of this syndrome. In terms of chronobiological studies, much of the earlier work was limited by both small sample sizes and a tendency to consider SAD as a unitary disorder, both of which contributed to inconsistencies across studies. More recent work, which has implemented careful measurement of circadian phase in larger samples, with a greater allowance for individual differences in the target phenotypes, has elucidated the picture of circadian dysregulation in significant subgroups of SAD patients. The importance of matching treatment protocols to a particular individual's circadian pattern has been an important clinical advance that has further emerged from this work.34,37
Neurotransmitter studies support a role for both serotonin and dopamine in the affective and/or appetitive symptoms of SAD. In the case of serotonin, there is significant evidence for an intrinsic seasonal rhythm of serotonin metabolism and turnover that is likely to contribute to seasonality of mood and food intake, significant evidence for altered serotonin receptor and transporter activity in SAD patients during winter depressive episodes, and clear sensitivity of SAD patients to depletion of the serotonin precursor tryptophan when remitted following light therapy. No strong genetic associations between SAD and serotonin genes have been found to date, although much larger studies with greater attention to intermediate phenotypes, use of multiple markers per gene, and consideration of gene-gene and gene-environment interactions are required to examine this question more robustly. Dopamine dysfunction might contribute to several aspects of SAD, including altered light responsivity at the level of the retina and both hypoarousal and overeating at the level of the central nervous system. The D4 receptor gene is of great interest in this regard, in that the hypofunctional 7-repeat allele of DRD4 has been linked to both affective and appetitive symptoms in SAD. As discussed above, this same allele is an excellent candidate to study altered ERG responses in this population. The fact that this 7R allele has been positively selected for in recent human evolution85 adds an intriguing twist to this story given several evolutionary models of SAD.4-9
There are several other areas that hold great promise for future investigation. For example, in addition to examining the genetic basis of retinal ERG changes as alluded to above, there is a great need to study the role of the melanopsin system, and its genetic and phenotypic variants, in mediating the circadian changes seen in many SAD patients. As the genetic and molecular mechanisms underlying various clock genes becomes clearer, applying these findings to understand individual differences in circadian physiology in SAD patients and matched controls should further improve our treatment of these patients. The use of genetic data to predict treatment response is largely unexplored to date.
Ultimately, many features of SAD make it an ideal focus for pathophysiological studies, suggesting that many significant new findings will emerge from the next decade of work in this area.
Selected abbreviations and acronyms
- 5-HTTLPR
serotonin transporter gene repeat length polymorphism
- ERG
electroretinography
- m-CCP
meta-chlorophenyl-piperazine
- SAD
seasonal affective disorder
- SCN
suprachiasmatic nucleus
REFERENCES
- 1.Rosenthal NE., Sack DA., Gillin JC., et al. Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 2.Kasper S., Wehr TA., Bartko JJ., Gaist PA., Rosenthal NE. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Arch Gen Psychiatry. 1989;46:823–833. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- 3.Magnusson A. An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatr Scand. 2000;101:176–184. [PubMed] [Google Scholar]
- 4.Rosenthal NE., Genhart M., Jacobsen FM., Skwerer RG., Wehr TA. Disturbances of appetite and weight regulation in seasonal affective disorder. Ann N Y Acad Sci. 1987;499:216–230. doi: 10.1111/j.1749-6632.1987.tb36213.x. [DOI] [PubMed] [Google Scholar]
- 5.Sher L. The role of genetic factors in the etiology of seasonality and seasonal affective disorder: an evolutionary approach. Med Hypotheses. 2000;54:704–707. doi: 10.1054/mehy.1999.0932. [DOI] [PubMed] [Google Scholar]
- 6.Eagles JM. Seasonal affective disorder: a vestigial evolutionary advantage? Med Hypotheses. 2004;63:767–772. doi: 10.1016/j.mehy.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Levitan RD., Masellis M., Basile VS., et al. The dopamine-4 receptor gene associated with binge eating and weight gain in women with seasonal affective disorder: an evolutionary perspective. Biol Psychiatry. 2004;56:665–669. doi: 10.1016/j.biopsych.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Davis C., Levitan RD. Seasonality and seasonal affective disorder (SAD): an evolutionary viewpoint tied to energy conservation and reproductive cycles. J Affect Disord. 2005;87:3–10. doi: 10.1016/j.jad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Levitan RD., Masellis M., Lam RW., et al. A birth-season/DRD4 gene interaction predicts weight gain and obesity in women with seasonal affective disorder: a seasonal thrifty phenotype hypothesis. Neuropsychopharmacology. 2006;31:2498–2503. doi: 10.1038/sj.npp.1301121. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal NE., Bradt GH., Wehr TA. Seasonal Pattern Assessment Questionnaire. Bethesda, MD: National Institute of Mental Health; 1987 [Google Scholar]
- 11.Rosen LN., Targum SD., Terman M., et al. Prevalence of seasonal affective disorder at four latitudes. Psychiatry Res. 1990;31:131–144. doi: 10.1016/0165-1781(90)90116-m. [DOI] [PubMed] [Google Scholar]
- 12.Michalak EE., Lam RW. Seasonal affective disorder: the latitude hypothesis revisited. Can J Psychiatry. 2002;47:787–788. doi: 10.1177/070674370204700820. [DOI] [PubMed] [Google Scholar]
- 13.Magnusson A., Axelsson J. The prevalence of seasonal affective disorder is low among descendants of Icelandic emigrants in Canada. Arch Gen Psychiatry. 1993;50:947–51. doi: 10.1001/archpsyc.1993.01820240031004. [DOI] [PubMed] [Google Scholar]
- 14.Levitt AJ., Boyle MH. The impact of latitude on the prevalence of seasonal depression. Can J Psychiatry. 2002;47:361–367. doi: 10.1177/070674370204700407. [DOI] [PubMed] [Google Scholar]
- 15.Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J Clin Endocrinol Metab. 1991;73:1276–1280. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- 16.Checkley SA., Murphy DG., Abbas M., et al. Melatonin rhythms in seasonal affective disorder. Br J Psychiatry. 1993;163:332–337. doi: 10.1192/bjp.163.3.332. [DOI] [PubMed] [Google Scholar]
- 17.Partonen T., Vakkuri O., Lamberg-AHardt C., Lonnqvist J. Effects of bright light on sleepiness, melatonin, and 25-hydrocyvitamin D(3) in winter seasonal affective disorder. Biol Psychiatry. 1996;39:865–872. doi: 10.1016/0006-3223(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 18.Danilenko KV., Putilov AA., Russkikh GS., Duffy LK., Ebbesson SO. Diurnal and seasonal variations of melatonin and serotonin in women with seasonal affective disorder. Arctic Med Res. 1994;53:137–145. [PubMed] [Google Scholar]
- 19.Koorengevel KM., Beersma DGM., den Boer JA., van den Hoofdakker RH. A forced desynchrony study of circadian pacemaker characteristics in seasonal affective disorder. J Biol Rhythms. 2002;17:463–475. doi: 10.1177/074873002237140. [DOI] [PubMed] [Google Scholar]
- 20.Wehr TA., Duncan WC Jr., Sher L., et al. A circadian signal of change of season in patients with seasonal affective disorder. Arch Gen Psychiatry. 2001;58:1108–1114. doi: 10.1001/archpsyc.58.12.1108. [DOI] [PubMed] [Google Scholar]
- 21.Provencio l., Rodriguez IR., Jiang G., Hayes WP., Moreira EF., Rollag MD. A novel human opsin in the inner retina. J Neu rosci. 2000;15;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provencio I., Rollag MD., Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 23.van der Horst GT., Muijtjens M., Kobayashi K., et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;98:672–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 24.Lewy AJ., Sack RL., Singer CM., White DM., Hoban TM. Winter depression and the phase-shift hypothesis for bright light's therapeutic effects: history, theory, and experimental evidence. J Biol Rhythyms. 1988;3:121–134. doi: 10.1177/074873048800300203. [DOI] [PubMed] [Google Scholar]
- 25.Lewy AJ., Sack RL., Miller LS., Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–354. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- 26.Lewy AJ., Bauer VK., Cutler NL., et al. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55:890–896. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- 27.Sack RL., Lewy AJ., White DM., Singer CM., Fireman MJ., Vandiver R. Morning vs evening light treatment for winter depression. Arch Gen Psychiatry. 1990;47:343–351. doi: 10.1001/archpsyc.1990.01810160043008. [DOI] [PubMed] [Google Scholar]
- 28.Eastman CI., Gallo LC., Lahmeyer HW., Fogg LF. The circadian rhythm of temperature during light treatment for winter depression. Biol Psychiatry. 1993;34:210–220. doi: 10.1016/0006-3223(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 29.Wirz-Justice A., Krauchï K., Brunner DP., et al. Circadian rhythms and sleep regulation in seasonal affective disorder. Acta Neuropsychiatrica. 1995;7:41–43. doi: 10.1017/S0924270800037522. [DOI] [PubMed] [Google Scholar]
- 30.Thompson C., Childs PA., Martin NJ., Rodin I., Smythe PJ. Effects of morning phototherapy on circadian markers in seasonal affective disorder. Br J Psychiatry. 1997;170:431–435. doi: 10.1192/bjp.170.5.431. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal NE., Levendosky AA., Skwerer RG., et al. Effects of light treatment on core body temperature in seasonal affective disorder. Biol Psychiatry. 1990;27:39–50. doi: 10.1016/0006-3223(90)90018-w. [DOI] [PubMed] [Google Scholar]
- 32.Murray G., Michalak EE., Levitt AJ., Levïtan RD., Enns MW., Morehouse R., Lam RW. O sweet spot where art thou? Light treatment of Seasonal Affective Disorder and the circadian time of sleep. J Affect Disord. 2006;90:227–231. doi: 10.1016/j.jad.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Avery DH., Dahl K., Savage MV., et al. Circadian temperature and Cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression [published erratum appears in Biol Psychiatry 1997;42:636]. Biol Psychiatry. 1997;1:1109–1123. doi: 10.1016/S0006-3223(96)00210-7. [DOI] [PubMed] [Google Scholar]
- 34.Lewy AJ., Lef 1er BJ., Emens JS., Bauer VK. The circadian basis of winter depression. PNAS. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terman M., Terman JS., Quitkin FM., McGrath PJ., Stewart JW., Rafferty B. Light therapy for seasonal affective disorder. A review of efficacy. Neuropsychopharmacology. 1989;2:1–22. doi: 10.1016/0893-133x(89)90002-x. [DOI] [PubMed] [Google Scholar]
- 36.Gaynes BN., Ekstrom D., Hamer RM., et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 37.Terman JS., Terman M., Lo ES., Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 38.Burgess HJ., Fogg LF., Young MA., Eastman CI. Bright light therapy for winter depression - is phase advancing beneficial? Chronobiol Int. 2004;21:759–775. doi: 10.1081/cbi-200025979. [DOI] [PubMed] [Google Scholar]
- 39.Partonen T., Treutlein J., Alpman A., et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39:229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson A., Svennerholm L., Winblad B. Seasonal and circadian monoamine variations in human brains examined post mortem. Acta Psychiatr Scand, 1980;(suppl 280):75–85. [PubMed] [Google Scholar]
- 41.Brewerton TD. Seasonal variation of serotonin function in humans: research and clinical implications. Ann Clin Psychiatry. 1989;1:153–164. [Google Scholar]
- 42.Lacoste V., Wirz-Justice A. Seasonal variation in normal subjects: an update of variables current in depression research. In: Rosenthal, NE, Blehar M, eds. Seasonal Affective Disorders and Phototherapy. New York, NY; Guilford Press; 1989:167–229. [Google Scholar]
- 43.Joseph-Vanderpool JR., Jacobsen FM., Murphy DL., Hill JL., Rosenthal NE. Seasonal variation in behavioural responses to m-CPP in patients with seasonal affective disorder and controls. Biol Psychiatry. 1993;33:496–504. doi: 10.1016/0006-3223(93)90003-v. [DOI] [PubMed] [Google Scholar]
- 44.Coiro V., Volpi R., Marchesi C., et al. Abnormal serotonergic control of prolactin and Cortisol secretion in patients with seasonal affective disorder. Psychoneuroendocrinology. 1993;18:551–556. doi: 10.1016/0306-4530(93)90032-g. [DOI] [PubMed] [Google Scholar]
- 45.Yatham LN., Michalon M. Hormonal responses to dl-fenfluramine challenge are not blunted in seasonal affective disorder. Psychoneuroendocrinology. 1995;20:433–438. doi: 10.1016/0306-4530(94)00072-7. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz PJ., Murphy DL., Wehr TA., et al. Effects of metachlorophenylpiperazine infusions in patients with seasonal affective disorder and healthy control subjects. Diurnal responses and nocturnal regulatory mechanisms. Arch Gen Psychiatry. 1997;54:375–385. doi: 10.1001/archpsyc.1997.01830160103013. [DOI] [PubMed] [Google Scholar]
- 47.Levitan RD., Kaplan AS., Brown GM., et al. Hormonal and subjective responses to intravenous m-chlorophenylpiperazine in women with seasonal affective disorder. Arch Gen Psychiatry. 1998;55:244–249. doi: 10.1001/archpsyc.55.3.244. [DOI] [PubMed] [Google Scholar]
- 48.Anand A., Charney DS., Delgado PL., McDougle CJ., Heninger GR., Price LH. Neuroendocrine and behavioral responses to intravenous mchlorophenylpiperazine (mCPP) in depressed patients and healthy comparison subjects [see comments]. Am J Psychiatry. 1994;151:1626–1630. doi: 10.1176/ajp.151.11.1626. [DOI] [PubMed] [Google Scholar]
- 49.Brewerton TD., Mueller EA., Lesem MD., et al. Neuroendocrine responses to m-chlorophenylpiperazine and L-tryptophan in bulimia. Arch Gen Psychiatry. 1992;49:852–861. doi: 10.1001/archpsyc.1992.01820110016002. [DOI] [PubMed] [Google Scholar]
- 50.Levitan RD., Kaplan AS., Joffe R., Levitt A., Brown G. Hormonal and subjective responses to intravenous mCPP in bulimia nervosa.. Arch Gen Psychiatry. 1997;54:521–527. doi: 10.1001/archpsyc.1997.01830180027004. [DOI] [PubMed] [Google Scholar]
- 51.Jimerson DC., Wolfe BE., Metzger ED., Finkelstein DM., Cooper TB., Levine JM. Decreased serotonin function in bulimia nervosa. Arch Gen Psychiatry. 1997;54:529–534. doi: 10.1001/archpsyc.1997.01830180043005. [DOI] [PubMed] [Google Scholar]
- 52.Blundell JE. Is there a role for serotonin (5-hydroxytryptamine) in feeding? Int J Obes. 1977;1:15–42. [PubMed] [Google Scholar]
- 53.Young SN., Smith SE., Pihl R., Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology. 1985;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 54.Neumeister A., Praschak-Rieder N., Hesselmann B., et al. Rapid tryptophan depletion in drug-free depressed patients with seasonal affective disorder. Am J Psychiatry. 1997;154:1153–1155. doi: 10.1176/ajp.154.8.1153. [DOI] [PubMed] [Google Scholar]
- 55.Smith KA., Fairburn CG., Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349:915–919. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- 56.Leyton M., Ghadirian AM., Young SN., et al. Depressive relapse following acute tryptophan depletion in patients with major depressive disorder. J Psychopharmacol. 2000;14:284–287. doi: 10.1177/026988110001400317. [DOI] [PubMed] [Google Scholar]
- 57.Lam RW., Zis AP., Grewal A., Delgado PL., Charney DS., Krystal JH. Effects of rapid tryptophan depletion in patients with seasonal affective disorder in remission after light therapy. Arch Gen Psychiatry. 1996;53:41–44. doi: 10.1001/archpsyc.1996.01830010043007. [DOI] [PubMed] [Google Scholar]
- 58.Neumeister A., Praschak-Rieder N., Besselmann B., Rao ML., Gluck J., Kasper S. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54:133–138. doi: 10.1001/archpsyc.1997.01830140043008. [DOI] [PubMed] [Google Scholar]
- 59.Neumeister A., Praschak-Rieder N., Hesselmann B., et al. Effects of tryptophan depletion in fully remitted patients with seasonal affective disorder during summer. Psychol Med. 1998;28:257–264. doi: 10.1017/s0033291797006375. [DOI] [PubMed] [Google Scholar]
- 60.Lam RW., Bowerîng TA., Tarn EM., et al. Effects of rapid tryptophan depletion in patients with seasonal affective disorder in natural summer remission. Psychol Med. 2000;30:79–87. doi: 10.1017/s003329179900152x. [DOI] [PubMed] [Google Scholar]
- 61.Smith KA., Fairburn CG., Cowen PJ. Symptomatic relapse in bulimia nervosa following acute tryptophan depletion. Arch Gen Psychiatry. 1999;56:171–176. doi: 10.1001/archpsyc.56.2.171. [DOI] [PubMed] [Google Scholar]
- 62.Rosenthal NE., Genhart MJ., Caballero B., et al. Psychobiological effects of carbohydrate- and protein-rich meals in patients with seasonal affective disorder and normal controls. Biol Psychiatry. 1989;25:1029–1040. doi: 10.1016/0006-3223(89)90291-6. [DOI] [PubMed] [Google Scholar]
- 63.Wurtman RJ. Nutrients that modify brain function. Sci Am. 1982;246:50–59. doi: 10.1038/scientificamerican0482-50. [DOI] [PubMed] [Google Scholar]
- 64.Willeit M., Praschak-Rieder N., Neumeister A., et al. [123I]-beta-CITSPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry. 2000;47:482–489. doi: 10.1016/s0006-3223(99)00293-0. [DOI] [PubMed] [Google Scholar]
- 65.Lehto S., Tolmunen T., Joensuu M., et al. Midbrain binding of [123l]norbeta-ClT in atypical depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1251–1255. doi: 10.1016/j.pnpbp.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Sohn C., Lam RW. Update on the biology of seasonal affective disorder: CME 3: review article. CNS Spectrums. 2005;10:635–646. doi: 10.1017/s109285290001960x. [DOI] [PubMed] [Google Scholar]
- 67.Lesch KP., Bengel D., Heïls A., et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 68.Rosenthal NE., Mazzantï CM., Barnett RL., et al. Role of serotonin transporter promoter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Mol Psychiatry. 1998;3:175–177. doi: 10.1038/sj.mp.4000360. [DOI] [PubMed] [Google Scholar]
- 69.Sher L., Hardin TA., Greenberg BD., Murphy DL., Li Q., Rosenthal NE. Seasonality associated with the serotonin transporter promoter repeat length polymorphism. Am J Psychiatry, 1999;156:1837. doi: 10.1176/ajp.156.11.1837. [DOI] [PubMed] [Google Scholar]
- 70.Johansson C., Willeit M., Levitan R., et al. The serotonin transporter promoter repeat length polymorphism, seasonal affective disorder and seasonality. Psychol Med. 2003;33:785–792. doi: 10.1017/s0033291703007372. [DOI] [PubMed] [Google Scholar]
- 71.Willeit M., Praschak-Rieder N., Neumeister A. A polymorphism (5HTTLPR) in the serotonin transporter promoter gene is associated with DSMIV depression subtypes in seasonal affective disorder. Mol Psychiatry. 2003;8:942–946. doi: 10.1038/sj.mp.4001392. [DOI] [PubMed] [Google Scholar]
- 72.Neumeister A., Konstantinidis A., Stastny J., et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- 73.Lenzinger E., Neumeister A., Praschak-Rieder N., et al. Behavioral effects of tryptophan depletion in seasonal affective disorder associated with the serotonin transporter gene? Psychiatry Res. 1999;85:241–246. doi: 10.1016/s0165-1781(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 74.Neumeister A., Turner EH., Matthews JR., et al. Effects of tryptophan depletion vs catecholamine depletion in patients with seasonal affective disorder in remission with light therapy. Arch Gen Psychiatry. 1998;55:524–530. doi: 10.1001/archpsyc.55.6.524. [DOI] [PubMed] [Google Scholar]
- 75.Lam RW., Tarn EM., Grewal A., Yatham LN. Effects of alpha-methyl-paratyrosine-induced catecholamine depletion in patients with seasonal affective disorder in summer remission. Neuropsychopharmacology. 2001;25(5 suppl):S97–S101. doi: 10.1016/S0893-133X(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 76.Hébert M., Beattie CW., Tarn EM., Yatham LN., Lam RW. Electroretinography in patients with winter seasonal affective disorder. Psychiatry Res. 2004;127:27–34. doi: 10.1016/j.psychres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Nir I., Harrison JM., Haque R., et al. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asghari V., Sanyal S., Buchwaldt S., Paterson A., Jovanovic V., Van Toi HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- 79.Levitan RD., Masellis M., Lam RW., et al. Childhood inattention and dysphoria and adult obesity associated with the dopamine D4 receptor gene in overeating women with seasonal affective disorder. Neuropsychopharmacology. 2004;29:179–186. doi: 10.1038/sj.npp.1300314. [DOI] [PubMed] [Google Scholar]
- 80.Neumeister A., Willeit M., Praschak-Rieder N., et al. Dopamine transporter availability in symptomatic depressed patients with seasonal affective disorder and healthy controls. Psychol Med. 2001;31:1467–1473. doi: 10.1017/s003329170105434z. [DOI] [PubMed] [Google Scholar]
- 81.Meador-Woodruff JH., Damask SP., Wang J., Haroutunian V., Davis KL., Watson SJ. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29. doi: 10.1016/0893-133X(95)00150-C. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz P., Murphy D., Wehr TA., et al. Effects of meta-chlorophenylpiperazine infusions in patients with seasonal affective disorder and healthy control subjects. Diurnal response and nocturnal regulatory mechanisms. Arch Gen Psychiatry. 1997;54:375–385. doi: 10.1001/archpsyc.1997.01830160103013. [DOI] [PubMed] [Google Scholar]
- 83.Anderson JL., Vasile RG., Mooney JJ., Bloomingdale KL., Samson JA., Schildkraut JJ. Changes in norepinephrine output following light therapy for fall/winter seasonal depression. Biol Psychiatry. 1992;32:700–704. doi: 10.1016/0006-3223(92)90299-f. [DOI] [PubMed] [Google Scholar]
- 84.Rudorfer MV., Skwerer RG., Rosenthal NE. Biogenic amines in seasonal affective disorder: effects of light therapy. Psychiatry Res. 1993;46:19–28. doi: 10.1016/0165-1781(93)90004-z. [DOI] [PubMed] [Google Scholar]
- 85.Ding YC., Chi HC., Grady DL., et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proc Natl Acad Sci U S A. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]