Abstract
The finding that bright light can suppress melatonin production led to the study of two situations, indeed, models, of light deprivation: totally blind people and winterdepressives. The leading hypothesis for winter depression (seasonal affective disorder, or SAD) is the phase shift hypothesis (PSH). The PSH was recently established in a study in which SAD patients were given low-dose melatonin in the afternoon/evening to cause phase advances, or in the morning to cause phase delays, or placebo. The prototypical phase-delayed patient as well as the smaller subgroup of phase-advanced patients, optimally responded to melatonin given at the correct time. Symptom severity improved as circadian misalignment was corrected. Orcadian misalignment is best measured as the time interval between the dim light melatonin onset (DLMO) and mid-sleep. Using the operational definition of the plasma DLMO as the interpolated time when melatonin levels continuously rise above the threshold of 10 pglmL, the average interval between DLMO and mid-sleep in healthy controls is 6 hours, which is associated with optimal mood in SAD patients.
Keywords: circadian rhythm, melatonin, seasonal affective disorder, winter depression, phase angle difference, dim light melatonin onset, light
Abstract
El hallazgo que la luz brillante puede suprimir la producción de melatonina condujo al estudio de dos situaciones, o mejor dicho modelos, de privación de luz: las personas totalmente ciegas y los depresivos invernales. La principal hipótesis para la depresión invernal (trastorno afectivo estacional, TAE) es la hipótesis del cambio de fase (HCF). La HCF se estableció recientemente a partir de los resultados de un estudio en pacientes con TAE que recibieron bajas dosis de melatonina en la tarde/noche para causar un avance de fase, o en la mañana para provocar retardo de fase, o placebo. El paciente prototípico con retardo de fase, como también el pequeño subgrupo de pacientes con avance de fase, respondieron en forma óptima a la melatonina administrada en el momento correcto. La gravedad de los síntomas mejoró en la medida que se corrigió el desajuste circadiano. El desajuste circadiano se puede definir mejor como el intervalo de tiempo entre el inicio de la producción de melatonina con la luz tenue (dim light melatonin onset, DLMO) y la mitad del sueño. Al utilizar la definición operacional de la DLMO como el tiempo interpolado cuando los niveles de melatonina se elevan continuamente sobre el umbral de 10 pglmL, el intervalo promedio entre DLMO y la mitad del sueño en controles sanos fue de 6 horas, lo que se asoció con un ánimo óptimo en pacientes con TAE.
Abstract
La découverte que la lumière intense peut inhiber la sécrétion de mélatonine a mené à l'étude de deux situations qui sont des modèles de la privation de lumière : la cécité totale et le trouble affectif saisonnier hivernal (TAS). L'hypothèse d'un décalage de phase (HDP) est la théorie principale pour le TAS. Une étude récente a permis de confirmer l'HDPpar administration à des patients ayant un TAS de faibles doses de mélatonine l'après-midi ou le soir pour provoquer une avance de phase, ou le matin pour un retard de phase. Un placebo a aussi été administré. Les patients ayant un retard de phase caractéristique, ainsi que le sous-groupe plus petit des patients ayant une avance de phase, ont répondu de façon optimale lorsque la mélatonine était administrée au bon moment. La sévérité des symptômes s'est améliorée quand la désynchronisation circadienne a été corrigée. La meilleure mesure de cette désynchronisation consiste en l'intervalle de temps entre le début de la sécrétion de mélatonine en éclairage faible (SMEF) et le milieu de la durée du sommeil. La définition opérationnelle de la SMEF consiste au moment (extrapolé) à partir duquel la concentration plasmatique de mélatonine reste systématiquement au-dessus du seuil de 10 pglml. Selon cette définition, l'intervalle moyen entre la SMLF et le milieu de la durée du sommeil est de 6 h chez les témoins sains, et est associée à l'humeur optimale chez les patients ayant un TAS.
The phase shift hypothesis
Winter depression (seasonal affective disorder, or SAD) has proved to be a useful model for evaluating the role of circadian rhythms in psychiatric and sleep disorders. The successful treatment of the first patient,1 as well as the first controlled study using bright light,2 assumed SAD to be a disorder of seasonal biological rhythms. Both studies were based on the finding that bright light could suppress melatonin production in humans.1 Accordingly, bright light exposure was scheduled in the morning and late afternoon/evening in order to mimic a spring photoperiod. The investigators involved in these early studies diverged into two groups: our group focused on a circadian approach to SAD3 while the other group did not.4,5 The circadian approach was based on the phase shift hypothesis (PSH) which states that most patients with SAD become depressed in the winter, at least in part because of a phase delay in circadian rhythms relative to the sleep/wake cycle.6-9 The PSH further postulates that a smaller subgroup of SAD patients becomes depressed in the winter because of a phase advance.
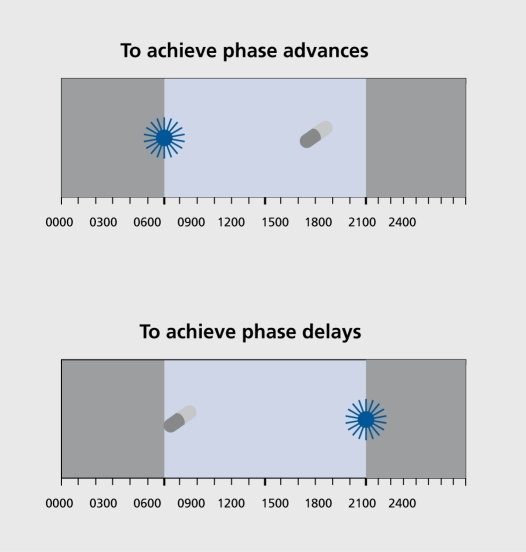
In 1987, based on our hypothesized phase response curve (PRC) to light and prior preliminary light studies in humans, we reported that bright light scheduled in the morning causes a phase advance (a shift to an earlier time) and that bright light scheduled in the evening causes a phase delay (a shift to a later time), using the dim light melatonin onset (DLMO, that is, the time of the beginning of melatonin production in dim light) as the marker for circadian phase position.8 We also reported that seven of eight SAD patients preferentially responded to the antidepressant effects of morning light, whereas one patient preferentially responded to evening light.8 The combination of morning and evening bright light counteracted each other. There was a statistically significant, but small, delay in the DLMO of the patients compared with the controls at baseline. The clinical recommendations following this report published 20 years ago9 remain state-of-the-art and unchanged, except that light intensity can be increased to 10 000 lux, offering some shortening of minimal exposure duration. Accordingly, these recommendations are reprinted in Table I SAD patients and controls were phase shifted with bright light according to Figure 1 (which also includes how to use melatonin administration to cause similar phase shifts).
Figure 1. Use of bright light and low-dose melatonin to treat circadian phase disorders. Adapted from ref 10: Lewy AJ, Sack RL. The role of melatonin and light in the human circadian system. In: Buijs R, Kalsbeek A, Romijn H, Pennartz C, Mirmiran M, eds. Progress in Brain Research, Vol, 111, Hypothalamic Integration of Circadian Rhythms. Amsterdam: Elsevier; 1996:205-216. Copyright © Elsevier 1996.
Table I. Treatment guidelines for patients with seasonal affective disorder. Adapted from ref 9: Lewy AJ. Treating chronobiologic sleep and mood disorders with bright light. Psychiatric Annals, 1987; 17:664-669. Copyright © Charles Slack 1987.
| Treatment guidelines for patients with seasonal affective disorder |
| • If patients do not have early-morning awakening, schedule 1to 2 hours of 2500=10 000 lux exposure immediately upon awakening. |
| • If patients begin treatment on the weekend, they may not have to rise earlier to accommodate the morning light exposure; early rising may retard the response for a few days. |
| • The response begins 2 to 4 days after beginning light therapy and is usually complete within 2 weeks. |
| • These patients should minimize any advance in their sleep time and should avoid bright light in the evening. |
| • If patients do not respond to treatment, they may need a longer duration of morning light. |
| • If patients respond only transiently or begin to complain of early morning awakening or severe fatigue in the evening, they may be becoming overly phase advanced due to too much morning light. The duration of morning light should be reduced but still begun immediately upon awakening or some late evening light exposure could be added. |
| • Some patients may respond to an immediate “energizing” effect of bright light exposure (this may be a placebo effect), which if not administered too late in the evening might be helpful. |
| • Once a response has been achieved, the duration and frequency of light exposures can be reduced” Always begin light exposure immediately upon awakening or a little later if patients become overly phase advanced. |
| • If there is still no response, a trial of evening bright light (7 9 pm) may be necessary. These patients should minimize any delay in their sleep time and should avoid bright light in the morning. |
| • Appropriate precautions should be taken to avoid any possibility of eye discomfort or injury (eg, an eye history and exam if indicated, instructions never to stare at the sun, use of safe artificial light sources, and recommendation of follow up checkups). |
The dim light melatonin onset
The dim light melatonin onset (DLMO) Is now the most commonly used marker for circadian phase position in humans.11 Either plasma or saliva is collected usually every 30 minutes between 6 PM and bedtime.12,13 The current recommendation for dim light is light that is too dim to allow reading without a book-light pointed directed at the page. Dim light should begin at about 5 PM. The DLMO can be operationally defined as the interpolated time when melatonin levels continuously rise above 10 pg/mL in plasma or 3 pg/mL in saliva. In some cases, thresholds of 2 pg/mL in plasma and 0.7 pg/mL in saliva are used; melatonin usually reaches these thresholds about 1hour earlier than the 10 pg/mL (3 pg/mL) thresholds (Figure 2). The DLMO appears to be a better marker for circadian phase position than core body temperature, even when the latter is measured in a constant routine.11 Furthermore, posture, sleep, activity, and meals do not need to be controlled when using the DLMO as a marker for phase position of the endogenous circadian pacemaker. Salivary DLMOs obtained in the home may soon become a standard procedure for the clinician. Space constraints do not permit a critical review of the literature in which the DLMO was initially considered to be the marker of just one component of a complex circadian oscillator.14-17
Figure 2. The dim light melatonin onset (DLMO) in plasma is operationally defined as the interpolated time when melatonin levels continuously rise above the threshold of either 10 pg/mL or 2 pg/mL (which usually occurs about 1hour earlier). In this figure the DLMO2 is at about 20:30 and the DLMO10 is about 21:30.
Testing the PSH using melatonin administration
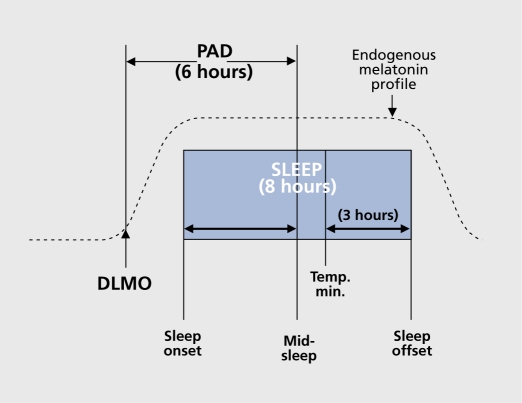
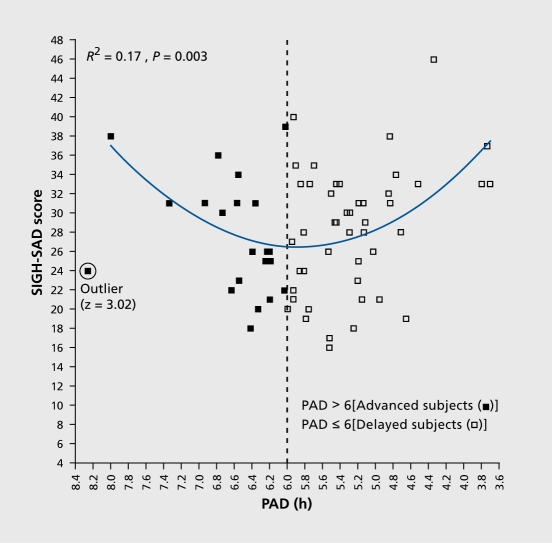
In order to provide a critical and falsifiable test of the PSH, we administered melatonin to cause phase shifts and thus avoided the large placebo component that accompanies light treatment. According to the melatonin PRC,18,19 melatonin administered in the morning (AM) causes a phase delay, and melatonin administered in the afternoon/evening (PM) causes a phase advance. That is, the melatonin PRC is about 12 hours out of phase with the light PRC. Therefore, according to the PSH, most SAD patients should preferentially respond to PM melatonin. After a baseline week in which subjects were permitted to sleep only between consistent bedtimes and wake times of their choosing and a baseline DLMO assessment, subjects were assigned to one of three regimens (AM melatonin, PM melatonin, or placebo capsules only). The melatonin dose varied slightly according to the year and was divided into 3 to 4 capsules, 2 hours apart; the total dose was 0.225 to 0.3 mg per day, depending on the year.20 Patients took 7 to 8 capsules per day, depending on the year. At the end of the third treatment week, capsules were stopped and the next day a post-treatment DLMO assessment was undertaken. The 29item SIGH-SAD (21-item Ham-D plus eight items characteristic of SAD) was administered weekly for 3 weeks. When this study was initially formulated, it was thought that the phase-advanced subgroup was too small to significantly influence, testing the hypothesis that PM melatonin would be more antidepressant than AM melatonin. This may explain why there was no statistically significant difference between these two treatments. However, more informative findings were revealed after phase typing, based on an operational definition of normal phase alignment as a 6-hour interval between the DLMO and midsleep, that is, a phase angle difference (PAD) of 6 h (Figure 3). Although the clock time of a phase marker is important, PAD is more useful because: (i) it removes confounding effects from sleep/wake cycles that occur at the different times; (ii) it is almost impossible for subjects or their raters to infer PAD from knowledge of sleep times; and (iii) psychiatric symptoms are more likely to be related to internal circadian misalignment. Plotting baseline SIGH-SAD scores against PAD revealed a statistically significant (P=0.003) parabola with an R2 of 0.17 and a minimum (vertex) of PAD 5.88 h (Figure 4). In psychiatric studies, an R2 of 0.17 is considered meaningful, given the inherent noise in behavioral ratings. Six hours was hypothesized to be the “sweet spot” (the therapeutic interval representing optimal mood), because this is the average PAD for healthy controls. PAD 6 could be used to operationally distinguish at baseline those subjects who were phase delayed (PAD ≥ 6) from those subjects who were phase advanced (PAD >6).The parabola revealed greater depression severity in 68 subjects who deviated from PAD 6 (in either direction).
Figure 3. Schematic diagram of normal phase relationships (rounded to the nearest integer) between sleep phase markers, the 10 pg/mL plasma dim light melatonin onset (DLMO) and the core body temperature minimum derived from historical controls. A DLMO-midsleep phase angle (PAD) difference of 6 h is the hypothesized interval for optimal circadian alignment in SAD (seasonal affective disorder) patients. Sleep times were determined using actigraphy. Adapted from ref 20: Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006:103:74147419. Copyright © National Academy of Sciences 2006.
Figure 4. Pretreatment SIGH-SAD depression score as a function of PAD (the interval between the DLMO and midsleep, as shown in Figure3). (The circled data point from a 36-year-old female SAD subject who was assigned to placebo treatment was the only one that met outlier criteria [z =3.02] and was therefore removed from all subsequent analyses and did not substantially affect any of the above findings [no outliers were detected in any other analyses].) The parabolic curve (minimum =5.88) indicates that PAD accounts for 17% of the variance in SIGH-SAD scores (F [2, 65] =6.43). A significant linear correlation was found for the absolute deviation from the parabolic minimum (r=0.39, R2 =0. 15, df =65, P =0.001), confirming the validity of the parabolic curve fit. SAD, seasonal affective disorder; PAD, phase angle difference. Adapted from ref 20: Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006:103:74147419. Copyright © National Academy of Sciences 2006.
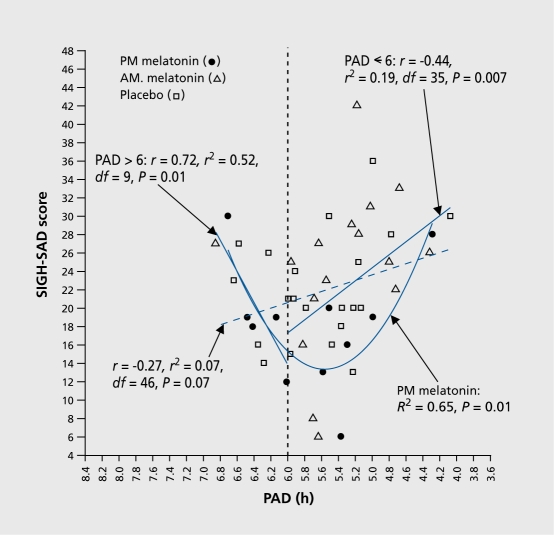
Using PAD 6 at baseline to retrospectively phase type subjects, analyses revealed that the prototypical phase delayed group represented only two thirds of the subjects. Post-treatment, the parabola was statistically significant for the prototypical delayed group (i 2=0.f 9; F=0.002; minimum =6), whereas the group as a whole has a less significant parabolic fit (R2=0.11; P=0.02; minimum =6). Eleven subjects in the prototypical group (that is, those who were phase delayed at baseline) had been assigned to PM melatonin (the equivalent of morning light, the treatment of choice). A plot of their SIGHSAD scores against PAD revealed an R2 of 0.65, P<0.001and parabolic minimum of 6 (Figure 5). That Is, 65% of the variance In the behavioral ratings could be explained by the clrcadlan misalignment component. Patients who became overly phase advanced on the PM melatonin and shifted across the sweet spot were more depressed than those who had shifted closer to PAD 6 (Figure 6).
Figure 5. Post-treatment SIGH-SAD score as a function of PAD. The parabolic curve (minimum =6. 18) indicates that PAD accounts for 11% of the variance in SIGH-SAD scores [F (2, 65)=3.96] for all subjects and 19% for phase-delayed subjects [F (2,45)=5.19]. Absolute deviations from the parabolic minima (6. 18 and 5.85, respectively) were statistically significant (advanced and delayed subjects: r=0.29, R2=0.09 df=65, P=0.02; delayed subjects: r=0.48, R2=0.23, df=65, P=0.001). SAD, seasonal affective disorder; PAD, phase angle difference Adapted from ref 20: Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci USA. 2006: 103:7414-7419. Copyright © National Academy of Sciences 2006.
Figure 6. Post-treatment SIGH-SAD score as a function of PAD in delayed subjects. (The parabolic curve and related statistics for the delayed subjects are provided in Figure 4). The linear correlation between PAD and SIGH-SAD score (diagonal hatched line) did not reach statistical significance, confirming that the parabolic curve in Figure 4 for delayed subjects (R2=0.19, P=0.009) is the better fit for these data. Directional linear correlations for underand overshifters (to the right and left of PAD 6, respectively) were both statistically significant. The parabolic curve for subjects receiving PM melatonin indicates that PAD accounts for 65% of the variance in SIGH-SAD scores (F [2, 8] =7.57; minimum =5.56); the correlation between the absolute deviation from the parabolic minimum was also statistically significant (r=0.75, R2=056, df=8, P=0.001 SAD, seasonal affective disorder; PAD, phase angle difference. Adapted from ref 20: Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006:103:74147419. Copyright © National Academy of Sciences 2006.
In this figure (which also Includes prototypical patients treated with AM melatonin and placebo), we did not find a statistically significant linear correlation, as reported by the Terman research group with respect to the decrease in depression ratings change scores plotted against phase advances in the DLMO.22 Therefore, while there is some consistency between their study and ours, there are some clear differences leading to differing treatment recommendations. Terman group claims the key to understanding the circadian mechanism of light lies in its ability to cause a phase advance; the greater the phase advance, the greater the antidepressant effect. This does not take into account the possibility that some patients may do better with evening light so as to provide a corrective phase delay. Furthermore, the Terman group recommends that patients awaken earlier than usual in order to schedule light at an earlier circadian time. However, an earlier wake time would shorten PAD, and, according to our findings, would compromise light's antidepressant effect in the prototypical phase-delayed patient. Moreover, we would predict that these prototypical patients would do less well if overly phase advanced by light scheduled too early; it had been known for some time that as long as the light was not scheduled too many hours before dawn, the earlier light exposure was scheduled relative to the DLMO, the greater was its phase-advancing effect.
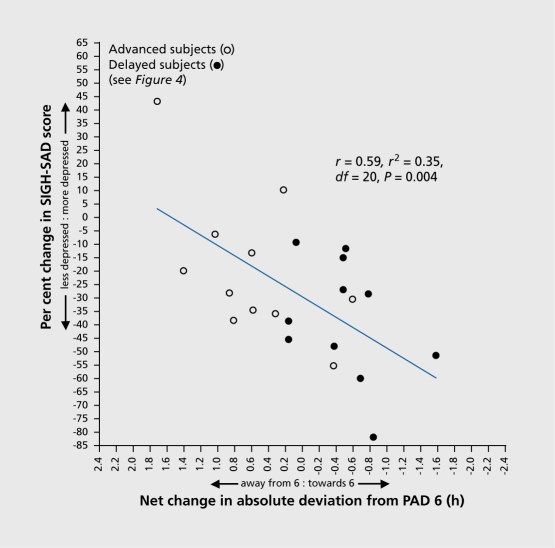
In our study, we also compared SIGH-SAD change scores versus changes in PAD (Figure 7). This figure requires some explanation. If a subject had a pretreatment PAD of 4 and a post-treatment PAD of either 5 or 7, this would represent a change closer to PAD 6 of 1. If the same subject had a post-treatment PAD of 8, this would represent a change closer to PAD 6 of 0. If the same subject had a post-treament PAD of either 9 or 3, this would represent a change closer to PAD 6 of -1, that Is, the PAD Is f hour further away from PAD 6, after treatment than before treatment. In this analysis, It does not matter whether the deviation from 6 is in one direction or the other, given what was found In the previous analyses, le, that subjects were more depressed as the deviation from 6 increased In either direction. In the change-score figure, we plotted pre- vs post-treatment percent change in SIGH-SAD score against the pre- vs post-treatment change closer to or further away from PAD 6 in all patients who received either AM melatonin (administered to cause a phase delay) or PM melatonin (administered to cause a phase advance). The prediected linear regression was highly significant (R2=0.345, r=0.59, P=0.004).
Figure 7. Percent change in SIGH-SAD score as a function of net change in absolute deviation toward and away from PAD 6 in PM melatonin treated advanced and delayed subjects. Pretreatment vs posttreatment shifts with respect to PAD 6 account for 35% of the variance. SAD, seasonal affective disorder; PAD, phase angle difference Adapted from ref 20: Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006:103:74147419. Copyright © National Academy of Sciences 2006.
Thus, SAD may be the first psychiatric disorder in which symptom severity has been demonstrated to correlate with a physiological marker before and in the course of treatment in the same patients. Accordingly, circadian misalignment may constitute a surrogate marker for the symptoms of SAD. The above analyses also indicate that circadian misalignment seems to be the causal term in the correlation with symptom severity. This remarkable finding is further confirmed by retrospectively comparing the behavioral response to the correct treatment vs the incorrect treatment or placebo. Patients who were phase delayed at baseline and were assigned to PM melatonin received the correct treatment, while those who were assigned to AM melatonin received the incorrect treatment; patients who were phase advanced at baseline and were assigned to AM melatonin received the correct treatment, while those who were assigned to PM melatonin received the incorrect treatment. The pre- to posttreatment decrease in depression ratings was 20% more in the correctly treated groups as compared with the other two groups, which is remarkably impressive for a fixed-dose trial of an antidepressant (Figure 8). The most conservative effect size was 0.61, which also is quite robust for an antidepressant. Given that most studies of antidepressants cannot disguise side effects and that melatonin was administered in such a way that sleepiness, its only side effect, was not present, melatonin appears to be of enormous therapeutic value, particularly once the dosing regimen is optimized. We used melatonin only as a means to test the PSH for light and the inclusion of AM melatonin was primarily as a control for PM melatonin. Future studies should continue to use the Instructions we provided to the patients which minimized their expectations and helped to lessen the placebo response (about 13%, compared with the usual 30% In antidepressant studies).
Figure 8. Percent change in (SIGH-SAD) depression score after correct treatment incorrect treatment and placebo, as well as incorrect treatment and placebo combined (correct treatment such as giving PM melatonin to phase-delay SAD patients, etc; see text for details of the composition of these treatment groups). Baseline SIGH-SAD scores for the three treatment groups (correct treatment incorrect treatment and placebo) were 28.9+1.0, 28.8+1.3, and 26.6+1.4, respectively. The Kruskal-WallisHtest (χ2=5.83, df=2, P=0.05) was statistically significant, but not the one-way ANOVA (F =2.96 on [2, 65], P=0.06). By using the Welch two-sample f test to compare differences in the change scores of the correct-treatment group with those of the other groups, correct treatment significantly decreased depression ratings more than the other groups: incorrect (19.1%: t =2.09, df =40.8, P =0.04); placebo (20.9%: t=2. 60, df =34.2, P=0.01); the latter two groups combined (19.9%: t=2.65, df =32.1, P=0.01). Pretreatment to post-treatment percent changes were significant for all groups: correct (t =5.43, df = 16, P<0.001), incorrect (f =2.20, df =26, P=0.04), placebo (t=2.50, df =23, P=0.02), and the latter two groups combined (t =3.25, df =50, P =0.002). Effect sizes (ES) are shown for pretreatment to posttreatment percent change scores for each group; also shown are the more conservative ES for differences in change scores between the correct treatment group and the other groups. (Before phase typing, percent change in the pm treated group was -28.5 + 5.6, and percent change in the am treated group was -15.5 + 8.0, although there were no statistically significant differences between the three treatment groups in percent changes in SIGH-SAD scores [see above]). SAD, seasonal affective disorder Adapted from ref 20: Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006:103:74147419. Copyright © National Academy of Sciences 2006.
Circadian alignment appears to cause at most about 35% to 65% of the variance In symptom severity In SAD. The PSH may also be applied to sleep and other psychiatric disorders. The PSH for these other disorders suggests that they are caused at least in part by a phase shift in circadian rhythms as marked by the DLMO with respect to the sleep/wake cycle. While we regard the PSH confirmed in SAD, the PSH remains to be tested in other sleep and psychiatric disorders.
In our recent study,20 we reported that the weekly SIGHSAD ratings continuously declined over the 4 weeks of the study only in the correctly treated group. These are plotted in the figure along with those of the incorrectly treated group combined with the placebo group; the slopes (not shown) are significantly different (Figure 9). The treatment response appears to be clinically relevant, if not statistically significant, at weeks 1and 2. Patients who can sense improvement soon after beginning treatment are more likely to comply and continue until the maximum benefit is achieved. This is a serious problem with antidepressants, along with their accompanying side effects.
Figure 9. Previously unpublished analyses based on data from the study by Lewy et al, 2006.20 SIGH-SAD and HAM-D scores of the groups receiving melatonin treatment given at the correct time vs. the incorrect time or placebo are shown by week. Although a two-sample t-test showed significant differences in the SIGHSAD scores of the treatment groups only at week 3 (P=0.04), the response appears to start between week 1and week 2. A two-sample t-test showed significant differences in the HAM-D scores of the two groups beginning at week 2 and increasing at week 3 (P=0.05, P=0.03, respectively), though these differences are also apparent at week 1. Additionally, regressions were run over the SIGH-SAD and HAM-D scores (P =0.04 and P =0.03, respectively) of each individual's weekly assessments, and a twosample t-test confirmed that the slopes of the subjects receiving correctly-timed melatonin were different from those receiving incorrectly-timed melatonin or placebo. SAD, seasonal affective disorder.
Using the correlation with phase angle difference (PAD) to refine symptom assessment
We are in the process of analyzing the data for the purpose of determining which of the 29 items of SIGHSAD account for the statistically significant findings for all of the main analyses of our recent study20 Unexpectedly, the group of eight additional SAD items was not statistically significant when used instead of the 294tem scale. This suggests that nonseasonal major depressive disorder, as measured by the 21-item HAM-D, might have a substantial circadian component related to the PSH. Furthermore, we have found that all of the main analyses using just three items substituted for the entire scale (that has a tenfold greater range) results in almost identical findings. These three items on 1-5 scales were: (1) self -reported symptoms of depression; (ii) self -reported symptoms of anxiety; and (iii) objective assessment of agitation of the subject by the rater at the time of the interview. Thus, anxiety disorders and mixed depressive/anxiety disorders should be evaluated for the PSH. Since depression and anxiety are frequently a part of sleep disorders, sleep disorders should also be tested for the PSH, as well as substance abuse disorders. As these iterative analyses proceed, we might be able to define a circadian endophenotype. However, we hesitate to use this term, as the range in PAD in healthy controls is the same as in our SAD patients, and the means are not much different. Therefore, the circadian component is a necessary but not sufficient cause of SAD. Why some people become symptomatic when their DLMO becomes delayed or advanced with respect to mid-sleep while others do not Is an Important research question. However, the answer to this may not affect treatment, and would not diminish the clinical importance of the circadian component in SAD: no matter how many necessary-but-not-sufficient causes there may be, correction of just one of these could produce a successful clinical outcome.
Implications for analyses of extant and new data sets
It is hoped that the work presented here will alert researchers to another way of conceptualizing a biological marker, in addition to the current concept of using a biological marker to distinguish between patients and healthy controls, which can often be made by merely taking a good history, that is, identifying patients who are anxious, depressed, obsessivecompulsive, paranoid, psychotic, substance abusers or poor sleepers, etc. Indeed, a symptom severity biological marker may be more useful than a chemical test for a DSM diagnosis, particularly if the former informs the type of treatment and provides a way of monitoring treatment in addition to assessing subjective and objective signs and symptoms. Given how relatively inexpensive and noninvasive the salivary DLMO is compared with other markers, neuroimaging for one example, and given how safe and inexpensive low-dose melatonin and light are compared with most other allopathic treatment modalities, there is ample justification for future investment in circadian research.
Our recent study20 establishes the PSH and the circadian component as a necessary but not sufficient cause of a substantial component of SAD, as well as a biological marker. It is hoped that the three criteria met by the circadian component for the latter designation will clarify what is important for other biological markers to demonstrate, something like Koch's postulates. We22 have recently described these; in the same patients:
Symptom severity correlates with the biological marker before treatment.
Symptom severity correlates with the biological marker in the course of treatment.
Symptom change scores in symptom severity correlate with the change in the biological marker. In our recent study,20 we concluded that (in order of certainty):
The prototypical SAD patient is phase delayed, whereas a less well defined subgroup may be phase advanced; (ii) the circadian component (at least for the prototypical patients) is substantial, and it is consistent with the PSH and a hypothesized therapeutic window for optimal circadian alignment; and (iii) the work presented here will be useful as a template for reanalyzing extant data sets and for implementing new studies of nonseasonal depression, as well as other sleep and psychiatric disorders, in which a circadian component might be present.
Accordingly, we tested three a priori hypotheses using the extant data from our 1998 SAD light treatment study. We restricted our analyses to the baseline data due to the known profound placebo component associated with light. Using the DLMOs as provided in the data set as well as diary-recorded sleep onset and offset times averaged across the 7 days of the baseline week, we tested the following hypotheses,13 plotting 29-item SIGH-SAD scores against PAD:
A parabola but not a linear regression would be statistically significant.
The minimum (vertex) would occur at PAD 6, rounded to nearest integer.
Two-thirds of the patients would be phase delayed (PAD <6).
The results confirmed our three hypotheses, as can be seen in Table II For disorders other than SAD, PAD 6 may not necessarily represent optimal circadian alignment. However, it is nevertheless important to operationally define the circadian alignment in terms of the interval between DLMO and midsleep. It may also be heuristically useful to consider PAD 6, or some other therapeutic window, in disorders other than SAD.
Table II. Baseline analyses23 of the extant data set24 replicated the results of the original study.20 .
| Lewy et al, 200620 | Replication Study | |
| R2, P | R2=M,P <.003 | R2 =22, P <.006 |
| Vertex | 5.88 h | 5.73 h |
| Z≤6 | 71% | 65% |
Once again, we encourage other researchers to make use of this work in reanalyzing extant data sets and in the design of future studies. Interestingly, we noticed that Figure 2 of the Terman paper21 contained data from which the proportion of phase delayed vs phase advanced patients at baseline could be estimated. In this figure, DLMO is plotted against sleep midpoint. The linear regression equation (r=0.66) is y=1.01x-5.93. Rounding to the nearest integer this equation becomes y=x-6 (or y-x=6). Since y is DLMO and x is midsleep, the line estimates PAD 6. Therefore, all data points to the left of this line are probably PAD <6 (phase delayed according to our criteria) and all data points to the right of this line are PAD >6 (phase advanced according to our criteria). Despite the limitations of this type of analysis, it is clear that the predominant group of patients are phase delayed and that there is a smaller, but substantial, subgroup of patients who we would categorize as phase advanced at baseline, which is consistent with the findings in our PNAS paper,20 although their presumably phase-advanced subgroup appears to comprise more than one third of the patients. Before closing, we should note that the work of others in the field of circadian rhythms and depression was recently reviewed.25 We should also note that agomelatine, a melatonin agonist with serotonergic actions, appears to be effective in major depressive disorder.26 Furthermore, at least animal studies suggest anxiolytic effects.27 These findings are consistent with what we have reported above.
For the clinicians, particularly those practicing in the US, it is important to note that melatonin is easily available to consumers without a prescription. Our impression is that some SAD patients prefer light, some prefer melatonin, and some prefer the combination, at least for the very shortest days of the year. Until more clinical labs are capable of measuring melatonin in saliva, clinicians must proceed without this test and the recommendations of Table I remain state-of-the-art. Since most patients with SAD are of the phasedelayed type, morning light should usually be tried first. Failure to respond is apparent within the first week of treatment, after which bright light should be switched to the evening. If morning bright light causes too much of a phase advance, patients will start to complain of early-morning awakening.
Selected abbreviations and acronyms
- PSH
phase shift hypothesis
- DLMO
dim light melatonin onset
- SAD
seasonal affective disorder
- PRC
phase response curve
- PAD
phase angle difference
We thank the research subjects, the nursing staff of the Oregon Health & Science University (OHSU) Clinical and Translational Research Center, Diana Arntz and Kathryn Woods. This work was supported by Public Health Service Grants R01MH55703, R01MH56874, R01AG21826, and R01HD42125 (to AJ.L) and 5 M01RR000334 (to the Clinical and Translational Research Center of OHSU). AJ.L. was supported by the National Alliance for Research on Schizophrenia and Depression 2000 Distinguished Investigator Award. J.S.E. was supported by Public Health Service Grant K23 RR017636-01.
Contributor Information
Alfred J. Lewy, Oregon Health & Science University, Department of Psychiatry, Sleep and Mood Disorders Laboratory Portland, Oregon, USA.
Jennifer N. Rough, Oregon Health & Science University, Department of Psychiatry, Sleep and Mood Disorders Laboratory Portland, Oregon, USA.
Jeannine B. Songer, Oregon Health & Science University, Department of Psychiatry, Sleep and Mood Disorders Laboratory Portland, Oregon, USA.
Neelam Mishra, Oregon Health & Science University, Department of Psychiatry, Sleep and Mood Disorders Laboratory Portland, Oregon, USA.
Krista Yuhas, Oregon Health & Science University, Department of Psychiatry, Sleep and Mood Disorders Laboratory Portland, Oregon, USA.
Jonathan S. Emens, Oregon Health & Science University, Department of Psychiatry, Sleep and Mood Disorders Laboratory Portland, Oregon, USA.
REFERENCES
- 1.Lewy AJ., Kern HA., Rosenthal NE., Wehr TA. Bright artificial light treatment of a manic-depressive patient with a seasonal mood cycle. Am J Psychiatry. 1982;139:1496–1498. doi: 10.1176/ajp.139.11.1496. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal NE., Sack DA., Gillin JC., et al. Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 3.Lewy AJ., Sack RL., Singer CM. Assessment and treatment of chronobiologic disorders using plasma melatonin levels and bright light exposure: the clockgate model and the phase response curve. Psychopharmacol Bull. 1984;20:561–565. [PubMed] [Google Scholar]
- 4.Rosenthal NE., Skwerer RG., Jacobson FM., Hardin TA., Wehr TA. Phototherapy: the NIMH experience. In: Thompson C, SilverstoneT, eds. Seasonal Affective Disorder. London, UK: CRC Clinical Neuroscience; 1988 [Google Scholar]
- 5.Wehr TA., Jacobsen FM., Sack DA., Arendt J., Tamarkin L., Rosenthal NE. Phototherapy of seasonal affective disorder: time of day and suppression of melatonin are not critical for antidepressant effects. Arch Gen Psychiatry. 1986;43:870–875. doi: 10.1001/archpsyc.1986.01800090060008. [DOI] [PubMed] [Google Scholar]
- 6.Lewy AJ., Sack RL., Singer CM. Treating phase typed chronobiologic sleep and mood disorders using appropriately timed bright artificial light. Psychopharmacol Bull. 1985;21:368–372. [PubMed] [Google Scholar]
- 7.Lewy AJ., Sack RL., Singer CM., White DM. The phase shift hypothesis for bright light's therapeutic mechanism of action: theoretical considerations and experimental evidence. Psychopharmacol Bull. 1987;23:349–353. [PubMed] [Google Scholar]
- 8.Lewy AJ., Sack RL., Miller S., Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–354. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- 9.Lewy AJ. Treating chronobiologic sleep and mood disorders with bright light. Psychiatric Annals. 1987;17:664–669. [Google Scholar]
- 10.Lewy AJ., Sack RL. The role of melatonin and light in the human circadian system. In: Buijs R, Kalsbeek A, Romijn H, Pennartz C, Mirmiran M, eds. Progress in Brain Research. Vol, 111, Hypothalamic Integration of Circadian Rhythms. Amsterdam: Elsevier; 1996:205–216. doi: 10.1016/s0079-6123(08)60409-4. [DOI] [PubMed] [Google Scholar]
- 11.Klerman EB., Gershengorn HB., Duffy JF., Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 12.Lewy AJ., Sack RL. The dim light melatonin onset (DLMO) as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 13.Lewy AJ., Cutler NL., Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 14.lllnerová H., Vanecek J. Two-oscillator structure of the pacemaker controlling the circadian rhythm of N-acetyltransferase in the rat pineal gland. J Comp Physiol [A]. 1982;145:539–548. [Google Scholar]
- 15.Parry BL., Berga SL., Kripke DF., et al. Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry. 1990;47:1139–1146. doi: 10.1001/archpsyc.1990.01810240059010. [DOI] [PubMed] [Google Scholar]
- 16.Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J Clin Endocrinol Metabol. 1991;73:1276–1280. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- 17.Wehr TA., Schwartz PJ., Turner EH., Feldman-Naim S., Drake CL., Rosenthal NE. Bimodal patterns of human melatonin secretion consistent with a two-oscillator model of regulation. Neurosci Lett. 1995;194:105–108. doi: 10.1016/0304-3940(95)11740-n. [DOI] [PubMed] [Google Scholar]
- 18.Lewy AJ., Ahmed S., Jackson JML., Sack RL. Melatonin shifts circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 19.Lewy AJ., Bauer VK., Ahmed S., et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 20.Lewy AJ., Lefler BJ., Emens JS., Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terman JS., Terman M., Lo E-S., Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Lewy A. Current understanding and future implications of the circadian uses of melatonin, a neurohormone discovered by Aaron B. Lerner. J Invest Dermatol. 2007;127:2082–2085. doi: 10.1038/sj.jid.5701004. [DOI] [PubMed] [Google Scholar]
- 23.Lewy A., Woods K., Kinzie J., Emens J., Songer J., Yuhas K. DLMO/M id-sleep interval of six hours phase types SAD patients and parabolically correlates with symptom severity. Sleep. 2007;30(Abstract supplement):A63–A64. [Google Scholar]
- 24.Lewy AJ., Bauer VK., Cutler NL., et al. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55:890–895. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- 25.Wirz-Justice A. Biological rhythm disturbances in mood disorders. Intl Clin Psychopharmacol. 2006;21:S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy SH., Emsley R. Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2006;16:93–100. doi: 10.1016/j.euroneuro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Millan MJ., Brocco M., Gobert A., Dekeyne A. Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology. 2005;177:1–12. doi: 10.1007/s00213-004-1962-z. [DOI] [PubMed] [Google Scholar]