Abstract
Alcohol dependence is a frequent, chronic, relapsing, and incurable disease with enormous societal costs. Thus, alcoholism therapy and research into its outcome are of major importance for public health. The present article will: (i) give a brief overview of the epidemiology, pathogenesis, and treatment outcomes of alcohol dependence; (ii) introduce the basic principles of outpatient long-term therapy of alcohol-dependent patients; and (iii) discuss in detail process-outcome research on Outpatient Long-term intensive Therapy for Alcoholics (OLITA). This successful biopsychosocial approach to the treatment of alcoholisms shows a 9-year abstinence rate of over 50%, a re-employment rate of 60%, and a dramatic recovery from comorbid depression, anxiety disorders, and physical sequelae. The outcome data are empirically based on treatment processes that have proven high predictive validity and give concrete information about where to focus the therapeutic efforts. Thus, process-outcome research on OLITA can serve for the development of new therapeutic guidelines on adapting individual relapse prevention strategies.
Keywords: alcohol, alcoholism therapy, addiction, chronic psychiatric disease, integrated long-term treatment, therapeutic alliance, therapy process and outcome
Abstract
La dependencia al alcohol es una enfermedad frecuente, crónica, recurrente e incurable con enormes costos sociales. Por lo tanto, la terapia del alcoholismo y la investigación acerca de ia evolución son de la mayor importancia para la salud pública. El presente artículo: 1) dará una breve visión acerca de cómo ha evolucionado la epidemiología, la patogénesis y la terapéutica de la dependencia al alcohol, 2) introducirá los principios básicos del tratamiento a largo plazo de pacientes ambulatorios con dependencia al alcohol y 3) discutirá en detalle la investigación del proceso y evolución de la Terapia Intensiva a Largo Plazo de Pacientes Alcohólicos Ambulatorios (OLITA). Esta exitosa aproximación biopsicosocial al tratamiento del alcoholismo muestra un porcentaje de abstinencia a nueve años sobre el 50%, una frecuencia de reincorporación laboral del 60% y una importante recuperación de la depresión, los trastornos de ansiedad y las secuelas físicas comórbidas. Los datos de la evolución se basan empíricamente en procesos terapéuticos que han probado una alta validez predictiva y dan información concreta acerca de dónde dirigir los esfuerzos terapéuticos. De esta forma, la investigación del proceso-evolución de OLITA puede servir para el desarrollo de nuevas guías terapéuticas para la adaptación individual a las estrategias para la prevención de recaídas.
Abstract
La dépendance alcoolique est une maladie fréquente, chronique, récidivante et incurable entraînant d'énormes coûts sociétaux. Le traitement de l'alcoolisme et la recherche sur les effets de ce traitement revêtent donc une importance majeure en termes de santé publique. L'article qui suit: (i) propose un bref aperçu de l'épidémiologie, de la pathogenèse et des résultats thérapeutiques de la dépendance alcoolique; (ii) introduit les principes de hase du traitement à long ternie en ambulatoire des patients alcooliques; (iii) discute en détail des résultats du programme thérapeutique intensif OLITA (Outpatient Long-term Intensive Therapy for Alcoholics). Cette approche bio-psychologique du traitement de l'alcoolisme s'est avérée efficace, montrant un taux d'abstinence de plus de 50 % sur 9 ans, un taux de réemploi de 60 % et une récupération très importante à la suite d'une dépression comorbide, de troubles anxieux ou de séquelles physiques. Les résultats, basés empiriquement sur des procédures thérapeutiques dont la valeur prédictive élevée a été démontrée, indiquent concrètement où porter les efforts thérapeutiques. Ainsi, la recherche concernant les effets des processus de la prise en charge OLITA peut servir à l'élaboration de nouvelles recommandations thérapeutiques pour adapter en les individualisant les stratégies de prévention de la rechute.
Epidemiology, pathogenesis, and long-term course of chronic alcohol dependence
Alcoholism is a chronic and relapsing disorder that imposes enormous costs on society, is one of the leading causes of death in industrialized countries, and is among the strongest cost drivers with respect to service use.1-5 Thus, the development of successful treatment approaches and their intensive analysis is of major importance for public health. Alcohol dependence is one of the most frequent psychiatric disorders, with a 12-month prevalence of at least 3%, a lifetime prevalence of 8% to 14%, and a maleifemale ratio of 2-5:1.6-11 Both, the course and the treatment of alcoholism are complicated by a high rate of comorbld psychiatric disorders, most importantly personality disorders (approximately 30% to 60%), anxiety (20% to 30%), and mood disorders (20% ).9,12-15 The alcohol-associated burden of disease is tremendous. Alcohol is third only to tobacco consumption and hypertension as a cause of disease and premature death in Europe. Alcohol consumption causes 6.1% of deaths, 12.3% of lost years caused by premature death, and 10.7% of all disability-adjusted life years (DALYs) - this is a measure for the estimation of the number of healthy life years lost by disease and premature death. Among young persons, alcohol constitutes the major cause of death; eg, more than 25% of deaths of European men between 15 and 29 years of age are attributable to alcohol.16,17
Even though the specific causes and complex etiological processes are only partly understood, five basic factors can be identified that play a major role for the development of alcohol dependence: (i) a strong genetic disposition, with the estimations of heritability ranging between 50% and 64%; (ii) irreversible damage of the so-called motivational or reward system (parts of the limbic system, above all hippocampus, amygdala, caudate nucleus, ventral tegmental area, parts of the frontal lobe and nucleus accumbens); (iii) specific changes in the interactions of centrally and peripherally acting neurotransmitters and hormones, eg, γ-aminobutyric acid (GABA), glutamate, dopamine, opioids, epinephrine, norepinephrine, serotonin, acetylcholine, cannablnolds, cortlcotropln-releaslng factor (CRF), and neuropeptide Y. Dysregulatlons in these transmitter systems are responsible for acute alcohol intoxication, alcohol dependence, and the withdrawal syndrome as a consequence of long-term alcohol consumption; (iv) a strong impairment of the psychobiological stress tolerance; (v) long years of overlearnlng of self-destructive behavioral processes (for review see refs 5,18-62).
Data concerning the long-term course and prognosis of chronic alcohol dependence are alarming. Longitudinal studies that investigated follow-up periods between 4 and 35 years identified the following prognostic characteristics: 63-76
In the long term, alcohol dependence is associated with significantly increased mortality rates between 15% and 60%. Thus, the mortality risk for persons with alcoholism is 2.5 to 9 times higher than for persons without alcoholism.
With only 5% to 30% of the samples from beginning of the studies, a small percentage maintained long-term abstinence; most patients either relapsed (25% to 60%), died (15% to 60%), or alternated with phases of abstinence, reduced consumption or relapse (10% to 16%).
Predictors for an unfavorable course are: chronicity, severe physical sequelae, a comorbid dissocial personality disorder, frequent excessive drinking in the past, separation from the partner, and unemployment; predictors of a good prognosis are: stable partnership, reemployment, long treatment duration, long-term participation in self-help groups after a preceding addiction therapy.
The recovery process proceeds quickly during the early years of abstinence. However, recovery takes in total 10 years or longer. The relapse risk is not significantly decreased nor stable before the third year of abstinence.
Outcome research on alcoholism therapy
A review of the current state of outcome research shows that there have not been any sensational therapeutic improvements during the last decades.
For more than 30 years, meta-analyses and literature reviews have consistently shown that alcoholism treatment is successful and cost-effective in the short term.77-84 Good evidence exists that 12-step treatment and diverse programs of cognitive behavioral therapy (CBT) are equally effective in achieving abstinence rates of approximately 25% to 30% during the year after treatment (for examples see refs 85,86). However, most treatment studies demonstrate substantial methodological shortcomings. Treatment outcomes are normally based on subjective statements of patients concerning their state of current alcohol consumption and abstinence. On the rare occasions that studies have corroborated subjective outcome data with objective laboratory data, the results are rather inexact and fragmented. Finally, the results of the few valid investigations of long-term outcome are inconsistent: objective information on drinking status indicates that only 6% to 18% of patients are abstinent at 2-year followup.87 In contrast, studies relying on self-report data suggest that approximately 30% of patients are abstinent 2 to 3 years after treatment.88,89
There is no evidence for a sufficient efficacy of a primarily pharmacotherapeutic treatment of alcoholism. Whereas the alcohol deterrent disulfiram has proven to be an adjunctive of psychotherapeutic alcoholism therapy for more than 50 years,90-95 many studies have found efficacy of the anticraving substances acamprosate and naltrexone over the last 15 years.96-100 However, the results of a recent large-scale multicenter study challenge the additional efficacy of anticraving medications over behavior therapy.101 Anton et al studied treatment outcomes of a large sample (N=1383) of alcohol-dependent patients who were treated for 16 weeks and re-examined after 12-month follow-up. The authors investigated whether different combinations of naltrexone, acamprosate, and cognitive behavior therapy differ with regard to the outcome “number of abstinent days.” Whereas acamprosate did not show any efficacy, the combinations “naltrexone plus medical management” and “naltrexone plus medical management and behavior therapy” were not more successful than a simple combination of behavior therapy, placebo medication, and medical management.101
A sobering conclusion can be drawn when interpreting these results critically, and taking into account a recent literature review that has compiled studies showing that the alcohol deterrent disulfiram is superior to the newer anticraving medications.90 Even though seemingly innovative psychotherapy concepts have been presented and praised every now and then, and a number of new medications have been launched, until now no treatment concept has been found that yields superior outcome data than the well-known and clinically often practiced combination of broad-spectrum behavior therapy and medical management.
Considering the high prevalence and chronicity, the fluctuating and devastating course, the increased mortality, and the low long-term abstinence rates, a challenging understanding of alcoholism treatment emerges. Alcohol dependence is among a group of chronic diseases such as chronic polyarthritis, hypertension, bronchial asthma, and diabetes mellitus that require a flexible, intensive, and lifelong treatment.4,94,102 Consequently, the question arises as to why therapists, therapy researchers, and social insurance agencies still recommend the so-called brief interventions as seemingly successful therapeutic options for individuals with alcohol dependence. Brief interventions may constitute treatment alternatives for individuals with risky consumption and alcohol abuse, and for these patients they can achieve outcomes with medium effect sizes. However, they are ineffective in the treatment of alcohol-dependent patients.103-105
Principles of an outpatient long-term treatment of alcohol-dependent patients
The basic principles of an innovative biopsychosocial treatment approach are derived from the evidence of epidemiology, pathogenesis, course, and treatment outcome of alcohol dependence102,106,107:
Strict abstinence orientation. Alcohol dependence is an irreversible and incurable disease. Only consequent long-term abstinence can stop disease progression and enhance the recovery process. Treatment approaches that aim at so-called “controlled drinking” are contraindicated for alcohol-dependent patients.
Supportive, nonconfronting therapist behavior. During the first months of abstinence, alcohol-dependent patients demonstrate a strong impairment of the psychobiological stress system which only recovers slowly Whereas confronting and emotionally stressful therapeutic interventions like cue exposure are harmful, the supportive, client-centered, and cognitive behavioral therapeutic strategies have proven efficient.
Chronic disease - intensive, lifelong treatment. Chronic alcohol dependence is associated with a strong genetic disposition, irreversible neurobiological damage, and decades of self-destructive learning processes. Only long-term and comprehensive therapies, followed by lifelong attendance of checkup sessions and self-help group participation, can guarantee long-term recovery.
A relapse is an emergency. Alcohol dependence is a severe psychiatric disease demonstrating high rates of physical and psychiatric comorbid disorders, a vast number of social problems, and a significantly increased mortality risk. Similarly to relapses in other severe diseases, an alcohol relapse has to be interpreted as an emergency that requires immediate crisis intervention. Any delay clearly means a poorer prognosis.
OLITA: a successful biopsychosocial approach to the treatment of alcoholism
Outpatient Long-term Intensive Therapy for Alcoholics (OLITA) is a four-step biopsychosocial outpatient therapy program for severely affected alcohol-dependent patients, aiming at immediate social reintegration within the sheltered setting of psychotherapeutic treatment and medical care. Therefore, basic elements of psychiatric patient care, client-centered and cognitive-behavioral psychotherapy, as well as classical addiction therapy, are integrated into a comprehensive, intensive and long-term treatment approach (Tables I and II). In order to take into account both the impaired stress tolerance of the patients during early abstinence and the chronicity of the disease, the OLITA concept combines high intensity (ie, high frequency of therapy contacts) and long duration of therapy26,108 Following inpatient detoxification, the treatment extends over 2 years. The OLITA pilot study started in 1993 and was terminated successfully in 2003 after 10 years and the completion of 180 patients assigned to recruitment cohorts 1-6.94,106 The main therapeutic elements of OLITA are: (1) frequent contacts, Initially dally, with a slow reduction of contact frequency up to the end of the second year; (ii) therapist rotation; (iii) support of social reintegration and aggressive aftercare; (iv) induction of alcohol intolerance through application of alcohol deterrents (inhibitors of acetaldehyde dehydrogenase); (v) explicit control: supervised intake of alco hoi deterrents and regular urine analysis for alcohol and other drugs of abuse. The therapeutic phases of OLITA consist of the inpatient period (detoxification; 2 to 3 weeks; daily individual sessions, 15 minutes), the outpatient period i (intensive phase; 3 months; daily individual sessions, 15 minutes), the outpatient period II (stabilizing phase; 3 to 4 months according to individual need; three times a week individual sessions, 15 minutes), the outpatient period III (weaningoff phase; 6 months; twice a week individual sessions, 30 minutes), and outpatient period iV (aftercare phase; 12 months; once weekly group session; initially once weekly individual session, 30 minutes, which is gradually tapered off). After completion of the 2 years of therapy, patients participate in weekly to quarterly follow-up contacts and are offered to make use of both the emergency service and the crisis interventions of the therapeutic team.
Table I. The main therapeutic elements of OLITA, Outpatient Long-term Intensive Therapy for Alcoholics.
| • High-frequency short-term individual therapeutic contacts |
| Structured, guarded attachment by supportive, nondemanding short-term contacts; initially 15 minutes daily; including weekends and holidays; slow tapering off of contact frequency aiming at regular and permanent attendance of weekly group sessions. |
| • Emergency service and crisis interventions |
| In case of emergency, patients and their relatives can contact OLITA round the clock on any day of the year. |
| • Social reintegration and home visits |
| Specific assistance in rearranging a social network which supports an abstinent lifestyle: explicit cooperation with family members and friends; family and marital sessions; advice and support regarding occupation, authorities, housing problems, moving, job-seeking, financial and legal problems. |
| • Induction of alcohol intolerance |
| Use of calcium carbimide (Colme®) or disulfiram (Antabuse®), so-called alcohol deterrent medication (inhibition of the alcohol-metabolizing enzyme acetaldehyde dehydrogenase leads in case of alcohol consumption to accumulation of toxic acetaldehyde resulting in an “inner poisoning”, the so called “disulfiram ethanol reaction,” comprising extensive flushing, hyper- or hypotension, tachycardia, nausea, vomiting, anxiety). |
| • Introduction of control factors |
| Regular urine and blood analyses for alcohol and other drugs of abuse; if necessary additional breath tests. Supervised intake of deterrent medication and explicit exploitation of its psychological effects. |
| • Aggressive aftercare |
| Aggressive therapeutic interventions to immediately interrupt beginning and to prevent threatening relapses. Patients who miss a therapeutic contact are called on to continue therapy or to restart abstinence; examples of aggressive aftercare are spontaneous house visits, telephone calls, and involvement of close friends/relatives. |
| • Therapist rotation |
| An interdisciplinary cooperating team of 6 to 7 therapists is treating the patients (supervising psychiatrist psychologist physician, social worker; nurse and MD or PhD students). All therapists are equally responsible for all patients. The classical fixation of a single patient to a single therapist is abandoned. |
Table II. Practical realization of the treatment program.
| • Inpatient period: Detoxification |
| 2-3 weeks; daily individual sessions, 15 minutes each; |
| disulfiram, 100 mg daily, or calcium carbimide, 50 mg daily. |
| • Outpatient period I: Intensive phase |
| 3 months; daily individual sessions, 15 minutes each; |
| disulfiram, 100 mg daily; or calcium carbimide, 50 mg daily. |
| • Outpatient period II: Stabilizing phase |
| 3-4 months, according to individual need; 3 times a week individual sessions, 15 minutes each; disulfiram, 400 mg, 3 times a week. |
| • Outpatient period III: Weaning-off phase |
| 6 months; twice a week individual sessions, 30 minutes each; |
| disulfiram, 400 mg, twice a week. |
| • Outpatient period IV: Aftercare phase |
| 12 months; once-weekly group session; initially weekly individual sessions (30 minutes) which are gradually reduced; disulfiram, 400 mg, once a week; tapering off between months 13 and 20, individual extension possible. |
Patients in the OLITA program: soriodemographic and addiction severity characteristics
Inclusion criteria for OLITA are alcohol dependence according to DSM-IV, residence nearby, and health insurance-covered treatment costs. Exclusion criteria are presence of moderate to severe dementia and acute concurrent abuse or dependence on substances other than alcohol (with the exception of caffeine and nicotine). Thus far, 180 alcoholics (144 men, 36 women) have been treated with a 7-year follow-up success rate of over 50% abstinent patients despite a “negative selection,” with regard to severity of alcohol dependence, comorbidity, and social detachment, upon entering the program. Patients were on average 44±8 years old, had a duration of alcohol dependence of 18±7 years, approximately 7±9 prior inpatient detoxification treatments, and 1±1 failed inpatient long-term therapy. Almost 60% of the patients were unemployed. Psychiatric comorbidity amounted to 80%. About 60% of the patients suffered from severe sequelae of alcoholism, such as polyneuropathy, chronic pancreatitis, or liver cirrhosis. To illustrate addiction severity in our population, representative scores of the European Addiction Severity Index109,110 were 0.58 (±0.38) for medical status, 0.56 (±0.47) for economic status, 0.51 (±0.37) for job satisfaction, 0.83 (±0.11) for alcohol use, 0.59 (±0.30) for family relationships, and 0.46 (±0.21) for psychiatric status.
Long-term treatment outcomes
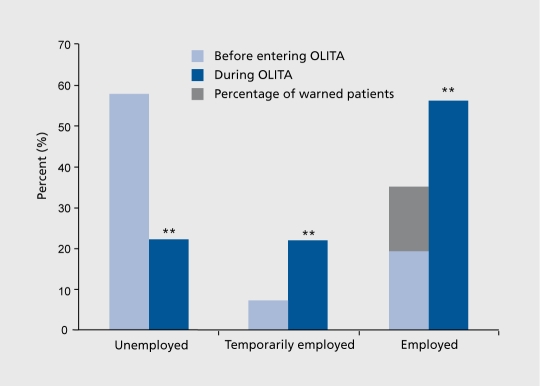
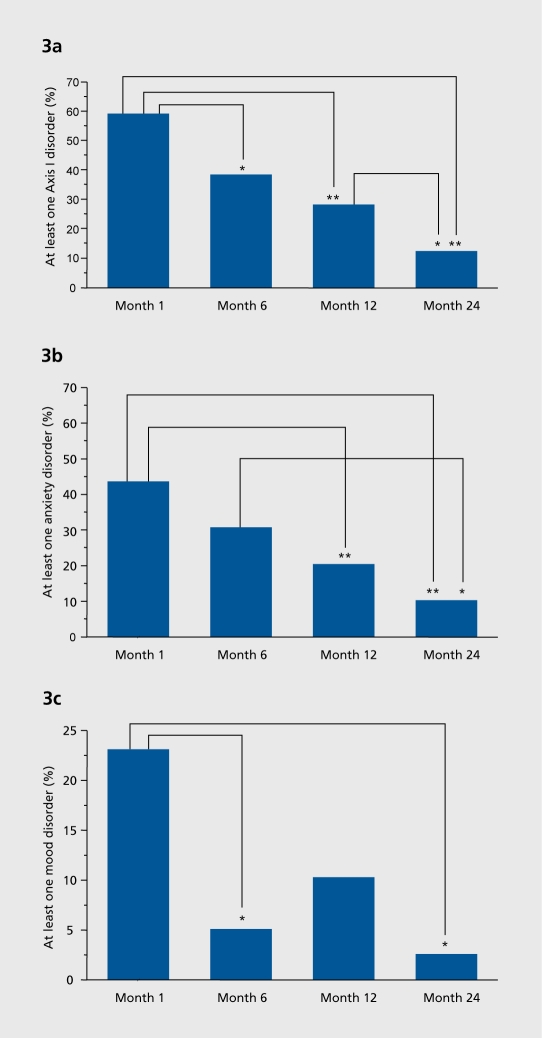
Considering this severely affected population of alcoholics, the long-term success rate of OLITA is incredibly high: More than 50% of the patients remain abstinent over up to 7 years of post-treatment follow-up (Figure 1). Based on this high abstinence rate, a tremendous improvement in psychological, biological, and social parameters of this patient group could be achieved. The unemployment rate of OLITA patients declined to 22% in an area (Göttingen) with a general unemployment rate of 17% (Figure 2). and the comorbid psychiatric disorders anxiety and depression decreased from approximately 60% to 13%. 76,94 Additionally, patients had a clear decrease in physical sequelae of alcoholism, ranging from liver disease to polyneuropathy Figure 3 a, b and c illustrate the highly significant reduction in psychiatric comorbidity Shown are all comorbid disorders (Figure 3a), anxiety disorders (Figure 3b), and mood disorders (Figure 3c) in percentage of the study population from month 1 of therapy to 2 years, ie, the termination of the program. The global decrease of comorbid disorders during therapy is characterized by two specific features of the recovery process. Firstly, anxiety disorders show a delayed remission, ie, they do not change significantly until the first year of therapy. Secondly, the early remission of mood disorders during the first 6 months harbors the risk of reccurence of major depression during long-term abstinence. These data suggest that effective treatment of dual diagnosis patients comprises two basic elements: (1) long-term duration as prerequisite of gradual remission of anxiety and protective factor against recidivism of mood disorders; (ii) comprehensive and careful integration of dual diagnosis interventions considering temporary impairments of coping skills and the imminent danger of overtaxing current patient resources. Simple addition of some treatment elements for comorbid disorders to short-term alcoholism therapy has no effect111 or even causes a negative outcome.112
Figure 1. The cumulative abstinence probability during the 9-year study is .52 for the complete sample (N=180); Kaplan-Meier estimates; cases are censored if they have not experienced a relapse by the end of follow-up.
Figure 2. Employment of OLITA patients (N=180); ** P<0.0001 versus situation upon entering OLITA. The gray shaded area shows the proportion of patients who were working before OLITA, but who had received official warnings from their employers.OLITA, Outpatient Long-term Intensive Therapy for Alcoholics.
Figure 3. Two-year course of comorbid axis I disorders during OLITA, Outpatient Long-term Intensive Therapy for Alcoholics ** P<0.01; * P<.05, P-values were adjusted for multiple comparisons according to the stepwise rejecting Holm procedure.121 Figure 3a. Two-year course of all comorbid axis I disorders. Figure 3b. Two-year course of anxiety disorders. Figure 3c. Two-year course of mood disorders.
A case-control study
Compared with thoroughly paralleled case controls who participated in alternative treatment programs, the outcome of OLITA patients is significantly better.102 Separate analysis of lapses (intake of alcohol followed by immediate cessation of drinking and continuation of the OLITA program) and relapses (intake of alcohol followed by “malignant” continuation of drinking) in OLITA patients reveals that the “true relapse rate” in OLITA patients is 30% as compared with 70% in controls. Relapses plus lapses in OLITA patients amounted to 60%. Thus, the immediate stop of lapses by means of crisis interventions has prevented the progression into relapses for 30% of the patients.
Mechanisms of recovery and irreversibility
The OLITA program offers the unique possibility of following a well defined population of alcoholics over a long period of strictly controlled alcohol abstinence. In this ideal setting, we were able to study alcohol-induced pathology, as well as kinetics and mechanisms of recovery Topics investigated include chromosomal aberrations, hematopoietic factors and circulating blood cells, stress hormones, sexual function and sex hormones, as well as neurocognitive functioning. Recently, we reported persistent alterations in many neuroendocrinological parameters, for example enduring disturbances of water/electrolyte homeostasis and thirst. These findings may prepare the ground for future pharmacological therapies. The underlying mechanisms of irreversibility could be directly or indirectly related to the phenomenon of dependence as well as of addictive behavior.23,26,31-35,51,113 Figure 4 shows the diurnal profile of epinephrine after 1 and 12 weeks of alcohol abstinence as an example of the biological basis of the patients' impaired stress tolerance during early abstinence. At both time points, data were obtained on three consecutive days from 7 AM to 3 PM from patients and controls in permanent supine position. Alcohol-dependent patients demonstrate extremely high levels of epinephrine at the beginning of abstinence that are still significantly higher than levels of healthy control subjects after 3 months of controlled abstinence (difference between alcohol-dependent patients and healthy control subjects: P<.0001 for the upper row, P<.01 for the lower row; N=11 for each group). The extent to which the stress response of the alcohol-dependent patients is impaired can be seen from the consistently higher stimulation of their epinephrine levels on all of the 6 days of assessment as compared with control subjects at the time point when the intravenous cannula was inserted (at 7 AM).
Figure 4. Diurnal profile of epinephrine during course of alcohol abstinence (see text for details).
Personalty disorder and chronicity of addiction as potent independent predictors of an unfavorable treatment outcome
A central issue of therapy research is to estimate the intensity of treatment needed on the basis of addiction severity of individuals. This approach is based on the assumption that patients whose addiction is less severe than others' might also benefit from less intensive treatment, whereas patients whose addiction is more severe need a more intensive therapy. However, it is far from clear which variables within the broad range of substance use data constitute the essential features of addiction severity14,69,86 The OLITA setting prepared the ground for a prospective longitudinal study that examined which components of addiction severity predict time to relapse for a subsample of 112 patients during 4-year follow-up.108 Among the various analyzed sociodemographic, psychiatric, and alcoholism-related patient characteristics, only the presence of a personality disorder (Wald=7.83, df=1, P= .005) and chronicity of addiction (Wald=5.17, df=1, P=.023) were independently associated with a decrease of cumulative 4-year abstinence probability. Chronicity was defined as the percentage of a patient's lifetime that he or she has been addicted (ie, duration of dependence divided by age at the beginning of therapy). As illustrated in Figure 5, patients with a comorbid personality disorder and/or higher chronicity of addiction had a lower abstinence probability and a shorter time to relapse than patients without personality disorder and/or with lower chronicity The four abstinence curves differ significantly (Breslow statistic=10.36, P=.02). Pairwise single comparisons of abstinence curves show that patients with both predictors are more at risk to relapse (.53, N=25, black line) than patients with no personality disorder and only low chronicity (.93, N=14, red line) (Breslow statistic=5.5, P=.02). Abstinence curves of patients who are handicapped only by personality disorder (.59, N=23, green line) or only by high chronicity (.60, N=11, gray line) approximate the abstinence curve of patients with both risk factors, indicating that these predictors independently cause a decrease of cumulative abstinence probability.
Figure 5. Prediction of cumulative abstinence probability during 4-year follow-up (Kaplan-Meier presentation). Interaction of the predictors personality disorder and chronicity (analysis of extreme groups).
Therapist rotation: a major element of OLITA
Apart from the regained quality of life of these patients, the general health care cost reduction is enormous. How can we explain the unusual success of our very structured, intensive, and comprehensive long-term treatment? A major “mechanism of action” of OLITA seems to be the therapist rotation.107 This element of OLITA represents a revolution in psychotherapy. The fact that six to seven therapists are equally responsible for each patient translates the ordinary two-way relation between therapist and patient into a most efficient multiway therapeutic network. Therapists stick to the rules of the program and the ideas of alcoholism treatment realized within the concept (congruence) and frequently repeat these rules and ideas (repetition). Thereby, a variety of individual therapists with a variety of different thoughts create a therapeutic atmosphere characterized by vivid and multifaceted variation. We hypothesize that these specific factors activate common factors of psychotherapy and that, as an element of OLITA, therapist rotation has a major contribution to its success.
How can we prove efficacy in a psychotherapeutic setting?
In contrast to pharmacological agents, psychotherapeutic effects are much more difficult to define or to measure. In addition, quality control for psychotherapy is widely missing. Therefore, and also to prove our hypotheses of how OLITA works, we have developed the VideoAssisted Monitoring of Psychotherapeutic Processes in Chronic Psychiatric Disease (VAMP). This diagnostic measure is a standardized, manualized, and video-based observational system that focuses mainly on the patients' behavior and makes it possible to assess treatment processes based on transcribed video recordings of therapy sessions.114 The scales evaluated in the VAMP are grouped into seven modules: (1) common psychotherapeutic factors; (ii) addictive behavior; (iii) disease concept; (iv) working atmosphere; (v) psychopathological symptoms; (vi) therapeutic alliance; and (vii) problem solving. A total of 64 patients have been analyzed over the past 4 years using the VAMP. Each patient had 17 videotapes of psychotherapeutic sessions within the 2 years of OLITA recorded. These videos are the basis of both, a macroanalytical and a microanalytical evaluation of therapeutic processes and their influence on long-term outcome. An ongoing project explores the use of the VAMP in a prospective longitudinal study investigating (i) processes of change during the first year of OLITA; (ii) associations between therapeutic processes and essential outcome variables (eg, abstinence, relapse, addiction severity, course of comorbidity, and neuropsychological regeneration).114 Therefore, treatment processes have been investigated at three time-points, t1 (week 3), t2 (month 6), and t3 (month 12) during the first year of OLITA.
Reliability analyses show that the scales of the VAMP have high interjudge reliability (median intraclass coefficient of 0.80) and internal consistency (median Cronbach's a 0.81). The construct validity is indicated by pronounced intercorrelation patterns of theoretically associated specific factors. Figures 6 and 7 demonstrate two examples. Relapse alertness (Figure 6) is strongly correlated with talk about relapse risk, disease concept, analytic processing and reflexion, experience of resources, as well as with functional and dysfunctional problem solving of current problems. However, correlations are only medium-sized with self-disclosure and implicit craving. Most interestingly, relapse alertness is only weakly associated and nearly independent of explicit craving, functional and dysfunctional problem solving of past problems and both general and abstinence self-efficacy. To perform construct validation of the VAMP therapeutic alliance scales (Figure 7), associations with the self-report measure Helping Alliance Questionnaire (HAQ) were analyzed.115,116 This 11-item questionnaire has well-established psychometric properties, is available as a patient form and a therapist form and measures how patient and therapist have experienced the quality of therapeutic alliance during the session just conducted. It is based on two underlying components of the therapeutic alliance, support by the therapist and collaborative teamwork with the therapist regarding treatment goals and tasks. In the present study, the HAQ was administered directly after a therapy session, and neither patient nor therapist or VAMP raters were allowed to inspect each others' ratings. The three VAMP scales, working atmosphere, therapeutic alliance-patient and therapeutic alliance-therapist are highly correlated, suggesting an underlying common factor. Compared with the rather small associations between patient, therapist, and observer alliance ratings that are reported in the general psychotherapy literature117 and in recent addiction therapy studies (eg, refs 118-120), both patient and therapist HAQ scores in our study show considerably higher correlations with each other and with VAMP observer ratings. This higher congruence, together with remarkably stable and high scores over 12 months in all used alliance measures,114 lead us to the speculation that the multiple relationships developed in the setting of therapist rotation might constitute a stronger therapeutic alliance than the two-way relationships in the dyadic therapy setting. Although these data are not yet a clear proof that therapist rotation is a major factor contributing to the long-term success of OLITA, they are the first empirical evidence on therapist rotation and may stimulate future investigations of this rather unexplored research topic.
Figure 6. Intercorrelational pattern of the VAMP scales at the beginning of therapy (n=64); relapse alertness (central construct, light blue) shows correlations of different sizes with process variables belonging to the groups of common psychotherapeutic factors (light gray), problem processing (dark gray) as well as addictive behavior (dark blue). OLITA, Outpatient Long-term Intensive Therapy for Alcoholics; VAMP: Video-Assisted Monitoring of Psychotherapeutic Processes In Chronic Psychiatric Disease.
Figure 7. Aspects of therapeutic alliance in OLITA: Correlational pattern between different observer-rated (dark blue, VAMP scales) and self-reported (light blue, HAQ) measures of therapeutic alliance at the beginning of therapy (n=64). OLITA, Outpatient Long-term Intensive Therapy for Alcoholics; VAMP: Video-Assisted Monitoring of Psychotherapeutic Processes In Chronic Psychiatric Disease; HAQ, Helping Alliance Questionnaire.115,116 .
Treatment processes in clinical practice: where to start?
Therapists in the addiction field daily face the difficulty to decide which of the many dysfunctional processes of their patients have priority and should be focused on at first. By integrating the VAMP scales with the highest predictive validity, the composite score Therapy Orientation by Process Prediction Score (TOPPS) was constructed. It includes the process variables experience of resources, abstinence self-efficacy, implicit craving, relapse alertness, relapse risk, disease concept, dysfunctional therapeutic engagement, and dysfunctional problem solving of current problems. The TOPPS strongly predicts 4-year abstinence probability at any of the 3 time-points (P<0.001).This result suggests employing the TOPPS in addiction therapy as a treatment guideline for adapting individual relapse prevention strategies. Therapists and addiction counselors can evaluate their patients according to the eight processes after individual therapy sessions as well as in team sessions. The ratings may be employed in form of a checklist that serves as a practical tool to plan, evaluate, reschedule, and regulate the course of therapy. Problems in one or more of the eight processes indicate to what extent a patient's current behavior constitutes a long-term risk factor for alcohol relapse. As a consequence, individually tailored relapse prevention strategies that target specifically the improvement of the problematic processes should be integrated into the treatment plan.
For possible interventions, a plethora of therapeutic elements are available in comprehensive addiction therapy, all of them realized within the OLITA program, eg, motivational interventions during inpatient detoxification, smooth transition from inpatient to outpatient treatment, high-frequency short-term individual therapeutic contacts, supportive psychotherapy during the first 6 months of abstinence, therapist rotation, social support, case management, regular urine and blood tests for alcohol and other drugs of abuse, supervised intake of alcohol deterrents, house visits, crisis interventions and assistance round the clock in case of emergency, “aggressive aftercare,” coping and problem-solving skills training including functional analyses, psychoeducation, and restructuring of dysfunctional thinking, eclectic cognitive-behavioral and psychopharmacological treatment of concurrent mental disorders, marital and family therapy, slow tapering of therapeutic contacts and weekly group sessions during the second year of treatment.
Conclusions and clinical implications
Alcohol dependence is a chronic, relapsing, and incurable disease that belongs to the most frequent psychiatric disorders. Personality disorder and chronicity constitute the essential features of addiction severity and result in low abstinence rates of short- and medium-term therapies after extended follow-up. A new understanding of alcoholism therapy recognizes alcohol dependence as a chronic disease such as hypertension, chronic polyarthritis, bronchial asthma, and diabetes mellitus. Similar to these diseases, alcohol dependence has to be treated with an unusually intensive biopsychosocial approach. Only comprehensive, integrated, and structured long-term therapy with a strict abstinence orientation, followed by lifelong attending of checkup sessions and self-help group participation will guarantee long-term recovery.
OLITA shows a 9-year abstinence rate of over 50%, a reemployment rate of 60%, and a dramatic recovery from comorbid depression, anxiety disorders, and physical sequelae. These outcome data are empirically based on treatment processes that have proven high predictive validity and give concrete information about where to focus the therapeutic efforts. Thus, process-outcome research on OLITA can serve for the development of new therapeutic guidelines for adapting individual relapse prevention strategies.
Selected abbreviations and acronyms
- HAQ
Helping Alliance Questionnaire
- OLITA
Outpatient Long-term Intensive Therapy for Alcoholics
- TOPPS
Therapy Orientation by Process Prediction Score
- VAMP
Video-Assisted Monitoring of Psychotherapeutic Processes in Chronic Psychiatric Disease
Contributor Information
Henning Krampe, Division of Clinical Neuroscience, Max-PIanck-Institute of Experimental Medicine, Goettingen, Germany.
Sabina Stawicki, Division of Clinical Neuroscience, Max-PIanck-Institute of Experimental Medicine, Goettingen, Germany.
Margret R. Hoehe, Genetic Variation Program, Max-PIanck-Institute for Molecular Genetics, Berlin, Germany.
Hannelore Ehrenreich, Division of Clinical Neuroscience, Max-PIanck-Institute of Experimental Medicine, Goettingen, Germany.
REFERENCES
- 1.Bray J., Zarkin G. Economic evaluation of alcoholism treatment. Alcohol Res Health. 2006;29:27–33. [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH., Marks JS., Stroup DF., Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Nalpas B., Combescure C., Pierre B., et al. Financial costs of alcoholism treatment programs: a longitudinal and comparative evaluation among four specialized centers. Alcohol Clin Exp Res. 2003;27:51–56. doi: 10.1097/01.ALC.0000047301.72437.10. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien CP., McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- 5.Uhl GR., Grow RW. The burden of complex genetics in brain disorders. Arch Gen Psychiatry. 2004;61:223–229. doi: 10.1001/archpsyc.61.3.223. [DOI] [PubMed] [Google Scholar]
- 6.Caetano R., Cunradi C. Alcohol dependence: a public health perspective. Addiction. 2002;97:633–645. doi: 10.1046/j.1360-0443.2002.00184.x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi F., Wittchen HU., Hoelting C., et al. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS). Psychol Med. 2004;34:597–611. doi: 10.1017/S0033291703001399. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC., McGonagle KA., Zhao S., et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Study. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 9.Regier DA., Farmer ME., Rae DS., et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 10.Rehm J., Room R., Vandenbrink W., Jacobi F. Alcohol use disorders in EU countries and Norway: an overview of the epidemiology. Eur Neuropsychopharmacol. 2005;15:377–388. doi: 10.1016/j.euroneuro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Wittchen H-U., Essau CA., von Zerssen D., Krieg J-C., Zaudig M. Lifetime and six-month prevalence of mental disorders in the Munich Follow-up Study. Eur Arch Psychiatry Clin Neurosci. 1992;241:247–258. doi: 10.1007/BF02190261. [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC., Crum RM., Warner LA., Nelson CB., Schulenberg J., Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 13.Ross HE. DSM-III-R alcohol abuse and dependence and psychiatric comorbidity in Ontario: Results from the Mental Health Supplement to the Ontario Health Survey. Drug Alcohol Depend. 1995;39:111–128. doi: 10.1016/0376-8716(95)01150-w. [DOI] [PubMed] [Google Scholar]
- 14.Verheul R. Co-morbidity of personality disorders in individuals with substance use disorders. Eur Psychiatry. 2001;16:274–282. doi: 10.1016/s0924-9338(01)00578-8. [DOI] [PubMed] [Google Scholar]
- 15.van den Bosch L., Verheul R. Patients with addiction and personality disorder: treatment outcomes and clinical implications. Curr Opin Psychiatry. 2007;20:67–71. doi: 10.1097/YCO.0b013e328011740c. [DOI] [PubMed] [Google Scholar]
- 16.John U., Hanke M. Alcohol-attributable mortality in a high per capita consumption country - Germany. Alcohol Alcohol. 2002;37:581–585. doi: 10.1093/alcalc/37.6.581. [DOI] [PubMed] [Google Scholar]
- 17.Rehm J., Taylor B., Patra J. Volume of alcohol consumption, patterns of drinking and burden of disease in the European region 2002. Addiction. 2006;101:1086–1095. doi: 10.1111/j.1360-0443.2006.01491.x. [DOI] [PubMed] [Google Scholar]
- 18.Berridge KC., Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 19.Bigelow GE. An operant behavioral perspective on alcohol abuse and dependence. In: Heather N, Peters TJ, Stockwell T, eds. International Handbook of Alcohol Dependence and Problems. Chichester, UK: John Wiley & Sons Ltd. 2001:299–316. [Google Scholar]
- 20.Breese GR., Chu K., Dayas CV., et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins RL., Bradizza CM. Social and cognitive learning processes. In: Heather N, Peters TJ, Stockwell T, eds. International Handbook of Alcohol Dependence and Problems. Chichester, UK: John Wiley & Sons. 2001:317–337. [Google Scholar]
- 22.Dackis C., O'Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- 23.Doering WK., Herzenstiel M-N., Krampe H., et al. Persistent alterations of vasopressin and NT-pro-ANP plasma levels in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2003;27:849–861. doi: 10.1097/01.ALC.0000065433.17403.DE. [DOI] [PubMed] [Google Scholar]
- 24.Drobes DJ., Saladin ME., Tiffany ST. Classical conditioning mechanisms in alcohol dependence. In: Heather N, Peters TJ, Stockwell T, eds. International Handbook of Alcohol Dependence and Problems. Chichester, UK: John Wiley & Sons Ltd. 2001:281–298. [Google Scholar]
- 25.Drummond DC., Tiffany ST., Glautier S., Remington B., eds. Addictive Behaviour: Cue Exposure Theory and Practice. Chichester, UK: John Wiley & Sons. 1995 [Google Scholar]
- 26.Ehrenreich H., Schuck J., Stender N., et al. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res. 1997;21:1285–1293. [PubMed] [Google Scholar]
- 27.Finn PR., Zeitouni NC., Pihl RO. Effects of alcohol on psychophysiological hyperreactivity to nonaversive and aversive stimuli in men at high risk for alcoholism. J Abnorm Psychol. 1990;99:79–85. doi: 10.1037//0021-843x.99.1.79. [DOI] [PubMed] [Google Scholar]
- 28.Flannery BA., Roberts AJ., Cooney N., Swift RM., Anton RF., Rohsenow DJ. The role of craving in alcohol use, dependence, and treatment. Alcohol Clin Exp Res. 2001;25:299–308. [PubMed] [Google Scholar]
- 29.Gifford E., Humphreys K. The psychological science of addiction. Addiction. 2007;102:352–361. doi: 10.1111/j.1360-0443.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 30.Greeley J., Oei T. Alcohol and tension reduction. In: Leonard KE, Blane HT, eds. Psychological Theories of Drinking and Alcoholism. 2nd ed. New York, NY: The Guilford Press. 1999:14–53. [Google Scholar]
- 31.Hasselblatt M., Martin F., Maul O., Ehrenreich H. Persistent macrocytosis following abstinence from chronic alcohol use. JAMA. 2001;286:2946. doi: 10.1001/jama.286.23.2946. [DOI] [PubMed] [Google Scholar]
- 32.Hasselblatt M., Krieg-Hartig C., Hüfner M., Halaris A., Ehrenreich H. Persistent disturbance of the hypothalamic-pituitary-gonadal axis in abstinent alcoholic men. Alcohol Alcohol. 2003;38:239–242. doi: 10.1093/alcalc/agg059. [DOI] [PubMed] [Google Scholar]
- 33.Hasselblatt M., Krampe H., Jacobs S., et al. Arginine challenge unravels persistent disturbances of urea cycle and gluconeogenesis in abstinent alcoholics. Alcohol Alcohol. 2006;41:372–378. doi: 10.1093/alcalc/agl032. [DOI] [PubMed] [Google Scholar]
- 34.Hüttner E., Matthies U., Nikolova T., Ehrenreich H. A follow-up study on chromosomal aberrations in lymphocytes of alcoholics during early, medium, and long-term abstinence. Alcohol Clin Exp Res. 1999;23:344–348. [PubMed] [Google Scholar]
- 35.Jahn H., Doering W., Krampe H., et al. Preserved vasopressin response to osmostimulation despite decreased basal vasopressin levels in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004;28:1925–1930. doi: 10.1097/01.alc.0000148110.34917.c3. [DOI] [PubMed] [Google Scholar]
- 36.Jones S., Bond A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Kendler KS. Twin studies of psychiatric illness: An update. Arch Gen Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- 38.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(suppl1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF., Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 40.Koob G., Kreek M. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koob GF., Sanna PP., Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 42.Lingford-Hughes A., Nutt D. Neurobiology of addiction and implications for treatment. Br J Psychiatry. 2003;182:97–100. doi: 10.1192/bjp.182.2.97. [DOI] [PubMed] [Google Scholar]
- 43.Liu X., Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lubman Dl., Yucel M., Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. 2004;99:1491–1502. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- 45.Marlatt GA., Gordon JR. Relapse Prevention. Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press. 1985 [Google Scholar]
- 46.Monti PM., Rohsenow DJ., Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95:S229–S36. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- 47.Moos RH. Theory-based processes that promote the remission of substance use disorders. Clin Psychol Rev. 2007;27:537–551. doi: 10.1016/j.cpr.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrakis IL., Limoncelli D., Gueorguieva R., et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1182. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- 49.Robbins TW., Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt LG., Dufeu P., Kuhn S., Smolka M., Rommelspacher H. Transition to alcohol dependence: Clinical and neurobiological considerations. Compr Psychiatry. 2000;41:90–94. doi: 10.1016/s0010-440x(00)80014-0. [DOI] [PubMed] [Google Scholar]
- 51.Schmitt M., Gleiter CH., Nichol JL., et al. Haematological abnormalities in early abstinent alcoholics are closely associated with alterations in thrombopoietin and erythropoietin serum profiles. Thromb Haemost. 1999;82:1422–1427. [PubMed] [Google Scholar]
- 52.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 53.Schuckit MA., Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- 54.Schumann G. Okey Lecture 2006: identifying the neurobiological mechanisms of addictive behaviour. Addiction. 2007;102:1689–1695. doi: 10.1111/j.1360-0443.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- 55.Spanagel R. Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol. 2003;17:507–518. doi: 10.1016/s1521-6918(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 56.Spanagel R., Heilig M. Addiction and its brain science. Addiction. 2005;100:1813–1822. doi: 10.1111/j.1360-0443.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 57.Volkow ND., Li T-K. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 58.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Weiss F., Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.vanderVorst H., Engels RCME., Dekovic M., Meeus W., Vermulst AA. Alcohol specific rules, personality and adolescents' alcohol use: a longitudinal person-environment study. Addiction. 2007;102:1064–1075. doi: 10.1111/j.1360-0443.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 61.West R. Theories of addiction. Addiction. 2001;96:3–13. doi: 10.1046/j.1360-0443.2001.96131.x. [DOI] [PubMed] [Google Scholar]
- 62.Zimmermann U., Spring K., Wittchen HU., et al. Arginine vasopressin and adrenocorticotropin secretion in response to psychosocial stress is attenuated by ethanol in sons of alcohol-dependent fathers. J Psychiatr Res. 2004;38:385–593. doi: 10.1016/j.jpsychires.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 63.Finney JW., Moos RH. The long-term course of treated alcoholism: I. Mortality, relapse and remission rates and comparisons with community controls. J Stud Alcohol. 1991;52:44–54. doi: 10.15288/jsa.1991.52.44. [DOI] [PubMed] [Google Scholar]
- 64.Hunter EE., Powell BJ., Penick EC., et al. Comorbid psychiatric diagnosis and long-term drinking outcome. Compr Psychiatry. 2000;41:334–338. doi: 10.1053/comp.2000.8997. [DOI] [PubMed] [Google Scholar]
- 65.Lewis CE., Smith E., Kercher C., Spitznagel E. Assessing gender interactions in the prediction of mortality in alcoholic men and women: a 20-year follow-up study. Alcohol Clin Exp Res. 1995;19:1162–1172. doi: 10.1111/j.1530-0277.1995.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 66.Liskow Bl., Powell BJ., Penick EC., et al. Mortality in male alcoholics after ten to fourteen years. J Stud Alcohol. 2000;61:853–861. doi: 10.15288/jsa.2000.61.853. [DOI] [PubMed] [Google Scholar]
- 67.Moos RH., Moos BS. Risk factors for nonremission among initially untreated individuals with alcohol use disorders. J Stud Alcohol. 2003;64:555–563. doi: 10.15288/jsa.2003.64.555. [DOI] [PubMed] [Google Scholar]
- 68.Ojesjo L., Hagnell O., Otterbeck L. The course of alcoholism among men in the Lundby longitudinal study, Sweden. J Stud Alcohol. 2000;61:320–322. doi: 10.15288/jsa.2000.61.320. [DOI] [PubMed] [Google Scholar]
- 69.Penick EC., Nickel EJ., Powell BJ., et al. The comparative validity of eleven alcoholism typologies. J Stud Alcohol. 1999;60:188–202. doi: 10.15288/jsa.1999.60.188. [DOI] [PubMed] [Google Scholar]
- 70.Powell BJ., Landon JF., Cantrell PJ., et al. Prediction of drinking outcomes for male alcoholics after 10 to 14 years. Alcohol Clin Exp Res. 1998;22:559–566. doi: 10.1111/j.1530-0277.1998.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 71.de Soto CB., O'Donnell WE., de Soto JL. Long-term recovery in alcoholics. Alcohol Clin Exp Res. 1989;13:693–697. doi: 10.1111/j.1530-0277.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 72.Timko C., DeBenedetti A., Moos B., Moos R. Predictors of 16-year mortality among individuals initiating help-seeking for an alcoholic use disorder. Alcohol Clin Exp Res. 2006;30:1711–1720. doi: 10.1111/j.1530-0277.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 73.Vaillant GE. The Natural History of Alcoholism - Revisited. Cambridge, Mass: Harvard University Press. 1995 [Google Scholar]
- 74.Vaillant GE. A long-term follow-up of male alcohol abuse. Arch Gen Psychiatry. 1996;53:243–249. doi: 10.1001/archpsyc.1996.01830030065010. [DOI] [PubMed] [Google Scholar]
- 75.Vaillant GE. A 60-year follow-up of alcoholic men. Addiction. 2003;98:1043–51. doi: 10.1046/j.1360-0443.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 76.Wagner T., Krampe H., Stawicki S., et al. Substantial decrease of psychiatric comorbidity in chronic alcoholics upon integrated outpatient treatment - results of a prospective study. J Psychiatr Res. 2004;38:619–635. doi: 10.1016/j.jpsychires.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Emrick CD. A review of psychologically oriented treatment of alcoholism: I. The use and interrelationships of outcome criteria and drinking behavior following treatment. Q J Stud Alcohol. 1974;35:523–549. [PubMed] [Google Scholar]
- 78.Finney JW., Monahan SC. The cost-effectiveness of treatment for alcoholism: a second approximation. J Stud Alcohol. 1996;57:229–243. doi: 10.15288/jsa.1996.57.229. [DOI] [PubMed] [Google Scholar]
- 79.Holder H., Longabaugh R., Miller WR., Rubonis AV. The cost effectiveness of treatment for alcoholism: a first approximation. J Stud Alcohol. 1991;52:517–540. doi: 10.15288/jsa.1991.52.517. [DOI] [PubMed] [Google Scholar]
- 80.McCrady BS., Langenbucher JW. Alcohol treatment and health care system reform. Arch Gen Psychiatry. 1996;53:737–746. doi: 10.1001/archpsyc.1996.01830080091014. [DOI] [PubMed] [Google Scholar]
- 81.Miller WR., Brown JM., Simpson TL., et al. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, eds. Handbook of Alcoholism Treatment Approaches: Effective Alternatives. 2nd ed. Boston, Mass: Allyn and Bacon. 1995:12–44. [Google Scholar]
- 82.Miller WR., Walters ST., Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 83.Miller WR., Wilbourne PL. Mesa Grande: A methodological analysis of clinical trials of treatment for alcohol use disorders. Addiction. 2002;97:265–277. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 84.Miller WR., Brown JM., Simpson TL., et al. What works? A summary of alcohol tretament outcome research. In: Hester RK, Miller WR, eds. Handbook of Alcoholism Treatment Approaches: Effective Alternatives. 3rd ed. Boston, Mass: Allyn and Bacon. 2003:13–63. [Google Scholar]
- 85.Moos RH., Finney JW., Ouimette PC., Suchinsky RT. A comparative evaluation of substance abuse treatment: I. Treatment orientation, amount of care, and 1-year outcomes. Alcohol Clin Exp Res. 1999;23:529–536. [PubMed] [Google Scholar]
- 86.Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH Posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- 87.Burtscheidt W., Woelwer W., Schwarz R., Strauss W., Gaebel W. Outpatient behaviour therapy in alcoholism: Treatment outcome after 2 years. Acta Psychiatr Scand. 2002;106:227–232. doi: 10.1034/j.1600-0447.2002.02332.x. [DOI] [PubMed] [Google Scholar]
- 88.Miller WR., Meyers RJ., Tonigan JS., Grant KA. Community reinforcement and traditional approaches: findings of a controlled trial. In: Meyers RJ, Miller WR, eds. A Community Reinforcement Approach to Addiction Treatment. Cambridge, Mass: Cambridge University Press. 2001:79–103. [Google Scholar]
- 89.Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH three-year drinking outcomes. Alcohol Clin Exp Res. 1998;22:1300–1311. doi: 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 90.Brewer C. Supervised disulfiram is more effective in alcoholics than naltrexone or acamprosate -or even psychotherapy: how it works and why it matters. Adicciones. 2005;17:285–296. [Google Scholar]
- 91.Ehrenreich H., Krampe H. Does disulfiram have a role in alcoholism treatment today? Not to forget about disulfiram's psychological effects. Addiction. 2004;99:26–27. doi: 10.1111/j.1360-0443.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- 92.Fuller RK., Gordis E. Does disulfiram have a role in alcoholism treatment today? Addiction. 2004;99:21–24. doi: 10.1111/j.1360-0443.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 93.Hughes JC., Cook CCH. The efficacy of disulfiram - a review of outcome studies. Addiction. 1997;92:381–395. [PubMed] [Google Scholar]
- 94.Krampe H., Stawicki S., Wagner T., et al. Follow-up of 180 chronic alcoholic patients for up to seven years after outpatient treatment: impact of alcohol deterrents on outcome. Alcohol Clin Exp Res. 2006;30:86–95. doi: 10.1111/j.1530-0277.2006.00013.x. [DOI] [PubMed] [Google Scholar]
- 95.Suh J., Pettinati H., Kampman K., O'Brien C. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006;26:290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- 96.Carmen B., Angeles M., Ana M., Maria AJ. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependences systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 97.O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 98.Kranzler HR., Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- 99.Mann K., Lehert P., Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- 100.Srisurapanont M., Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 101.Anton R., O'Malley S., Ciraulo D., et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 102.Ehrenreich H., Krampe H., Wagner T., et al. Outpatient long-term intensive therapy for alcoholics, “OLITA”: re-considering severe alcoholism, disease and treatment. Suchtmedizin in Forschung und Praxis. 2000;2:221–222. [Google Scholar]
- 103.Burke BL., Arkowitz H., Menchola M. The efficacy of motivational interviewing: A meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 104.Moyer A., Finney JW., Swearingen CE., Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 105.Vasilaki El., Hosier SG., Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol. 2006;41:328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- 106.Ehrenreich H., Mangholz A., Schmitt M., et al. OLITA: An alternative in the treatment of therapy-resistant chronic alcoholics. First evaluation of a new approach. Eur Arch Psychiatry Clin Neurosci. 1997;247:51–54. doi: 10.1007/BF02916253. [DOI] [PubMed] [Google Scholar]
- 107.Krampe H., Wagner T., Küfner H., et al. Therapist rotation - a new element in the outpatient treatment of alcoholism. Subst Use Misuse. 2004;39:135–178. doi: 10.1081/ja-120027769. [DOI] [PubMed] [Google Scholar]
- 108.Krampe H., Wagner T., Stawicki S., et al. Personality disorder and chronicity of Addiction, as independent outcome predictors in alcoholism treatment. Psychiatr Serv. 2006;57:708–712. doi: 10.1176/ps.2006.57.5.708. [DOI] [PubMed] [Google Scholar]
- 109.Gsellhofer B., Küfner H., Vogt M., Weiler D. European Addiction. Severity Index - EuropASI. Baltmannsweiler: Schneider-Verlag Hohengehren. 1999 [Google Scholar]
- 110.McLellan AT., Kushner H., Metzger D., et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 111.Bowen RC., D'Arcy C., Keegan D., Senthilselvan A. A controlled trial of cognitive behavioral treatment of panic in alcoholic inpatients with comorbid panic disorder. Addict Behav. 2000;25:593–597. doi: 10.1016/s0306-4603(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 112.Randall CL., Thomas S., Thevos AK. Concurrent alcoholism and social anxiety disorder: A first step toward developing effective treatments. Alcohol Clin Exp Res. 2001;25:210–220. [PubMed] [Google Scholar]
- 113.Bartels C., Kunert H-J., Stawicki S., Kroener-Herwig B., Ehrenreich H., Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored longterm abstinence. Alcohol Alcohol. 2007;42:92–102. doi: 10.1093/alcalc/agl104. [DOI] [PubMed] [Google Scholar]
- 114.Krampe H., Stawicki S., Ribbe K., et al. Development of an outcome prediction measure for alcoholism therapy by multimodal monitoring of treatment processes. J Psychiatr Res. 2007. In press doi: 10.1016/j.jpsychires.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 115.Alexander LB., Luborsky L. The Penn helping alliance scales. In: Green berg LS, Pinsof WM, eds. The Psychotherapeutic Process: a Research Handbook. New York, NY: The Guilford Press. 1986:325–366. [Google Scholar]
- 116.Luborsky L., McLellan AT., Woody GE., O'Brien CP., Auerbach A. Therapist success and its determinants. Arch Gen Psychiatry. 1985;42:602–611. doi: 10.1001/archpsyc.1985.01790290084010. [DOI] [PubMed] [Google Scholar]
- 117.Horvath AO. The therapeutic relationship: Research and theory. Psychother Res. 2005;15:3–7. [Google Scholar]
- 118.Belding M., Iguchi M., Morral A., McLellan A. Assessing the helping alliance and its impact in the treatment of opiate dependence. Drug Alcohol Depend. 1997;48:51–59. doi: 10.1016/s0376-8716(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 119.Fenton L., Cecero J., Nich C., Frankfurter T., Carroll K. Perspective is everything: The predictive validity of six working alliance instruments. Journal Psychother Pract Res. 2001;10:262–268. [PMC free article] [PubMed] [Google Scholar]
- 120.Meier P., Donmall M. Differences in client and therapist views of the working alliance in drug treatment. J Substance Use. 2006;11:73–80. [Google Scholar]
- 121.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]