Abstract
Although the pharmacology of amphetamine-like psychostimulants at dopamine transporters is well understood, addiction to this class of drugs has proven difficult to deal with. The reason for this disconnection is that while the molecular mechanism of amphetamine action is critical to reinforce drug use, it is only the first step in a sequence of widespread neuroplastic events in brain circuitry. This review outlines the affect of psychostimulants on mesocorticolimbic dopamine projections that mediate their reinforcing effect, and how this action ultimately leads to enduring pathological neuroplasticity in glutamatergic projections from the prefrontal cortex to the nucleus accumbens. Molecular neuroadaptations induced by psychostimulant abuse are described in glutamate neurotransmission, and from this information potential pharmacotherapeutic targets are identified, based upon reversing or countermanding psychostimulant-induced neuroplasticity.
Keywords: cocaine, glutamate, amphetamine, accumbens, prefrontal cortex, dopamine
Abstract
Aunque es bien conocida la farmacología de los psicoestimulantes tipo anfetamina a nivel de los transportadores de dopamina, ha sido difícil abordar la adicción a esta clase de drogas. La razón de esta discordancia se explica porque si bien el mecanismo molecular de la acción de la anfetamina es crítico para reforzar el uso de la droga, éste representa sólo el primer paso en una secuencia de numerosos acontecimientos neuroplásticos en los circuitos cerebrales. Esta revisión resume el efecto de los psicoestimulantes en las proyecciones mesocorticolímbicas de dopamina que median el efecto de refuerzo, y cómo esta acción en último término conduce a una neuroplasticidad patológica permanente en las proyecciones glutamatérgicas desde la corteza prefrontal hasta el núcleo accumbens. Se describen las neuroadaptaciones moleculares inducidas por el abuso de psicoestimulantes en la neurotransmisión glutamatérgica, y a partir de esta información se identifican potenciales blancos farmacoterapéuticos, en base a las modificaciones en la neuroplasticidad inducida por psicoestimulantes.
Abstract
En dépit d'une bonne compréhension de la pharmacologie des psychostimulants amphétaminoïdes au niveau des transporteurs de la dopamine, il semble difficile de faire face à l'addiction à cette classe de médicaments. Cette discordance s'explique ainsi : si le mécanisme moléculaire de l'action amphétaminique est essentiel pour renforcer l'action du médicament, il ne représente qu'une première étape dans une succession de nombreux événements neuroplastiques dans le circuit cérébral. Cette revue souligne l'effet des psychostimulants sur les projections méso-cortico-limbiques dopaminergiques qui médient l'effet de consolidation et explique comment cette action mène finalement à une neuroplasticité pathologique persistante dans les projections glutamatergiques, du cortex préfrontal au noyau accumbens. Les neuroadaptations moléculaires induites par l'abus des psychostimulants sont décrites en ce qui concerne la neurotransmission du glutamate, et des cibles pharmacothérapeutiques potentielles sont identifiées à partir de ces informations, basées sur la neuroplasticité réversible ou annulable induite par les psychostimulants.
Background
Cocaine and other amphetamine-like psychostimulants have been a significant part of the human pharmacopoeia for thousands of years.1,2 However, the appearance of these substances in Western societies has been relatively recent, cocaine having debuted as both a local anesthetic and a psychostimulant in the 19th century. Over the course of the next century, it became increasingly clear that the amphetamine-like psychostimulants carried serious abuse liability, as well as producing a prominent paranoia-like syndrome among many individuals who chronically used this class of drugs.3,4 The abuse liability of these drugs has resulted in sociological use patterns that have been described as epidemics, such as the methamphetamine epidemic in Japan in the 1950s, the cocaine epidemic in the United States in the 1980s, and the crack cocaine epidemic of the 1990s.5,6
The high abuse liability of this class of drugs relies on both pharmacological properties and the sociological characteristics of how the drugs are introduced into various societies around the world. This article will not significantly address the sociology of psychostimulant abuse, which involves diverse events ranging from the use of amphetamines by Japanese soldiers in World War II, to the formulation of crack as a less expensive version of cocaine in the United States, to the introduction of prescription formulations to regulate eating habits or to treat attention deficit-hyperactivity disorder. 5-8 Rather, we will review the basic pharmacology of amphetamine-like drugs, integrate these molecular mechanisms into the brain circuitry of reward, and describe how these drugs are thought to create pathological changes in reward and learning circuitry Finally, this knowledge will be amalgamated into a vision of future pharmacotherapies for treating psychostimulant addiction.
Basic pharmacology of amphetamine-like psychostimulants
The defining mechanism of action of amphetamine4ike psychostimulants as a class of drugs with high abuse liability is the ability to bind to dopamine transporters (DAT).9,10 Dopamine transporters are a member of a class of proteins that eliminate monoamines, including dopamine, from the synaptic cleft after neuronal release.11 This protein has a high affinity for dopamine relative to other monoamines, such as norepinephrine or serotonin, and while all the readily abused psychostimulants bind to DAT, they may also bind to the other monoamine transporters with greater or lesser affinity.9,12 To some extent, the relative profile of binding by individual drugs to the different transporter proteins explains different characteristics of the drugs. Most striking, for example, is 3,4-methylenedioxymethamphetamine (MDMA) which has a relatively higher affinity for serotonin transporters, and is thereby a mild hallucinogen and neurotoxic to serotonin axon terminals,13,14 while methamphetamine binds more avidly to DAT, which explains its greater toxicity at dopamine terminals, as well as its propensity to induce paranoid psychosis-like symptoms.15 While the binding to other monoamine transporters contributes to the antidepressant and hallucinogenic characteristics of some psychostimulants, it is the binding to DAT that provides the major influence on abuse liability, which is the focus of this review.
There are two major categories of interaction by ampetamine-like psychostimulants with DAT, but in all cases the end result is to inhibit the elimination of dopamine from the synapse and thereby increase the quantity and half-life of synaptic and extrasynaptic dopamine levels.16,17 The first mechanism is typified by cocaine and methylphenidate that bind to DAT, but are not transported into the presynaptic terminal as surrogate dopamine. Therefore, when these drugs bind to DAT the increase in extracellular dopamine relies primarily on normal synaptic release, which is more amenable to physiological feedback regulation.18 The second mechanism is typified by amphetamines, and involves not only binding to DAT, but also translocation into the cell in place of dopamine.9 In addition, these drugs enter dopamine synaptic vesicles, where the fact that these compounds are basically charged degrades the pH gradient necessary to sequester dopamine into the vesicle.19 This in turn results in a large buildup of dopamine in the cytosol, thereby reversing the direction of DAT to release dopamine into the extracellular space rather than facilitating its removal Regardless of the precise interaction with DAT by individual amphetamine-like psychostimulants, this class of drugs dramatically elevates extracellular dopamine, and this action is thought to be the initiating molecular event that reinforces drugseeking behaviors, ultimately culminating in addiction.20,21
How release of dopamine by psychostimulants initiates addiction
Dopamine release is physiologically employed to signal novel, motivationally relevant environmental events. Thus, when an organism encounters a novel stimulus, whether a positive stimulus such as a food reward or a negative stimulus such as a stressor, the activity of dopamine cells in the ventral tegmental area, and dopamine release in axon terminal fields in the prefrontal cortex, nucleus accumbens, and/or amygdale, are altered.22-24 An important characteristic of this brain-environment interaction is that the ability of a given stimulus to increase dopamine cell firing and release decreases with repeated presentation of the stimulus. However, it can be shown that if a motivationally neutral stimulus (such as a light or tone) is associated with the motivational event in such a manner that the neutral stimulus predicts arrival of the motivational event, the ability of the motivational stimulus to release dopamine is transferred to the neutral stimulus.22,25,26 Thus, the neutral stimulus now causes dopamine release in a manner predicting arrival of a motivationally relevant event. Based upon these data, the role for dopamine release in the mesocorticolimbic brain regions is twofold: (i) to cue the organism that a novel motivationally relevant event is occurring and that adaptive behavioral responses need to be engaged (eg, approach a reward or avoid a stress); (ii) once the behavioral response is established, dopamine release is antecedent to the appearance of the motivationally relevant event and is triggered by environmental associations that the organism has made with the event as part of learning the adaptive behavioral response. In this way, dopamine serves to alert and thereby prepare the organism for an impending important event.
The primary differences between psychostimulant-induced dopamine release and release associated with normal learning about important environmental events such as rewards and stressors is: (i) since psychostimulants block the elimination of dopamine through DAT, the level of dopamine achieved far exceeds what is possible from a biological stimulus; (ii) in contrast to biological stimuli that cease to release dopamine once an approach or avoidance response to that stimulus has been learned, psychostimulants continue to release large amounts of dopamine upon every administration (with the possible exception of extended binging that can temporarily deplete dopamine stores).27 Thus, with psychostimulants, each administration releases dopamine into mesocorticolimbic regions, causing further associations to be made between the drug experience and the environment. In this way, it is thought that the more a psychostimulant is administered, the more learned associations are made with the environment and the more effective the environment becomes at triggering craving and drug-seeking. It is this “overlearning” of drugseeking behaviors by progressive associations formed between repeated drug-induced dopamine release and the environment that is thought to lead to increased vulnerability to relapse.
How psychostimulant-induced dopamine release creates pathological neuroplasticity in cortical regulation of behavior
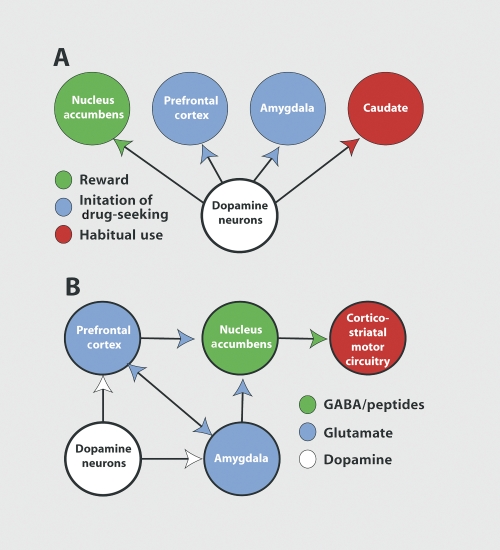
As outlined above, psychostimulant-induced dopamine release is responsible for reinforcing behaviors designed to seek and administer the drugs. The dopamine projections involved in this process are outlined in Figure 1A, and as indicated, the most critical projection in this regard is the projection from the ventral tegmental area dopamine cells to the nucleus accumbens.28-31 For example, if psychostimulant-induced release of dopamine in the nucleus accumbens is impaired, this affects the acquisition of drug-seeking behaviors, and can markedly influence the amount of drug taken in a well-trained subject. Thus, the learning of a task to obtain the drug and the amount of drug taken in a given session is strongly regulated by dopamine release in the accumbens. However, when an animal has been withdrawn from repeated psychostimulant use, and drug-seeking is initiated by an environmental stimulus such as a cue previously paired with drug delivery, or a novel stressor, it is dopamine release in the prefrontal cortex and amygdala, respectively, that mediates the reinstatement of drug-seeking.32,33 Thus, relapse can be induced by dopamine release in prefrontal and allocortical brain regions, and reflects the aforementioned physiological role of dopamine release as a predictive antecendent to stimulus (drug) delivery. What this implies is that chronic release of dopamine by repeated psychostimulant administration may be modifying cortical and allocortical regulation of behavior.
Figure 1. Models of the circuitry regulating the transition from psychostimulant reward to relapse. A. Dopamine projections and how chronic psychostimulant use produces a transition from reliance on accumbens dopamine for drug reinforcement, to reliance on the prefrontal and amygala dopamine to trigger relapse, to dopamine in the caudate in regulating habit responding. B. The circuitry in which dopamine projections are embedded that initiates relapse to drug-taking. Note that dopamine input to the amygdala and prefrontal cortex is critical, as is the glutamatergic output from these regions to the nucleus accumbens. GABA, γ-aminobutyric acid.
Figure 1B shows that the cortical and allocortical regulation of behavior is primarily mediated by glutamatergic projections. These projections are to subcortical structures, such as the nucleus accumbens and dopamine cells In the ventral tegmental area, as well as between the cortical and allocortical regions. Thus, when dopamine Is released Into the prefrontal cortex or amygdala by a drug-associated cue or stressor, this is thought to stimulate glutamatergic projections between the prefrontal cortex and amygdala, as well as glutamatergic outputs to the accumbens and ventral tegmental area.34 A variety of studies have linked this activation of corticofugal glutamate transmission with craving In psychostimulant addicts or drug-seeking In animal models of addiction. The neuroimaging literature clearly shows metabolic activation of regions of the prefrontal cortex, including portions of the anterior cingulate and ventral orbital cortices, and the amygdala during cue-induced craving for amphetamine-like psychostimulants.35-39 Interestingly, while a cue or low dose of psychostimlant markedly increases metabolic activity in the prefrontal cortex and amygdala, in the absence of a learned drug association the prefrontal cortex is hypoactive.40 The reduction in basal metabolic activity is taken to indicate a potential deficit in cognitive ability to regulate relapse, and recent cognitive testing in psychostimulant addicts confirms the presence of certain cognitive dysfunctions related to impulse control and switching behaviors in an adaptive manner to changing environmental circumstances.41-45 A strong role for activation of both the prefrontal cortex and amygdala has been confirmed in animal studies. Thus, pharmacological inhibition of either of these regions prevents the reinstatement of drug-seeking in animals withdrawn from drugs that have undergone extinction training.46-48 Moreover, a marked release of glutamate is measured in the nucleus accumbens of animals initiating drug-seeking in response to a stressor, and this glutamate is derived from increased activity in the projection from the prefrontal cortex to the nucleus accumbens.49,50 Accordingly, drug-seeking is abolished by inhibiting glutamate receptors in the accumbens.51-53
One final set of studies to be considered regarding cortical glutamate is the recent evidence that as drug-seeking becomes more compulsive there is a gradual shift to greater reliance on corticostriatal habit circuitry, and less involvement of prefrontal to accumbens circuitry.54 This possibility is supported by animal models in two ways: (i) if animals that have been trained to self-administer cocaine are left in abstinence for an extended period, drug-seeking is augmented,55 and in this case inhibition of the prefrontal cortex or amygdala no longer inhibits drug-seeking induced by drug-associated stimuli. However, inhibition of the dorsolateral striatum is still effective at blocking drug-seeking56; (ii) as training of an animal in drug-seeking paradigms progresses it is possible to show a gradual increase in dopamine released into the caudate in favor of release into the nucleus accumbens.57 This is illustrated in Figure 1A, showing that dopamine release into the caudate can regulate habitual behaviors. On one hand, these data point to the possibility that in treating compulsive relapse we should be focusing on regulation of corticostriatal habit circuitry, including glutamate input to the caudate from sensorymotor cortex and dopamine input from the substantia nigra. However, these studies have been conducted in rats in whom the frontal cortex is poorly evolved, and given the marked activation produced in the prefrontal cortex and amygdala by drug-associated stimuli in psychostimulant addicts, the conclusion that compulsive relapse is entirely derived from corticostriatal habit circuitry may be an oversimplification. Indeed, it has been argued that a primary role for therapy in treating addiction is to strengthen prefrontal regulation of drug-seeking behaviors, whether through psychosocial interventions or pharmacotherapy.27,58,59
Enduring psychostimulant-induced neuroplasticity in the prefrontal to accumbens glutamate projection
Given the apparent critical role played by glutamatergic afférents to the nucleus accumbens in initiating drugseeking or craving, recent studies have identified a number of enduring cellular changes in glutamate transmission that may be critical pathological neuroadaptations to psychostimulant use, and may serve as targets for pharmcotherapeutic intervention. In general the neuroplasticity can be categorized as postsynaptic, presynaptic and nonsynaptic (ie, residing predominantly in glia). However, since these processes are intimately related to each other, it is perhaps best to consider all the adaptations as changes in glutamate homeostasis, the end result of which is a psychostimulant-induced enduring change in the fidelity of communication between the prefrontal cortex and the nucleus accumbens, and the regulation by this projection of corticostriatal habit circuitry. It has been proposed that this loss of fidelity results in a weakening or loss in the capacity of psychostimulant addicts to cognitively intervene in habitual behaviors, thereby making drug-seeking more difficult to control and increasing the vulnerability to relapse.27
As mentioned above, drug-seeking is associated with a large release of prefrontal glutamate into the nucleus accumbens. The large release of glutamate during drugseeking is all the more remarkable because it was discovered using microdialysis. which is not a very sensitive measure of glutamate transmission.60 Indeed, when animals are trained to seek a biological reward, such as food, microdialysis cannot measure glutamate release.49 Thus, the large psychostimulant-induced release of glutamate has been hypothesized to be a pathological and perhaps critical mediator of relapse. This hypothesis is supported by the fact that treatments interrupting synaptic glutamate release also inhibit drug-seeking. This includes a variety of pharmacological treatments that have the potential to be developed into pharmacotherapeutic agents, as outlined below.
Perhaps in part a consequence of the massive synaptic glutamate release occurring during psychostimulantseeking behavior, a number of marked changes in postsynaptic glutamate transmission have been measured in animals withdrawn from chronic cocaine or amphetamine administration. Perhaps among the most dramatic is an increase in the density of dendritic spines in the nucleus accumbens.61 Importantly, this appears to be accompanied by an increase in the insertion of a-amino3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) glutamate receptors into the membrane of spiny neurons in the accumbens,62 and is associated with an increase in electrophysiological sensitivity to AMPA receptor stimulation (as measured by the AMPA:N-methyl D-aspartate [NMDA] ratio).63 Moreover, a number of other proteins regulating the fidelity of postsynaptic glutamate transmission are altered after chronic cocaine use, including proteins that regulate the structure and function of the protein scaffolding in which the glutamate receptors are embedded, including postsynaptic density (PSD)-95 and Homer proteins, among others.64,65 Also, in addition to AMPA ionotropic glutamate receptors, signaling through metabotropic glutamate receptors is downregulated.66,67 Finally, this psychostimulant-induced postsynaptic neuroplasticity is associated with changes in the biochemical machinery regulating spine formation, notably an increase in actin cycling and formation of Factin (a primary structural protein regulating spine morphology and the insertion of proteins into and out of the membrane).68 Taken together, these findings indicate that significant changes have been produced by psychostimulants in the way that synaptically released glutamate will be interpreted by postsynaptic cells. However, it is important to note that this knowledge is nascent and emerging. Thus, there remain many apparent contradictions in the literature regarding changes in specific proteins, and in the overall direction of synaptic grading (ie, is postsynaptic glutamate transmission augmented or inhibited by chronic psychostimulant administration).69 Therefore, for now it is probably not prudent to speculate on the type of drug development that may arise from this particular direction of research into psychostimulant-induced changes in glutamate signaling.
Ideas for pharmacotherapies based upon psychostimulant-induced plasticity in glutamate transmission
As outlined above, given our current state of knowledge it is more likely that pharmacotherapeutic restoration of normal glutamate release may be a more successful approach than manipulating postsynaptic proteins responsible for and/or associated with changes in the fidelity of postsynaptic glutamate transmission. In part, this is due to the relatively contradictory status of the emerging literature on postsynaptic plasticity. Moreover, it has been hypothesized that the adaptations in presynaptic glutamate release may be at least partly causal in the postsynaptic adaptations, posing the possibility that if the pathological release of glutamate can be successfully ameliorated, postsynaptic normalization may follow.27
Pharmacotherapeutic targets for regulating the pathological synaptic glutamate release seen in the accumbens of psychostimulant-seeking animals can be placed into two categories: (i) targets based upon psychostimulant induced changes in proteins regulating synaptic glutamate release; (ii) proteins that produce a general decrease in excitatory transmission. Compounds in the first category are likely to be the most specific for psychostimulant addiction, and perhaps carry the least number of unwanted side effects, while the latter category may be less selective not only regarding effects on other addictive drugs, but also in terms of unwanted side effects. Table I lists some potential pharmacotherapeutic targets according to these two categories.
Table I. List of compounds that affect glutamate neurotransmission with potential pharmacotherapeutic value in treating addiction to amphetamine-like psychostimulants. mGluR2/3, metabotropic glutamate receptors; GABA, γ-aminobutyric acid; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid.
| Directly reducing glutamate transmission | Indirectly inhibiting glutamate transmission | ||
| Compound | Target | Compound | Target |
| N-acetylcysteine | Cystine/glutamate exchanger | Baclofen | GABA-b receptor |
| β-lactam | Glutamate transporter | Topiramate | GABA-a and AMPA |
| mGluR2/3 agonist | mGluR2/3 | Vigabatrin | GABA transaminase |
| Modafinil | mGluR2/3 |
Neuroplasticity produced by chronic cocaine administration that could potentially contribute to pathological glutamate release includes downregulation of cystine-glutamate exchange, downregulation of glial glutamate transporters, and downregulation of release-regulating presynaptic metabotropic glutamate receptors (mGluR2/3). Importantly, these three changes are interrelated due to the cystine-glutamate exchanger and glutamate transporter regulating extrasynaptic glutamate tone on release regulating mGluR2/3.70,71 Drugs have been examined in animal models of psychostimulant addiction, and to a lesser extent in clinical trials with cocaine addicts that regulate one or more of these processes. For example, N-acetylcysteine upregulates cystine glutamate exchange, and has been shown in animal models to prevent synaptic glutamate release associated with drug-seeking, restore inhibitory tone on synaptic release through activation of mGluR2/3, and to inhibit the desire for cocaine in a double-blind cue-reactivity trial in non-treatment-seeking cocaine addicts.71-73 Also, mGluR2/3 agonists have proven effective at inhibiting cocaine seeking in animal models; however, unlike Nacetylcysteine, food-seeking was inhibited at only a 3- to 10-fold increase in dose relative to inhibiting cocaineseeking.74,75 Although no studies have yet evaluated regulating glutamate transport in drug-seeking models of psychostimulant addiction, recent reports of the use of β-lactam antibiotics to increase glutamate transporter membrane insertion poses an interesting possibility for pharmacologically overcoming the cocaine-induced downregulation of glutamate transporters. Finally, while the mechanism is not clear, modafinil has been reported to increase extracellular glutamate levels, which would restore tone on release inhibiting mGluR2/3.76 Notably, modafinil has been found to successfully decrease cocaine relapse in a number of clinical trials.77,78
The primary drugs in the category of nonspecific inhibitors of synaptic glutamate release include a variety of γ-aminobutyric acid (GABA)-mimetic compounds. These range from relatively specific agonists at GABAb receptors, such as baclofen, which inhibit synaptic glutamate release to a host of less selective compounds known to increase GABA transmission via interactions with synthetic or elimination mechanisms, such as topiramate or vigabatrin. For all of these compounds there is preclinical and clinical data to support some potential efficacy.79-85 However, as predicted, especially for the nonselective GABAmimetics untoward side effects, such as sedation, are reported.
Conclusions
This review has endeavored to transport the reader from the initiating molecular actions of amphetamine-like psychostimulants on dopamine systems in the brain to enduring neuroplasticity produced in glutamate transmission responsible for communicating from prefrontal and allocortical brain regions through the nucleus accumbens to motor regulatory systems. Moreover, by examining molecular neuroplasticity produced in excitatory synapses by chronic psychostimulant administration, it is possible to make some deductions about potential pharmacotherapeutic interventions. Indeed, there already exists an emerging literature supporting this approach in developing potential pharmacotherapies for treating psychostimulant addiction. Importantly, this is a nascent and emerging science, and while much has been discovered, the cutting edge of discovery into the neuroplasticity produced by psychostimulants is understandably contradictory. As further discoveries are made that allow us to understand the nature of these contradictions, it should follow that additional targets will emerge to provide potential novel pharmacotherapies for treating psychostimulant addiction.
Selected abbreviations and acronyms
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- DAT
dopamine transporters
- GABA
γ-aminobutyric acid
- MDMA
3,4-methylenedioxymethamphetamine
- mGluR2/3
metabotropic glutamate receptors
A portion of the research described in this article was supported by USPHS grants DA03906, DA12513, DA015851, DA11809 and DA015369.
REFERENCES
- 1.Aldrich M., Barker R. Cocaine: Chemical, Biological, Social and Treatment Aspects. Cleveland, Oh: CRC Press. 1976 [Google Scholar]
- 2.Aguayo LG., Guzman L., Perez C., Aguayo LJ., Silva M., Becerra J., Fuentealba J. Historical and current perspectives of neuroactive compounds derived from Latin America. Mini Rev Med Chem. 2006;6:997–1008. doi: 10.2174/138955706778195144. [DOI] [PubMed] [Google Scholar]
- 3.Ellinwood EH. Amphetamine psychosis. I. Description of the individuals and process. J Nerv Ment Dis. 1967;144:273–283. [Google Scholar]
- 4.Tatetsu S., Goto A., Fujiwara T. The Methamphetamine Psychosis. Tokyo: Igakushoin. 1956 [Google Scholar]
- 5.Suwanwela C., Poshyachinda V. Drug abuse in Asia. Bull Narc. 1986;38:41–53. [PubMed] [Google Scholar]
- 6.Hartman DM., Golub A. The social construction of the crack epidemic in the print media. J Psychoactive Drugs. 1999;31:423–433. doi: 10.1080/02791072.1999.10471772. [DOI] [PubMed] [Google Scholar]
- 7.Douglas JG., Munro JF. Drug treatment and obesity. Pharmacol Ther. 1982;18:351–373. doi: 10.1016/0163-7258(82)90037-7. [DOI] [PubMed] [Google Scholar]
- 8.Arria AM., Wish ED. Nonmedical use of prescription stimulants among students. Pediatr Ann. 2006;35:565–571. doi: 10.3928/0090-4481-20060801-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiden LS., Sabol KE., Ricuarte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 10.Ritz MC., Lamb RJ., Goldberg SR., Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 11.Sonders MS., Zhu S-J., Zahniser NR., Kavanaugh MP., Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritz MC., Cone EJ., Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- 13.White SR., Obradovic T., Imel KM., Wheaton MJ. The effects of methyl-enedioxymethamphetamine (MDMA, “ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 14.Morton J. Ecstasy: pharmacology and neurotoxicity. Curr Opin Pharmacol. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 15.McCann UD., Ricaurte GA. Amphetamine neurotoxicity: accomplishments and remaining challenges. Neurosci Biobehav Rev. 2004;27:821–826. doi: 10.1016/j.neubiorev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Wayment HK., Schenk JO., Sorg BA. Characterization of extracellular dopamine clearance in the medial prefrontal cortex: role of monoamine uptake and monoamine oxidase inhibition. J Neurosci. 2001;21:35–44. doi: 10.1523/JNEUROSCI.21-01-00035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JEG., Wieczorek W., WiIIner P., Kruk ZL. Parametric analysis of the effects of cocaine pretreatment on dopamine release in nucleus accumbens measured by fast cyclic voltammetry. Brain Res. 1996;678:225–232. doi: 10.1016/0006-8993(95)00188-v. [DOI] [PubMed] [Google Scholar]
- 18.White FJ., Wachtel SR., Johansen PA., Einhorn LC. Electrophysiological studies in the rat mesoaccumbens dopamine system: focus on dopamine receptor subtypes, interactions, and the effects of cocaine. In: Chiodo LA, Freeman AS, eds. Neurophysiology of Dopaminergic Systems: Current Status and Clinical Perspectives. Detroit, Mich: Lakeshore. 1987:317–365. [Google Scholar]
- 19.Sulzer D., Maidment NT., Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60:527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 20.Koob GF., Ahmed SH., Boutrel B., et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TE., Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 22.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 23.Spanagel R., Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 24.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 25.Schultz W. Predictive reward signal of dopamine neurons. Am J Physiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Phillips PE., Stuber GD., Heien ML., Wightman RM., Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 27.Kalivas PW., O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacol Rev. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 28.Caine SB., Koob GF. Effects of dopamine D1 and D2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacology Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- 29.Taylor JR., Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacol. 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- 30.Wise RA., Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 31.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 32.Capriles N., Rodaros D., Sorge RE., Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl). 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 33.See RE., Kruzich PJ., Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl). 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- 34.Kalivas PW., Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein RA., Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breiter HC., Gollub RL., Weisskoff RM., et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 37.Maas LC., Lukas SE., Kaufman MJ., et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 38.London ED., Bonson KR., Ernst M., Grant S. Brain imaging studies of cocaine abuse: implications for medication development. Crit Rev Neurobiol. 1999;13:227–242. doi: 10.1615/critrevneurobiol.v13.i3.10. [DOI] [PubMed] [Google Scholar]
- 39.Childress AR., Mozley PD., McElgin W., Fitzgerald J., Reivich M., O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND., Wang GJ., Ma Y., et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ernst M., Grant SJ., London ED., Contoreggi CS., Kimes AS., Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- 42.Paulus MR., Hozack NE., Zauscher BE., et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain SR., MuIIer U., Robbins TW., Sahakian BJ. Neuropharmacological modulation of cognition. Curr Opin Neurol. 2006;19:607–612. doi: 10.1097/01.wco.0000247613.28859.77. [DOI] [PubMed] [Google Scholar]
- 44.Dalley JW., Laane K., Pena Y., Theobald DE., Everitt BJ., Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl). 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- 45.Kantak KM., Udo T., Ugalde F., Luzzo C., Di Pietro N., Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology (Berl). 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- 46.McFarland K., Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaughlin J., See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl). 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 48.Kantak KM., Black Y., Valencia E., Green-Jordan K., Eichembaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McFarland K., Lapish CC., Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McFarland K., Davidge SB., Lapish CC., Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park WK., Bari AA., Jey AR., et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Ciano P., Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 53.Cornish J., Kalivas P. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89(1-5). doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Everitt BJ., Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 55.Grimm JW., Hope BT., Wise RA., Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuchs RA., Branham RK., See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito R., Dalley JW., Howes SR., Robbins TW., Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jentsch K., Taylor J. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacol. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 59.Volkow ND., Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 60.Timmerman W., Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 61.Robinson TE., Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Boudreau AC., Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kourrich S., Rothwell PE., Klug JR., Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swanson C., Baker D., Carson D., Worley P., Kalivas P. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: A potential role for Homer 1 b/c. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao WD., Gainetdinov RR., Arbuckle Ml., et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 66.Bowers MS., McFarland K., Lake RW., et al. Activator of G-protein signaling 3: a gatekeeper of cocaine sensitization and drug-seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xi ZX., Ramamoorthy S., Baker DA., Shen H., Samuvel DJ., Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 68.Toda S., Shen HW., Peters J., Cagle S., Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalivas PW., Hu XT. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29:610–616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 71.Moran MM., McFarland K., Melendez Rl., Kalivas PW., Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker DA., Xi ZX., Shen H., Swanson CJ., Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LaRowe S., Myrick H., Hedden S., et al. Cocaine desire is reduced by Nacetylcysteine. Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 74.Baptista MA., Martin-Fardon R., Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters J., Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl). 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 76.Ferraro L., Antonelli T., O'Connor WT., Tanganelli S., Rambert FA., Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998;253:135–138. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- 77.Dackis CA., Kampman KM., Lynch KG., Pettinati HM., O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 78.Ballon JS., Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 79.Paul M., Dewey SL., Gardner EL., Brodie JD., Ashby CR., Jr. Gamma-vinyl GABA (GVG) blocks expression of the conditioned place preference response to heroin in rats. Synapse. 2001;41:219–220. doi: 10.1002/syn.1078. [DOI] [PubMed] [Google Scholar]
- 80.Vocci F., Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 81.Cubells JF. Topiramate for cocaine dependence. Curr Psychiatry Rep. 2006;8:130–131. doi: 10.1007/s11920-006-0011-5. [DOI] [PubMed] [Google Scholar]
- 82.Brebner K., Childress AR., Roberts DC. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- 83.Ranaldi R., Poeggel K. Baclofen decreases methamphetamine self-administration in rats. Neuroreport. 2002;13:1107–1110. doi: 10.1097/00001756-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 84.Sofuoglu M., Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- 85.Haney M., Hart CL., Foltin RW. Effects of baclofen on cocaine selfadministration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1814–1821. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]